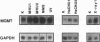Abstract
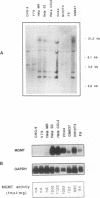
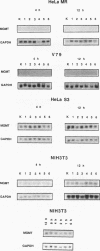
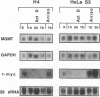
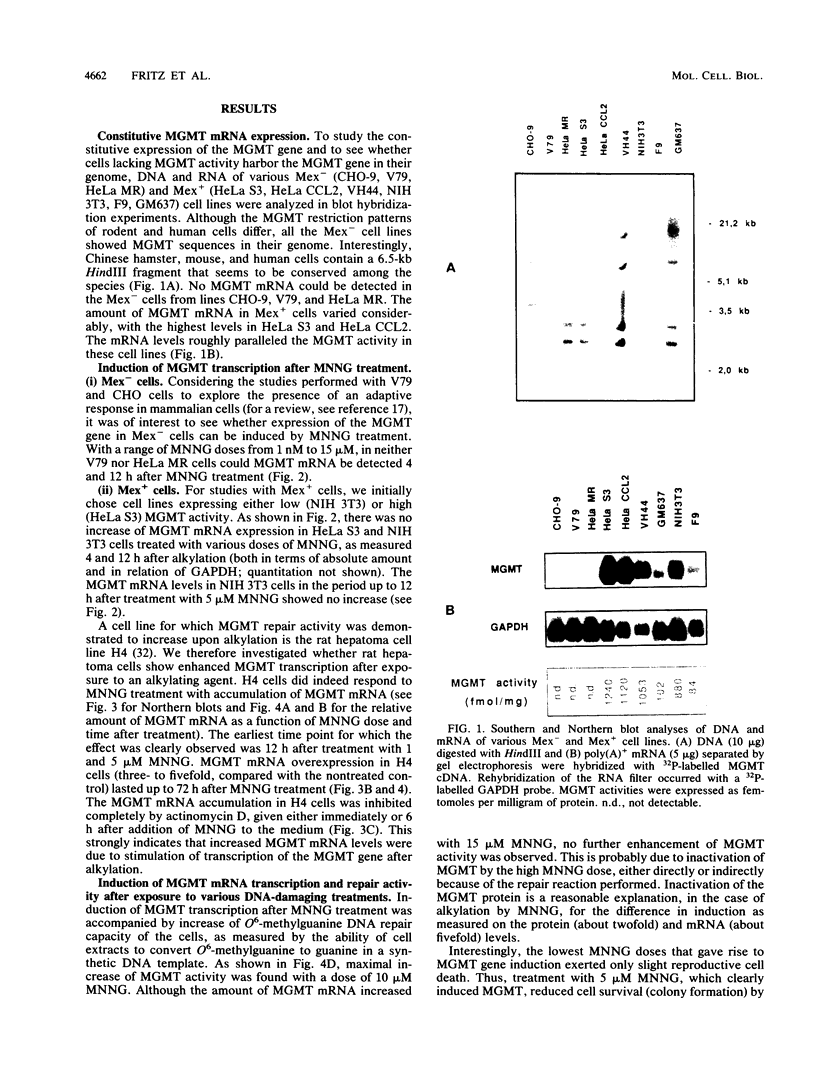
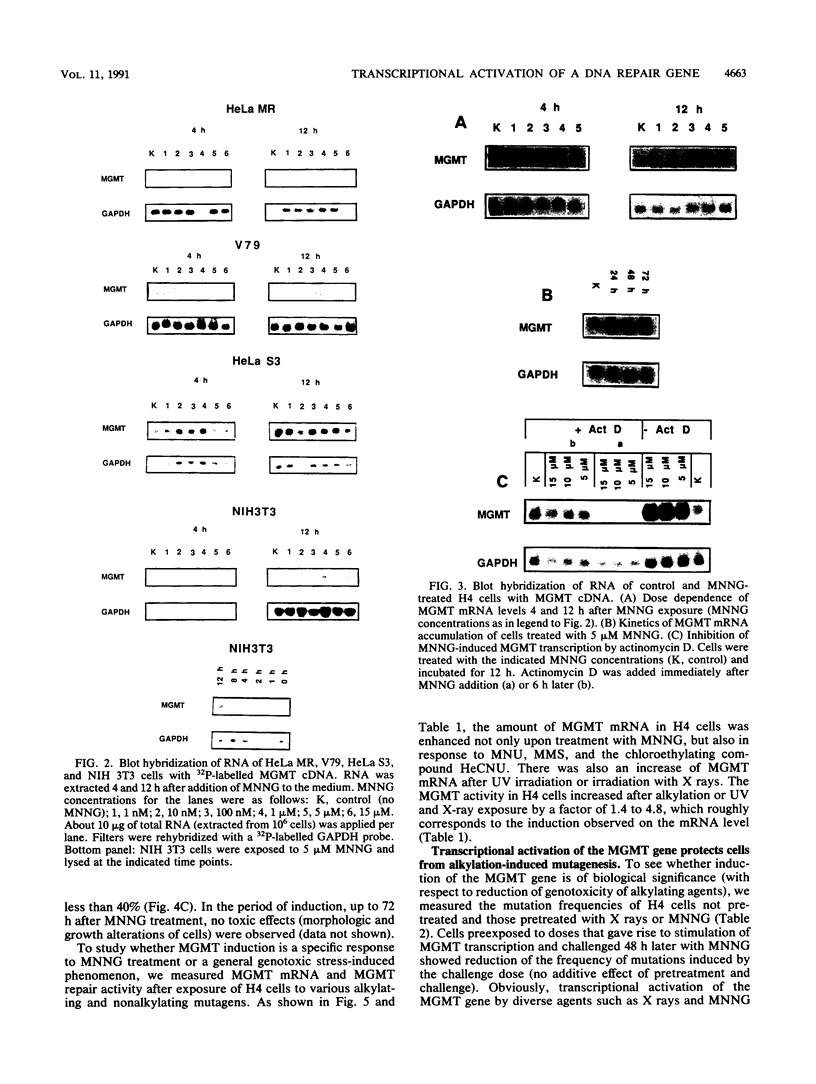
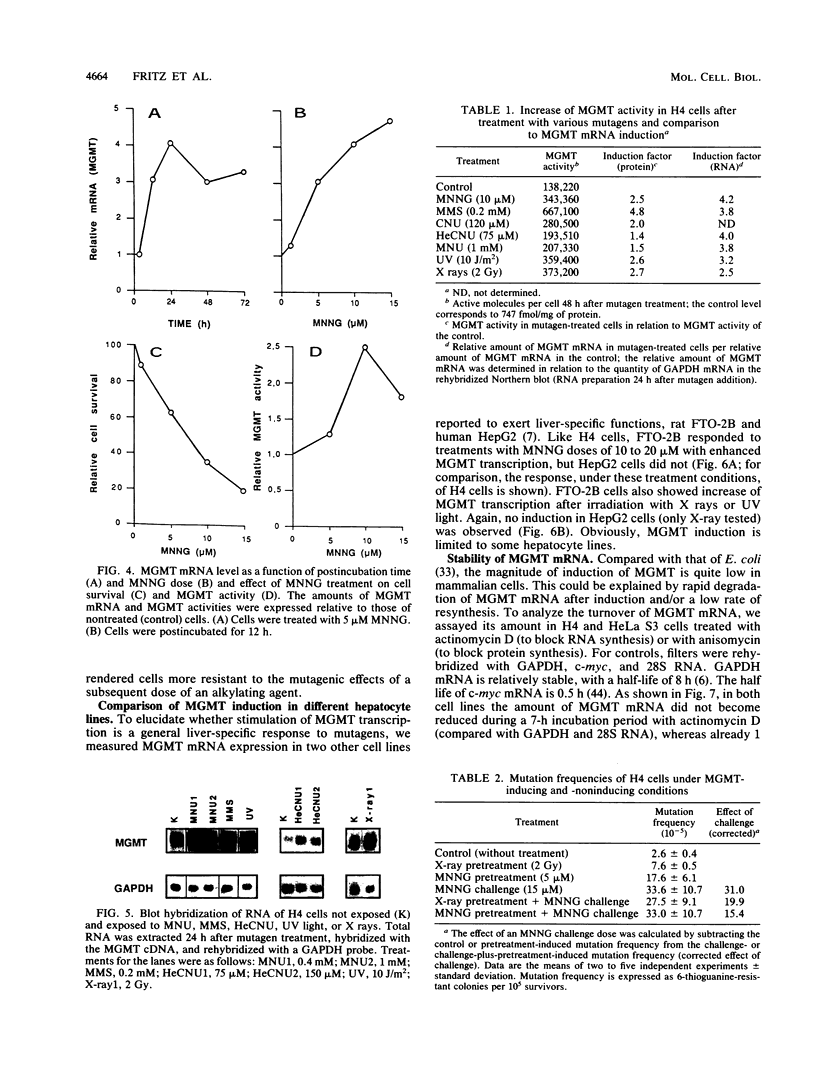
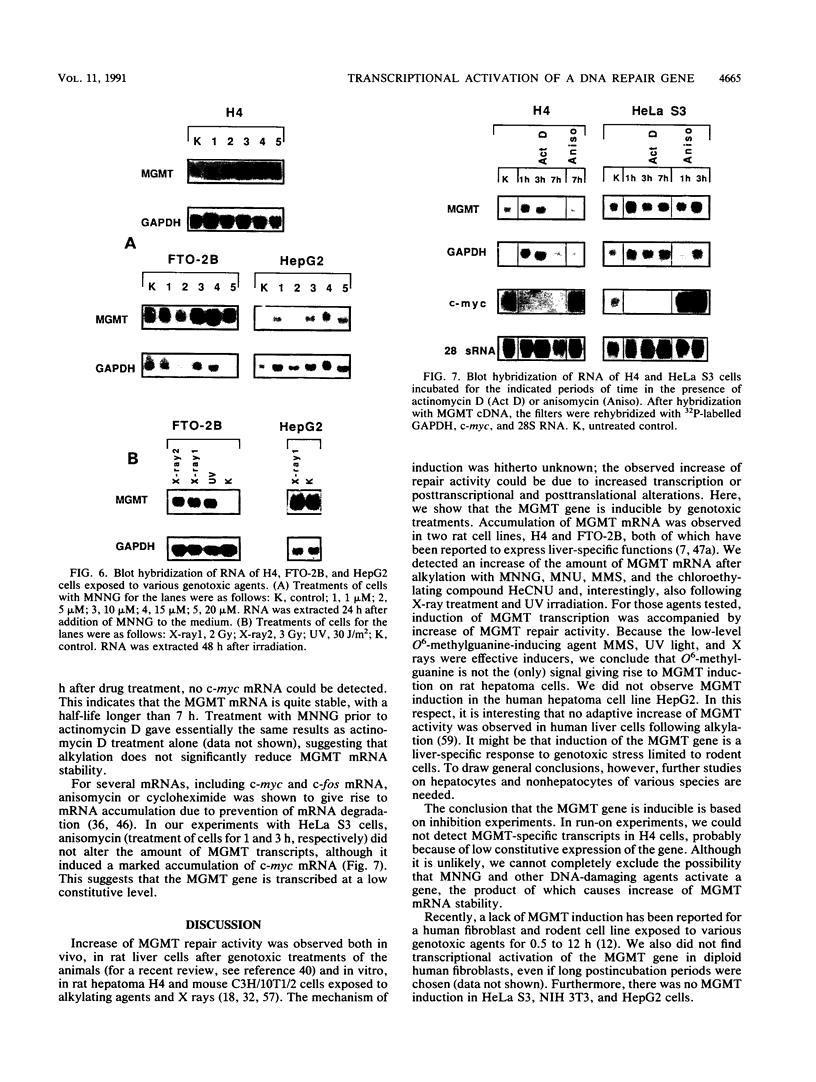
The inducibility of the mammalian O6-methylguanine-DNA methyltransferase (MGMT) gene encoding the MGMT protein (EC 2.1.1.63) responsible for removal of the procarcinogenic and promutagenic lesion O6-alkylguanine from DNA was examined by an analysis of transcription of the MGMT gene following exposure of repair-competent (Mex+) and repair-deficient (Mex-) cells to N-methyl-N'-nitro-N-nitrosoguanidine (MNNG). While human and rodent Mex- cells (CHO-9, V79, HeLa MR) showed no detectable MGMT mRNA despite the presence of the gene in their genome, the amount of it in several Mex+ lines (NIH 3T3, HeLa S3, HepG2) paralleled their MGMT activity. However, none of these cell lines showed an increase in the MGMT mRNA level after treatment with various concentrations of MNNG. In contrast, MNNG-treated rat hepatoma cells, H4IIE and FTO-2B, both Mex+, had three- to fivefold more MGMT mRNA than the corresponding untreated controls as measured 12 to 72 h after alkylation. N-Methyl-N-nitrosourea, methyl methanesulfonate, N-hydroxyethyl-N-chloroethylnitrosourea, UV light, and X rays caused a similar accumulation of MGMT mRNA in rat hepatoma cells. Studies with inhibitors of RNA and protein synthesis indicate that the induced increase in the amount of MGMT mRNA was due to enhanced transcription of the gene. Furthermore, they revealed the turnover of the MGMT mRNA to be relatively low (half-life, greater than 7 h). Mutagen-induced increase of transcription of MGMT mRNA in H4IIE cells was accompanied by elevation of MGMT repair activity and resulted in reduction of mutation frequency after a challenge dose of MNNG. Although induction of MGMT mRNA transcription has been observed in two rodent hepatoma cell lines so far, this appears to be the first demonstration of inducibility of a mammalian gene encoding a clearly define DNA repair function. The transcription activation of the MGMT gene protects cells from the mutagenic effects of methylating agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu Y. H., Craig A. W., O'Connor P. J. Repair of O6-methylguanine in rat liver DNA is enhanced by pretreatment with single or multiple doses of aflatoxin B1. Br J Cancer. 1981 Jun;43(6):850–855. doi: 10.1038/bjc.1981.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. P., O'Connor P. J., Margison G. P. Effect of acute doses of 2-acetylaminofluorene on the capacity of rat liver to repair methylated purines in DNA in vivo and in vitro. Cancer Res. 1982 Oct;42(10):4203–4209. [PubMed] [Google Scholar]
- Dani C., Piechaczyk M., Audigier Y., El Sabouty S., Cathala G., Marty L., Fort P., Blanchard J. M., Jeanteur P. Characterization of the transcription products of glyceraldehyde 3-phosphate-dehydrogenase gene in HeLa cells. Eur J Biochem. 1984 Dec 3;145(2):299–304. doi: 10.1111/j.1432-1033.1984.tb08552.x. [DOI] [PubMed] [Google Scholar]
- Darlington G. J. Liver cell lines. Methods Enzymol. 1987;151:19–38. doi: 10.1016/s0076-6879(87)51006-0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982 Nov 25;257(22):13776–13780. [PubMed] [Google Scholar]
- Foote R. S., Mitra S. Lack of induction of O6-methylguanine-DNA methyltransferase in mammalian cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 1984 Feb;5(2):277–281. doi: 10.1093/carcin/5.2.277. [DOI] [PubMed] [Google Scholar]
- Foote R. S., Mitra S., Pal B. C. Demethylation of O6-methylguanine in a synthetic DNA polymer by an inducible activity in Escherichia coli. Biochem Biophys Res Commun. 1980 Nov 28;97(2):654–659. doi: 10.1016/0006-291x(80)90314-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Papathanasiou M. A., Hollander M. C., Yarosh D. B. Expression of the O6-methylguanine-DNA methyltransferase gene MGMT in MER+ and MER- human tumor cells. Cancer Res. 1990 Dec 15;50(24):7908–7911. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Schalch H., Alamo I., Jr Coordinate induction of metallothioneins I and II in rodent cells by UV irradiation. Mol Cell Biol. 1988 Nov;8(11):4716–4720. doi: 10.1128/mcb.8.11.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Zmudzka B., Hollander M. C., Wilson S. H. Induction of beta-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol Cell Biol. 1989 Feb;9(2):851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M., Sultani-Makzoumi C. M., Boyle J. M. A search for adaptive or inducible responses to DNA damage in V79 Chinese hamster cells. Biochimie. 1982 Aug-Sep;64(8-9):687–692. doi: 10.1016/s0300-9084(82)80111-9. [DOI] [PubMed] [Google Scholar]
- Frosina G., Abbondandolo A. The current evidence for an adaptive response to alkylating agents in mammalian cells, with special reference to experiments with in vitro cell cultures. Mutat Res. 1985 Sep;154(2):85–100. doi: 10.1016/0165-1110(85)90021-1. [DOI] [PubMed] [Google Scholar]
- Frosina G., Laval F. The O6-methylguanine-DNA-methyltransferase activity of rat hepatoma cells is increased after a single exposure to alkylating agents. Carcinogenesis. 1987 Jan;8(1):91–95. doi: 10.1093/carcin/8.1.91. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K., Berger N. A. Comparison of O6-alkylguanine-DNA alkyltransferase activity based on cellular DNA content in human, rat and mouse tissues. Carcinogenesis. 1986 May;7(5):745–749. doi: 10.1093/carcin/7.5.745. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. C., Pegg A. E., Trump B. F., Harris C. C. O6-alkylguanine-DNA alkyltransferase activity in normal human tissues and cells. Cancer Res. 1984 Jul;44(7):2855–2857. [PubMed] [Google Scholar]
- Hayakawa H., Koike G., Sekiguchi M. Expression and cloning of complementary DNA for a human enzyme that repairs O6-methylguanine in DNA. J Mol Biol. 1990 Jun 20;213(4):739–747. doi: 10.1016/S0022-2836(05)80260-8. [DOI] [PubMed] [Google Scholar]
- Hesse S., Mezger M., Wiebel F. J. The capacity of rat hepatoma cell lines for O6-methylguanine-DNA repair correlates with their status of differentiation. Carcinogenesis. 1984 Jul;5(7):975–978. doi: 10.1093/carcin/5.7.975. [DOI] [PubMed] [Google Scholar]
- Hollander M. C., Fornace A. J., Jr Induction of fos RNA by DNA-damaging agents. Cancer Res. 1989 Apr 1;49(7):1687–1692. [PubMed] [Google Scholar]
- Kaina B. Cross-resistance studies with V79 Chinese hamster cells adapted to the mutagenic or clastogenic effect of N-methyl-N'-nitro-N-nitrosoguanidine. Mutat Res. 1983 Nov;111(3):341–352. doi: 10.1016/0027-5107(83)90031-3. [DOI] [PubMed] [Google Scholar]
- Kaina B. Enhanced survival and reduced mutation and aberration frequencies induced in V79 chinese hamster cells pre-exposed to low levels of methylating agents. Mutat Res. 1982 Mar;93(1):195–211. doi: 10.1016/0027-5107(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Kaina B. Studies on adaptation of V79 Chinese hamster cells to low doses of methylating agents. Carcinogenesis. 1983 Nov;4(11):1437–1443. doi: 10.1093/carcin/4.11.1437. [DOI] [PubMed] [Google Scholar]
- Kaina B., Van Zeeland A. A., Backendorf C., Thielmann H. W., Van de Putte P. Transfer of human genes conferring resistance to methylating mutagens, but not to UV irradiation and cross-linking agents, into Chinese hamster ovary cells. Mol Cell Biol. 1987 May;7(5):2024–2030. doi: 10.1128/mcb.7.5.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina B., van Zeeland A. A., de Groot A., Natarajan A. T. DNA repair and chromosomal stability in the alkylating agent-hypersensitive Chinese hamster cell line 27-1. Mutat Res. 1990 Mar;243(3):219–224. doi: 10.1016/0165-7992(90)90094-z. [DOI] [PubMed] [Google Scholar]
- Karran P., Arlett C. F., Broughton B. C. An adaptive response to the cytotoxic effects of N-methyl-N-nitrosourea is apparently absent in normal human fibroblasts. Biochimie. 1982 Aug-Sep;64(8-9):717–721. doi: 10.1016/s0300-9084(82)80117-x. [DOI] [PubMed] [Google Scholar]
- Laval F., Laval J. Adaptive response in mammalian cells: crossreactivity of different pretreatments on cytotoxicity as contrasted to mutagenicity. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1062–1066. doi: 10.1073/pnas.81.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P., Laval F. Enhancement of O6-methylguanine-DNA-methyltransferase activity induced by various treatments in mammalian cells. Cancer Res. 1986 Nov;46(11):5701–5705. [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mai S., Stein B., van den Berg S., Kaina B., Lücke-Huhle C., Ponta H., Rahmsdorf H. J., Kraemer M., Gebel S., Herrlich P. Mechanisms of the ultraviolet light response in mammalian cells. J Cell Sci. 1989 Dec;94(Pt 4):609–615. doi: 10.1242/jcs.94.4.609. [DOI] [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. doi: 10.1038/310697a0. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Butler J., Hoey B. O6-Methylguanine methyltransferase activity is increased in rat tissues by ionising radiation. Carcinogenesis. 1985 Dec;6(12):1699–1702. doi: 10.1093/carcin/6.12.1699. [DOI] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Margison G. P. Increased excision of O6-methylguanine from rat liver DNA after chronic administration of dimethylnitrosamine. Cancer Res. 1979 May;39(5):1798–1802. [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Pegg A. E. Methylation of the O6 position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Invest. 1984;2(3):223–231. doi: 10.3109/07357908409104376. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W., Bennett R. A. Effect of partial hepatectomy on removal of O6-methylguanine from alkylated DNA by rat liver extracts. Biochem J. 1981 Jul 1;197(1):195–201. doi: 10.1042/bj1970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Stimulation of transfer of methyl groups from O6-methylguanine in DNA to protein by rat liver extracts in response to hepatotoxins. Carcinogenesis. 1981;2(11):1195–1200. doi: 10.1093/carcin/2.11.1195. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg B., Spurr N., Karran P. cDNA cloning and chromosomal assignment of the human O6-methylguanine-DNA methyltransferase. cDNA expression in Escherichia coli and gene expression in human cells. J Biol Chem. 1990 Jun 5;265(16):9563–9569. [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Schwartz J. L. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980 Oct 30;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Schmerold I., Spath A. Induction of rat liver O6-alkylguanine-DNA alkyltransferase by bleomycin. Chem Biol Interact. 1986 Dec;60(3):297–304. doi: 10.1016/0009-2797(86)90060-8. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Lindahl T. A common mechanism for repair of O6-methylguanine and O6-ethylguanine in DNA. J Mol Biol. 1982 Jan 5;154(1):169–175. doi: 10.1016/0022-2836(82)90424-7. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Datta R., Hallahan D. E., Weichselbaum R. R., Kufe D. W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Snow E. T., Foote R. S., Mitra S. Base-pairing properties of O6-methylguanine in template DNA during in vitro DNA replication. J Biol Chem. 1984 Jul 10;259(13):8095–8100. [PubMed] [Google Scholar]
- Tano K., Shiota S., Collier J., Foote R. S., Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci U S A. 1990 Jan;87(2):686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein E. A., Cao E. H., Setlow R. B. Adaptive resynthesis of O6-methylguanine-accepting protein can explain the differences between mammalian cells proficient and deficient in methyl excision repair. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5117–5121. doi: 10.1073/pnas.79.17.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani G., Wani A. A., Gibson-D'Ambrosio R., Samuel M., Lowder E., D'Ambrosio S. M. Absence of DNA damage-mediated induction of human methyltransferase specific for precarcinogenic O6-methylguanine. Teratog Carcinog Mutagen. 1989;9(5):259–272. doi: 10.1002/tcm.1770090502. [DOI] [PubMed] [Google Scholar]
- Washington W. J., Foote R. S., Dunn W. C., Generoso W. M., Mitra S. Age-dependent modulation of tissue-specific repair activity for 3-methyladenine and O6-methylguanine in DNA in inbred mice. Mech Ageing Dev. 1989 Apr;48(1):43–52. doi: 10.1016/0047-6374(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Foote R. S., Mitra S., Day R. S., 3rd Repair of O6-methylguanine in DNA by demethylation is lacking in Mer- human tumor cell strains. Carcinogenesis. 1983;4(2):199–205. doi: 10.1093/carcin/4.2.199. [DOI] [PubMed] [Google Scholar]
- von Hofe E., Kennedy A. R. In vitro induction of O6-methylguanine-DNA methyltransferase in C3H/10T1/2 cells by X-rays is inhibited by nitrogen. Carcinogenesis. 1988 Apr;9(4):679–681. doi: 10.1093/carcin/9.4.679. [DOI] [PubMed] [Google Scholar]