Abstract
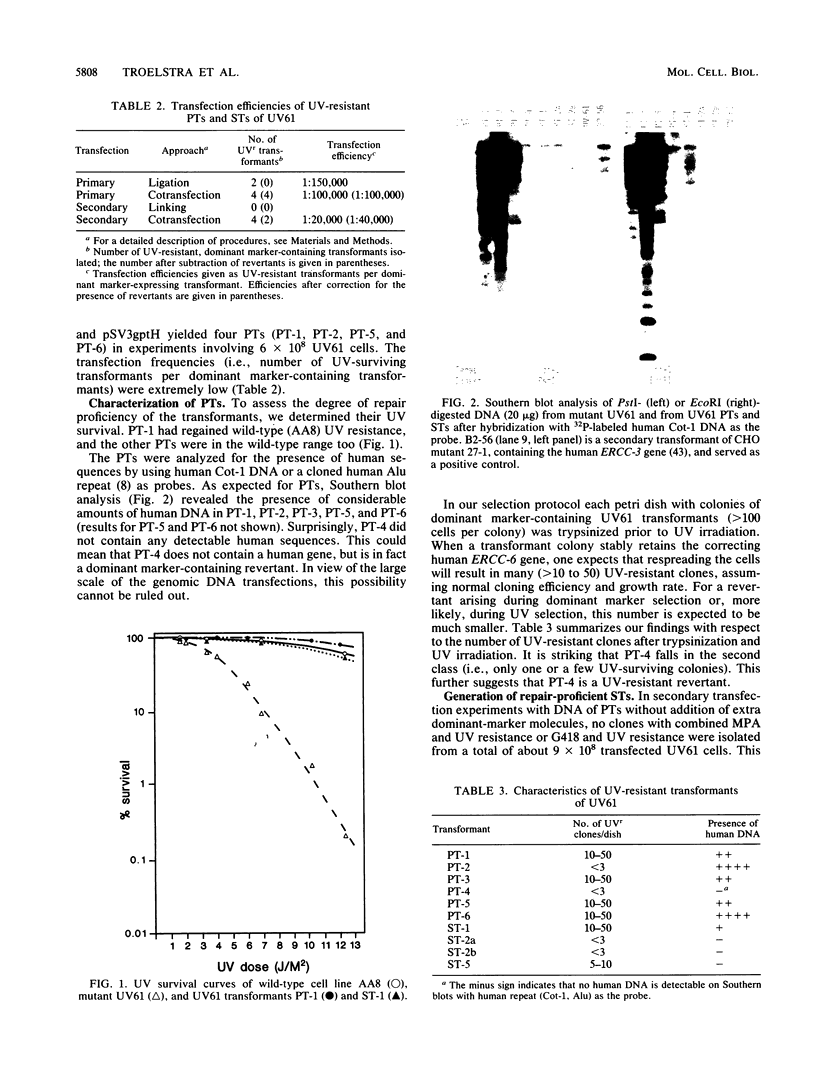
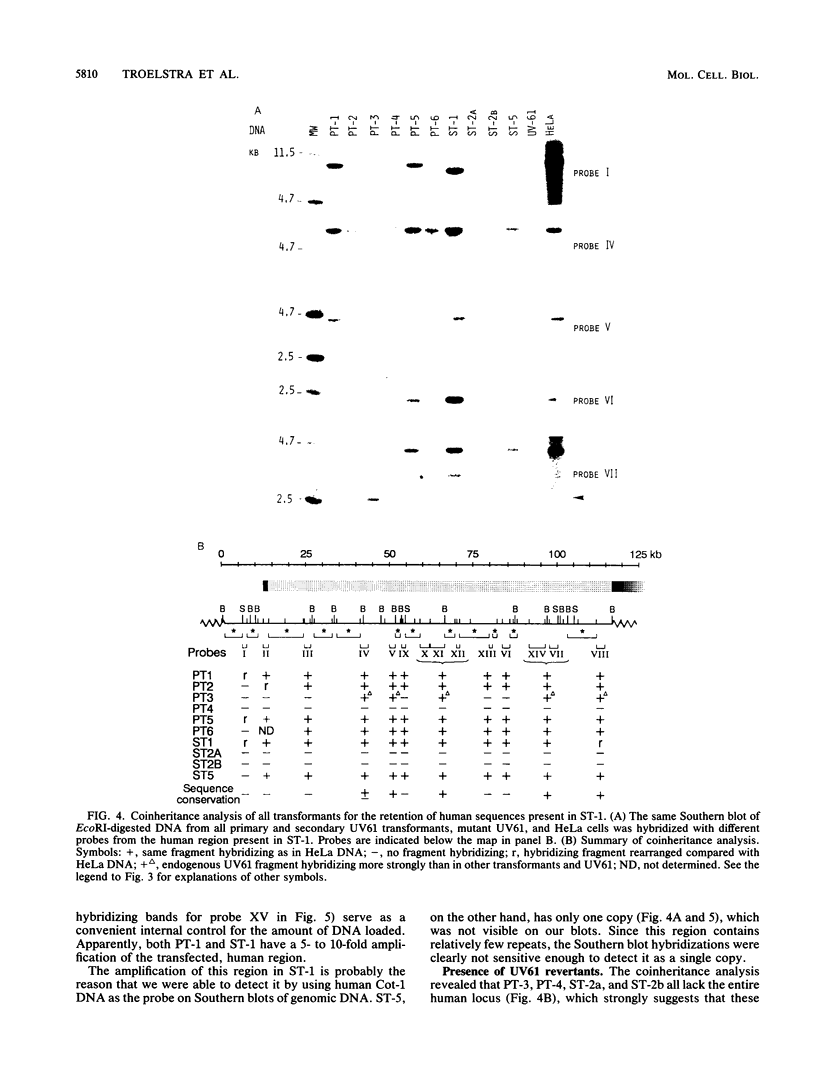
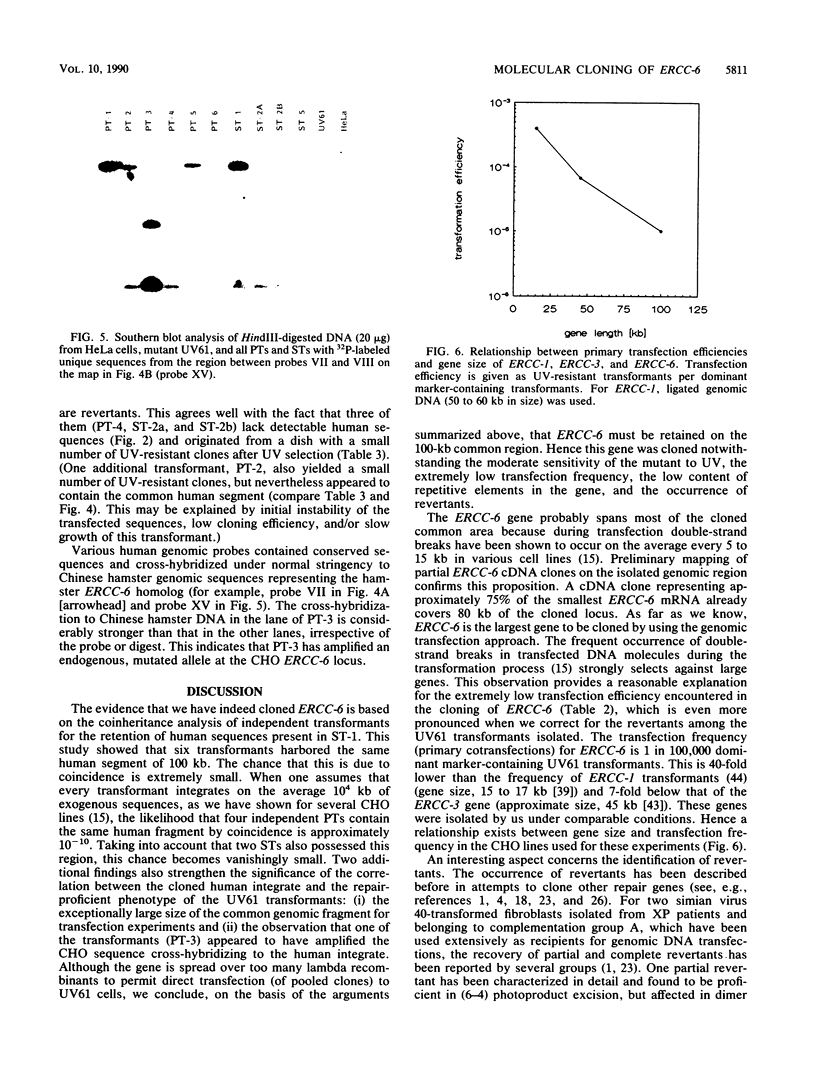
The UV-sensitive, nucleotide excision repair-deficient Chinese hamster mutant cell line UV61 was used to identify and clone a correcting human gene, ERCC-6. UV61, belonging to rodent complementation group 6, is only moderately UV sensitive in comparison with mutant lines in groups 1 to 5. It harbors a deficiency in the repair of UV-induced cyclobutane pyrimidine dimers but permits apparently normal repair of (6-4) photoproducts. Genomic (HeLa) DNA transfections of UV61 resulted, with a very low efficiency, in six primary and four secondary UV-resistant transformants having regained wild-type UV survival. Southern blot analysis revealed that five primary and only one secondary transformant retained human sequences. The latter line was used to clone the entire 115-kb human insert. Coinheritance analysis demonstrated that five of the other transformants harbored a 100-kb segment of the cloned human insert. Since it is extremely unlikely that six transformants all retain the same stretch of human DNA by coincidence, we conclude that the ERCC-6 gene resides within this region and probably covers most of it. The large size of the gene explains the extremely low transfection frequency and makes the gene one of the largest cloned by genomic DNA transfection. Four transformants did not retain the correcting ERCC-6 gene and presumably have reverted to the UV-resistant phenotype. One of these appeared to have amplified an endogenous, mutated CHO ERCC-6 allele, indicating that the UV61 mutation is leaky and can be overcome by gene amplification.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum M., Baumann I., Lohrer H., Rahmsdorf H. J., Herrlich P. A promising genomic transfectant into Xeroderma pigmentosum group A with highly amplified mouse DNA and intermediate UV resistance turns revertant. Biochem Biophys Res Commun. 1989 Apr 28;160(2):647–655. doi: 10.1016/0006-291x(89)92482-0. [DOI] [PubMed] [Google Scholar]
- Bootsma D., Keijzer W., Jung E. G., Bohnert E. Xeroderma pigmentosum complementation group XP-I withdrawn. Mutat Res. 1989 Sep;218(2):149–151. doi: 10.1016/0921-8777(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Busch D., Greiner C., Lewis K., Ford R., Adair G., Thompson L. Summary of complementation groups of UV-sensitive CHO cell mutants isolated by large-scale screening. Mutagenesis. 1989 Sep;4(5):349–354. doi: 10.1093/mutage/4.5.349. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Cortés F., Lutze L. H., Morgan W. F., Player A. N., Mitchell D. L. Unique DNA repair properties of a xeroderma pigmentosum revertant. Mol Cell Biol. 1987 Sep;7(9):3353–3357. doi: 10.1128/mcb.7.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Johnson R. T. DNA repair mutants in higher eukaryotes. J Cell Sci Suppl. 1987;6:61–82. doi: 10.1242/jcs.1984.supplement_6.4. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972 Jul 19;238(81):80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grossman L., Caron P. R., Mazur S. J., Oh E. Y. Repair of DNA-containing pyrimidine dimers. FASEB J. 1988 Aug;2(11):2696–2701. doi: 10.1096/fasebj.2.11.3294078. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Odijk H., Westerveld A. Differences between rodent and human cell lines in the amount of integrated DNA after transfection. Exp Cell Res. 1987 Mar;169(1):111–119. doi: 10.1016/0014-4827(87)90230-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Elliott G. C., Squires S., Joysey V. C. Lack of complementation between xeroderma pigmentosum complementation groups D and H. Hum Genet. 1989 Feb;81(3):203–210. doi: 10.1007/BF00278989. [DOI] [PubMed] [Google Scholar]
- MacInnes M. A., Bingham J. M., Thompson L. H., Strniste G. F. DNA-mediated cotransfer of excision repair capacity and drug resistance into chinese hamster ovary mutant cell line UV-135. Mol Cell Biol. 1984 Jun;4(6):1152–1158. doi: 10.1128/mcb.4.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Zdzienicka M. Z., van Zeeland A. A., Nairn R. S. Intermediate (6-4) photoproduct repair in Chinese hamster V79 mutant V-H1 correlates with intermediate levels of DNA incision and repair replication. Mutat Res. 1989 May;226(1):43–47. doi: 10.1016/0165-7992(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Orren D. K., Sancar A. The (A)BC excinuclease of Escherichia coli has only the UvrB and UvrC subunits in the incision complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5237–5241. doi: 10.1073/pnas.86.14.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Haseltine W. A. Isolation of UV-resistant revertants from a xeroderma pigmentosum complementation group A cell line. 1984 Sep 27-Oct 3Nature. 311(5984):390–392. doi: 10.1038/311390a0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Schultz R. A., Barbis D. P., Friedberg E. C. Studies on gene transfer and reversion to UV resistance in xeroderma pigmentosum cells. Somat Cell Mol Genet. 1985 Nov;11(6):617–624. doi: 10.1007/BF01534726. [DOI] [PubMed] [Google Scholar]
- Shiomi T., Hieda-Shiomi N., Sato K. Isolation of UV-sensitive mutants of mouse L5178Y cells by a cell suspension spotting method. Somatic Cell Genet. 1982 May;8(3):329–345. doi: 10.1007/BF01538891. [DOI] [PubMed] [Google Scholar]
- Stefanini M., Keijzer W., Westerveld A., Bootsma D. Interspecies complementation analysis of xeroderma pigmentosum and UV-sensitive Chinese hamster cells. Exp Cell Res. 1985 Dec;161(2):373–380. doi: 10.1016/0014-4827(85)90094-1. [DOI] [PubMed] [Google Scholar]
- Sung P., Prakash L., Matson S. W., Prakash S. RAD3 protein of Saccharomyces cerevisiae is a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8951–8955. doi: 10.1073/pnas.84.24.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Mooney C. L., Carrano A. V. Hypersensitivity to mutation and sister-chromatid-exchange induction in CHO cell mutants defective in incising DNA containing UV lesions. Somatic Cell Genet. 1982 Nov;8(6):759–773. doi: 10.1007/BF01543017. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Busch D. B., Brookman K., Mooney C. L., Glaser D. A. Genetic diversity of UV-sensitive DNA repair mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3734–3737. doi: 10.1073/pnas.78.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mitchell D. L., Regan J. D., Bouffler S. D., Stewart S. A., Carrier W. L., Nairn R. S., Johnson R. T. CHO mutant UV61 removes (6-4) photoproducts but not cyclobutane dimers. Mutagenesis. 1989 Mar;4(2):140–146. doi: 10.1093/mutage/4.2.140. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Brookman K. W. Genetic complementation between UV-sensitive CHO mutants and xeroderma pigmentosum fibroblasts. Mutat Res. 1985 Jun-Jul;150(1-2):423–429. doi: 10.1016/0027-5107(85)90139-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Collins C. C., Stewart S. A., Busch D. B., Weber C. A. Recent progress with the DNA repair mutants of Chinese hamster ovary cells. J Cell Sci Suppl. 1987;6:97–110. doi: 10.1242/jcs.1984.supplement_6.6. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Shiomi T., Salazar E. P., Stewart S. A. An eighth complementation group of rodent cells hypersensitive to ultraviolet radiation. Somat Cell Mol Genet. 1988 Nov;14(6):605–612. doi: 10.1007/BF01535314. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Somatic cell genetics approach to dissecting mammalian DNA repair. Environ Mol Mutagen. 1989;14(4):264–281. doi: 10.1002/em.2850140409. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Masurel R., Westerveld A., Odijk H., de Wit J., Bootsma D., van der Eb A. J., Hoeijmakers J. H. Molecular cloning and biological characterization of the human excision repair gene ERCC-3. Mol Cell Biol. 1990 Jun;10(6):2570–2581. doi: 10.1128/mcb.10.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Simons J. W. Identification of a new seventh complementation group of UV-sensitive mutants in Chinese hamster cells. Mutat Res. 1988 Sep;194(2):165–170. doi: 10.1016/0167-8817(88)90018-1. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Westerveld A., van Zeeland A. A., Simons J. W. Phenotypic heterogeneity within the first complementation group of UV-sensitive mutants of Chinese hamster cell lines. Mutat Res. 1988 Jan;193(1):31–41. doi: 10.1016/0167-8817(88)90005-3. [DOI] [PubMed] [Google Scholar]
- van Duin M., Koken M. H., van den Tol J., ten Dijke P., Odijk H., Westerveld A., Bootsma D., Hoeijmakers J. H. Genomic characterization of the human DNA excision repair gene ERCC-1. Nucleic Acids Res. 1987 Nov 25;15(22):9195–9213. doi: 10.1093/nar/15.22.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Duin M., van den Tol J., Warmerdam P., Odijk H., Meijer D., Westerveld A., Bootsma D., Hoeijmakers J. H. Evolution and mutagenesis of the mammalian excision repair gene ERCC-1. Nucleic Acids Res. 1988 Jun 24;16(12):5305–5322. doi: 10.1093/nar/16.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]