Abstract
While fluoro-deoxy-glucose (FDG) has emerged as an important radiotracer for imaging tumors, myocardial viability and infection, the role of other glucose analogues should also be explored. Tc-99m Glucoheptonate (GHA) has been used for imaging brain tumors and lung tumors. The uptake mechanism may be linked to GLUT-1 (Glucose transporter) and GLUT-4 expression similar to FDG. GHA is easily available and cheap. With the availability of single photon emission computed tomography/computed tomography (SPECT/CT), GHA imaging should be re-explored as a tumor agent and also for imaging myocardial viability.
Keywords: Tc-99m Glucoheptonate, fluoro-deoxy-glucose, SPECT-CT, PET-CT
INTRODUCTION
A recent editorial in the European Journal of Nuclear Medicine and Molecular Imaging—“PET and SPECT: Synergy rather than competition” by Giulino Mariani and William Strauss[1] has triggered this article. For over a decade, I have been proposing that Tc-99m glucoheptonate (GH) is the poor man's fluorodeoxyglucose (FDG). This has been stated in the preface of my book “Principles and Practice of Nuclear Medicine and Correlative Medical Imaging, with a foreword by Dr. Henry Wagner”.
Tc-99m GH was introduced in the 1970's in Dr. Henry Wagner's Nuclear Medicine Department at John's Hopkins, Baltimore USA, Rossman, Siegel, and Friedman in 1974;[2] Rossman, Strauss, Siegel, and Pitt in 1975[3,4] studied the utility of Tc-GH to image myocardial infarction. In mouse and canine models after ligation of a coronary artery, the greatest concentration of the tracer was noted in areas distal to the occlusion that had 20-40% of normal perfusion, which showed persistent ventricular akinesis, indicating severe persistent ischemia that would subsequently lead to infarction [Figure 1]. In their pre-occupation with myocardial infarct imaging, where they commented that Tc-GH is not an ideal agent for that purpose, they overlooked the utility of Tc-GH as an ischemia imaging agent; although the authors emphasized that concentration of GH in the myocardium depended on sufficient residual or collateral flow, and an alteration in the myocardial cell allowed the tracer to be actively transported across the membrane. In 1975, there was no knowledge of what alteration in the myocardial cell allowed GH to be actively transported across the ischemic muscle cell membrane. Today, we know these alterations (vide infra).
Figure 1.
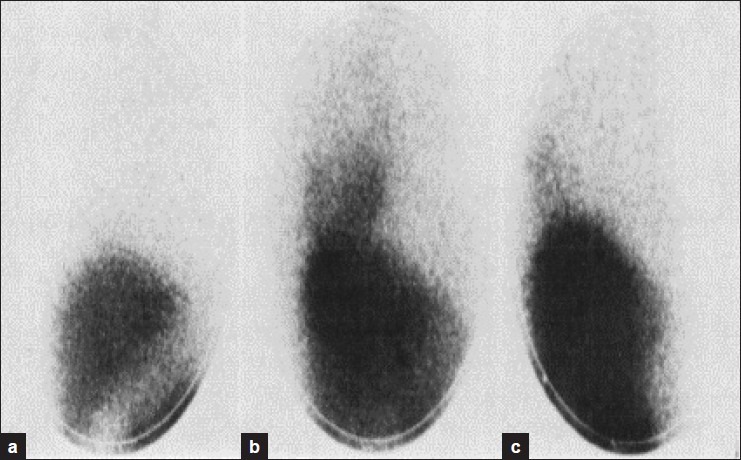
Accumulation of Tc GH in areas of persisted ischaemia in the dog myocardium. Rossaman, et al., 1974[2]
Arnold, et al.,[5] showed the utility of Tc-GH as a renal imaging agent.
GH for tumor imaging
In as early as 1975, Tanasescu, et al.,[6] showed the utility of Tc-GH as a brain scanning agent. Lιveillι, et al.,[7] described Tc-GH as an important advance in brain tumor detection. They noted progressive tracer concentration over time as shown by comparison of late (5-6 h) with early (1 h) images. They suggested that Tc-GH is taken up like a glucose analog by an active transport mechanism to be used as a substrate for energy by the metabolically active tumor tissue [Figure 2].
Figure 2.
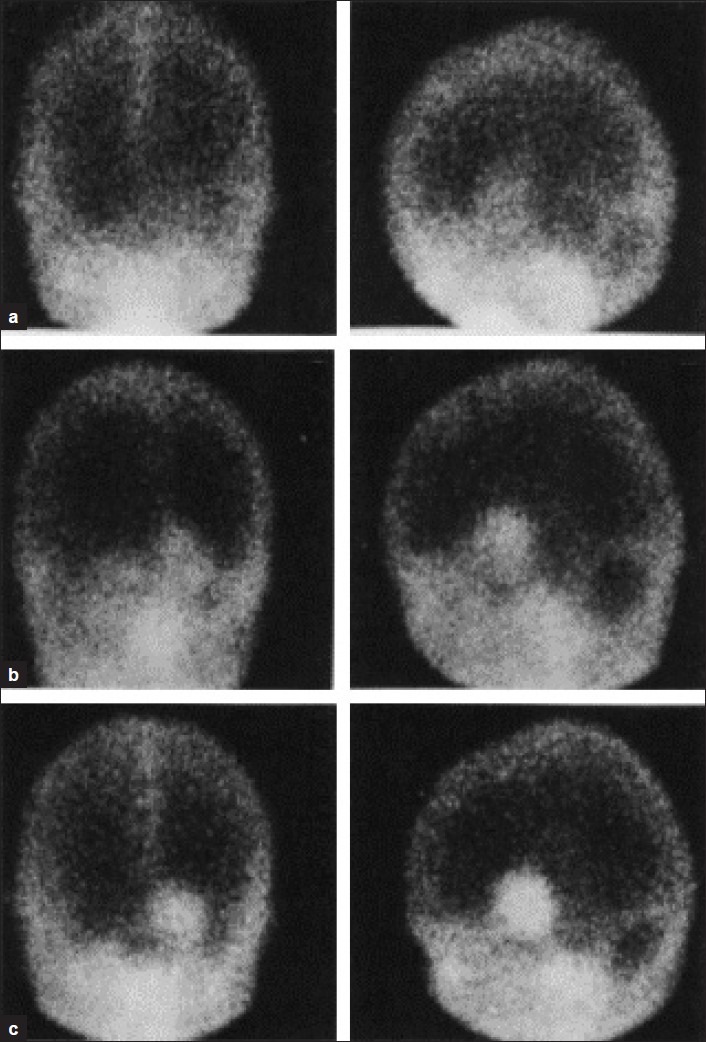
Tc-GH Actively transported in brain tumours progressive rise in 5 hr. images compared to 1 hr. images. Léveillé, et al., 1977[7]
Sty, et al.,[8] showed the utility of Tc-GH in brain tumors and metastases. Delayed images were useful to detect low-grade glyomas and other slowly growing avascular tumors in pediatric population. Sodee and Ballistrea[9] showed that Tc-GH Single-photon emission computed tomography (SPECT) is better than computed tomography (CT) with contrast and equally useful compared to magnetic resonance imaging (MRI). They observed that Tc-GH accumulated in viable components of the tumor and not in necrotic parts. In 1987, Kaplan, et al.,[10] compared Tl-201 2 mCi (30 min), Tc-GH 20 mCi (4 h), and Ga-67, 7 mCi (48 h) images and CT brain scan in 29 patients with malignant gliomas with pathological correlation in 7 autopsies. In all tumors, GH accumulation was more than Tl and Ga-67. While Tl-201 showed tumor only, GH showed tumor as well as surrounding edema not defined by CT. Steroid therapy did not interfere with tumor uptake of Tl and GH, unlike Ga-67 uptake, which was reduced [Figure 3].
Figure 3.
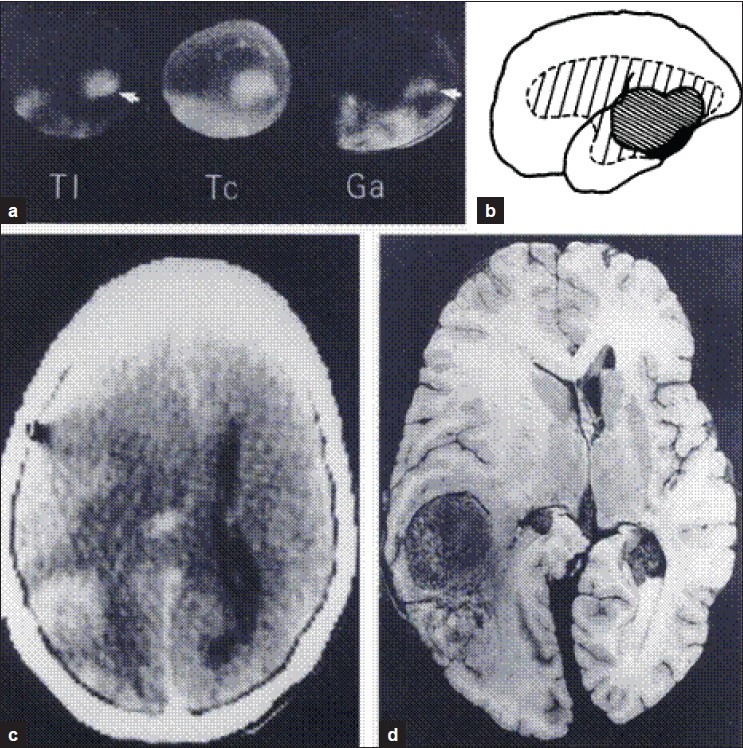
Tl-201, Tc-GH, Ga-67 brain images compared to CT scan and autopsy findings. Kaplan, et al., 1987[10]
Vorne, et al.,[11] showed accumulation of Tc-GH in 23 of 26 patients with primary lung cancer. The visualization of malignant tumors was much better in late (5 h) than in early (1 h) images. A total of 23 patients without lung disease gave negative Tc-GH results. Tc-GH accumulated in the viable parts of 2 lung tumors, but not in necrotic tissue. Uptake in benign lesions was not gradually progressive. The authors commented “Perhaps Tc-GH is taken up like a glucose analog by an active transport mechanism to be used as a substrate for energy by the metabolically active tumor tissue” [Figure 4].
Figure 4.
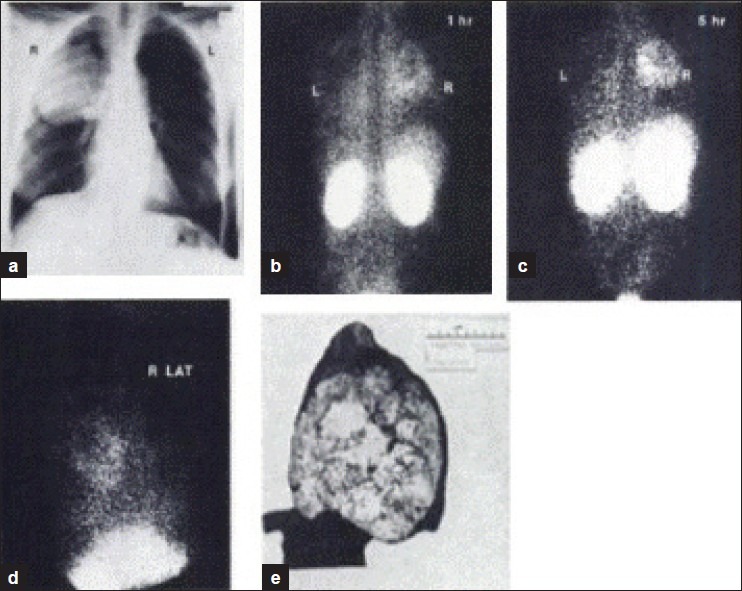
Squamous cell car cinome of right lung with Tc-GH accumulation. Vorne, et al., 1982[11]
Passmonte, et al.,[12] and Sauerland et al.,[13] also showed the utility of Tc-GH imaging in lung cancer and cervical lymph node metastases. Crane, et al.,[14] showed that Tc-GH imaging was comparable to the CT for brain metastases from small cell lung cancer. In 1981, Wim de Kieviet[15] studied the chemical structure [Figure 5] and tissue distribution of Tc-GH in stomach, intestines, liver, spleen, kidneys, bone, and bone marrow, thyroid, and blood in Wistar rats. At that time, there was no knowledge about glucose transporters to compare the behavior of GH with glucose or FDG.
Figure 5.
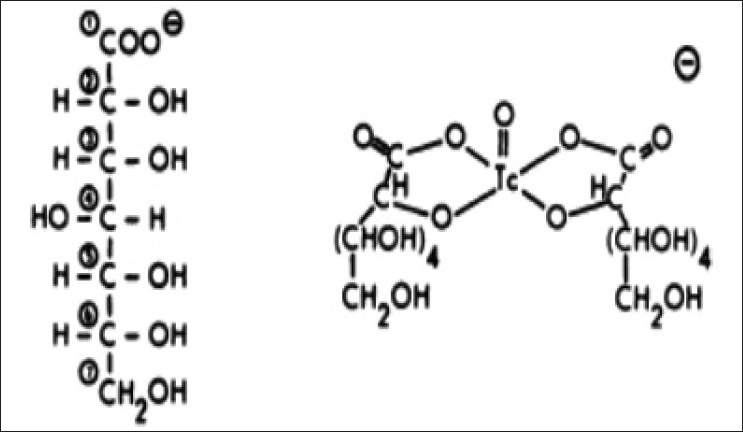
Structures of glucohepronate and Tc-99 Glucoheptonate
Glucose transporter (GLUT) expression in health and disease
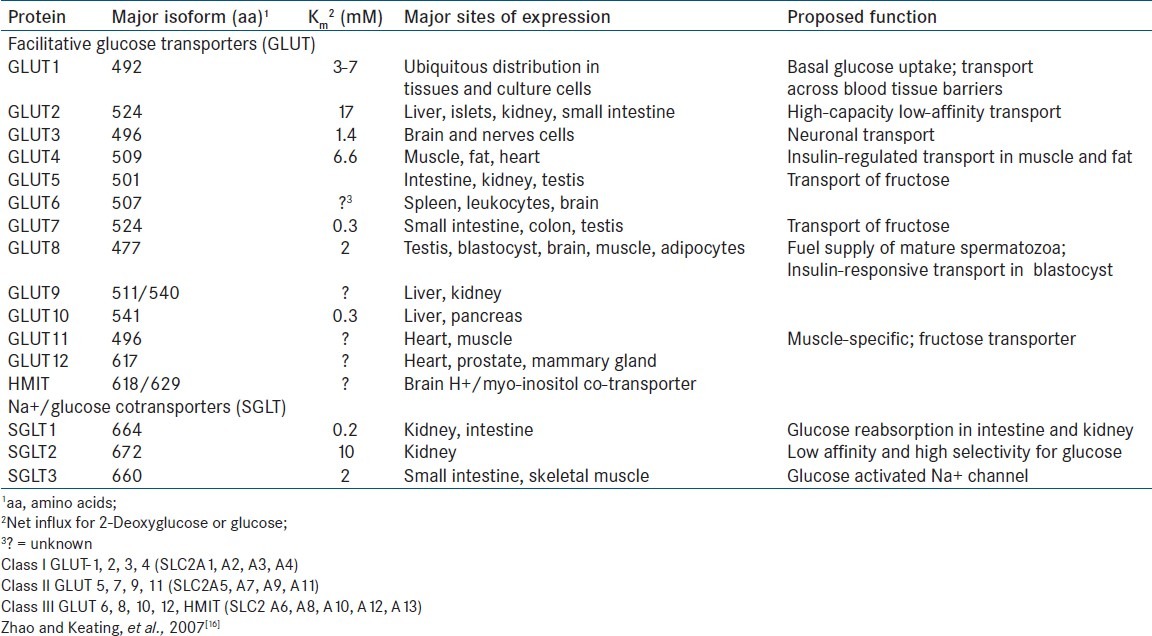
Oxidation of glucose is a major source of metabolic energy for mammalian cells. Two classes of glucose carriers have been described in mammalian cells: Na+ glucose cotransporters and facilitative glucose transporters. Intestinal transport of glucose was shown by Crene in 1960 to be by flux-coupling via sodium-glucose co-transporters. Facilitative glucose transporters have distinct tissue distribution as shown in Table 1.[16] Molecular biology of mammalian glucose transporters was reviewed by Bell, et al.,[17] Flier, et al.,[18] showed elevated levels of GLUT 1 transporter mRNA induced by RAS or SRE oncogenes.
Table 1.
Summary of the Properties of Facilitative Glucose Transporter and Na+/ Glucose co-Transporter Family Members
GLUT-1 is over-expressed on cell surface of many cancers.[19] GLUT-1 is also increased in hypoxic areas of tumors near necrotic regions.[20] All malignant cells exhibit increased GLUT-1, leading to increased FDG uptake (recognized by GLUT-1 as glucose). Phosphorylated FDG-6P is trapped within the cell without further metabolism, enabling FDG-PET imaging for visualization, staging, monitoring progress, and response to therapy.[21] FDG uptake in breast cancer cells is positively correlated to GLUT-1.[22] Effective therapy reduces FDG uptake substantially and often completely.[23]
The availability of metabolic tracers (FDG for glucose and palmitate for fatty acids) clarified the metabolic behavior of the normal heart. Aerobically, perfused myocardium prefers free fatty acids (FFAs) as major energy source.[24] In the postprandial state, glucose and insulin levels in the blood increase and FFA levels decrease and glucose uptake by the heart increases.[25] Lactate may be the main substrate after exercise.[26]
During myocardial ischemia, when blood flow and oxygen level are decreased, FFA utilization is decreased. Impaired fatty acid uptake persists much longer than the duration of ischemia. This is demonstrated by I-123 BMIPP SPECT imaging (cold spot due to decreased uptake). If cyclotron-produced I-123 becomes readily available SPECT/CT has great future for myocardial ischemia imaging. Hypoxia-induced factor 1α stimulates GLUT-1 expression and glucose uptake, glucose utilization is upregulated.[27]
Ischemia and hypoxia increase glucose uptake by the myocardium. During and following acute ischemia, glucose is the main energy source for anerobic glycolysis for several hours.[28,29] This is achieved by translocation of GLUT-1.[29,30] Clinically, sudden coronary occlusion with acute ischemia rapidly increases regional FDG uptake.[31]
GLUT-1 may act as a “stress-induced protein” stimulated by hypoxia inducible transcriptional factor, hypoxia inducible factor (HIF1α).[32] There is 50% increase in glucose uptake in ischemic myocardial regions.[33] HIF1α, in response to hypoxia, stimulates expression of GLUT-1 and GLUT-3 in articular chondrocytes and promotes anerobic glycolysis.[34]
Stunning of the myocardium following transient periods of ischemia was first described by Heyndrickx in 1975. Clinically, stunning has been observed after exercise induced ischemia, unstable angina, and after hypothermic ischemic arrest for cardiac surgery.[35,36] Ischemia-induced accumulation of free intracellular calcium causes a decreased myofilament responsiveness in calcium-dependent proteases.[37] Cardiac function completely recovers in time. Increase in FDG uptake is utilized for anerobic glycolysis and is not utilized for restoration of the glycogen pool.[38] GLUT-1, 2, 3, 4, and 8 are expressed in myocytes. The contribution of GLUT-3 and GLUT-8 to glucose transport appears to be less important than that of GLUT-4 and GLUT-1 in the myocardium.[39] Chronic ischemia is a result of intermittent reductions of coronary flow. Several distinct strategies are used by the heart to adapt to the repeated under perfusion.
Hibernating myocardium[40] is characterized by uncoupling of cardiac contractile function and blood flow. Myocytes show a perinuclear loss of contractile protein, alteration of all structural proteins and abnormally shaped and sized nuclei that demonstrate heterogenous distribution of chromatin.[41] Hibernating myocardium takes up FDG via increased GLUT-1 expression.[42] Human myocardial biopsies of hibernating myocardium showed intense GLUT-1 expression compared to normal heart regions. Most strikingly hibernating myocytes are filled with glycogen deposits.[42,43]. Doenst, et al.,[44] showed that the FDG taken up by these myocytes is converted to glycogen. Accumulation of glycogen is a characteristic of the fetal heart.[45]
GLUT-4 translocation in the myocardium can occur following ischemia and reperfusion mediated by p38 MAPkinase[46,47] SB 202190, an inhibitor of p38 MAPkinase attenuates the increased FDG6P accumulation in preconditioned hearts.
A and β adrenergic stimulation contributes to ischemia-induced GLUT-4 and GLUT-1 in isolated perfused rat hearts.[48] In canine heart model in vivo, moderate regional ischemia results in a 2-3 fold increase in glucose uptake mediated by a 2-fold increase in GLUT-4 and 40% increase in GLUT-1.[49] This translocation of GLUT-1 and GLUT-4 persists several hours longer than the duration of ischemia. Ischemia-induced increased myocardial FDG uptake is 10-fold or more higher than the regional variability of glucose uptake at rest in the non-ischemic myocardium. Qualitative analysis is presently quite useful until quantitative programs are developed.
GH and FDG as analog of glucose?
FDG is similar but not identical with glucose. While glucose is nearly totally reabsorbed from renal tubules in normal individuals, FDG is substantially excreted in the urine unchanged.
GH is filtered by the renal glomerulus and protein bound fraction is secreted by the renal tubules and some of it is actively secreted in the bile and intestines. About 12% activity remains in the renal cortex for up to 6 h, while most of the injected activity appears in the urine. Kidneys show GLUT-1, 2, 5, 9, and SGLT-1 and SGLT-2 expression.
There is sufficient persuasive evidence that GH behaves as a glucose analog, actively transported as a source of energy. The obvious unanswered question is—does GH utilize GLUTs similar to glucose or FDG? The most persuasive evidence came recently from an in vitro evaluation of apoptosis with Tc-99m GH[50] A549 lung cancer cell lines showed uptake of Tc-99 GH, whereas chemotherapeutically induced apoptosis in A549 cells led to decrease in Tc-GH uptake depending on the level of apoptosis.
Hypoxia imaging
Hypoxia imaging with radiolabeled nitroimidazole (e.g., F18 fluoromisonidazol PET), while suitable for tumor hypoxia, which is a persistent phenomenon; the relatively slow extraction and gradual accumulation requiring prolonged circulation time, make it unsuitable for imaging exercise-induced transient hypoxia. It is useful for imaging chronic persistent hypoxia in chronically ischemic and viable hibernating myocardium.[51] Since hypoxia stimulates GLUT1 over-expression, Tc-GH may severe as a surrogate marker of hypoxia in these situations.
Tc 99m-GH for myocardial ischemic imaging?
Recently, stress FDG PET has been successfully utilized to demonstrate myocardial ischemia.[52,53] Can Tc-GH be used for the same purpose? Since the best images with GH are obtained at 4 h, it is presumed that the “ischemic memory” may persist for 4 h after the GH is injected during exercise stress. Prospective studies are undergoing at Jaslok Hospital and Research Centre and Lilavati Hospital and Research Centre, Mumbai to determine the utility of Tc-GH for myocardial ischemia imaging similar to stress FDG PET. The economic advantage of Tc-GH SPECT over FDG PET is obvious; hence, the importance of the study.
Tc-99m GH SPECT for tumor imaging can be seriously considered as an alternative to FDG PET/CT. Effective chemo/radiotherapy reduced FDG uptake substantially and often completely.[19,23] It is worth exploring if delayed 5 h images with Tc-GH SPECT/CT can take the place of FDG PET/CT for the purpose of metabolic monitoring.
Since macrophages and neutrophils accumulate FDG more than normal cells, FDG PET/CT is extensively used for imaging infection/inflammation apart from malignancy.[54]
GLUT-1 and GLUT-3 expression in white blood cells (WBCs) in inflammatory tissues was studied with C-14 FDG and immunohistochemistry, and compared to tumor tissue, GLUT-1 was significantly higher in tumor than in WBCs, while GLUT-3 was only slightly higher in WBCs than in tumor. Strauss (1996)[55] had recognized the “false positive” FDG as a major problem in oncology. Hamacher and Coenen (2002) proposed fluoroethyl tyrosine (FET) PET as the preferred imaging modality for tumor imaging since it has no uptake in inflammatory cells. I-123 iodomethyl tyrosine SPECT/CT will be a better option than FET PET/CT.
Whether Tc-GH is accumulated in infection/inflammation need to be studied separately. Whether it will be able to differentiate infection from malignancy (current dilemma with FDG PET) can be addressed by multi-centric prospective studies in India.
Back-to-back studies on the same patient to compare Tc-GH SPECT/CT and FDG PET/CT will clinch the issue that has great financial implications especially in the third-world countries, since Tc-GH SPECT/CT will be more widely available and more affordable at one-tenth the cost of FDG PET/CT.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mariani G, Strauss HW. Positron emission and single-photon emission imaging: Synergy rather than competition. Eur J Nucl Med Mol Imaging. 2011;38:1189–90. doi: 10.1007/s00259-011-1767-3. [DOI] [PubMed] [Google Scholar]
- 2.Rossman DJ, Siegel ME, Frieman BH. Accumulation of Tc-glucoheptonate in acutely infarcted myocardium. J Nucl Med. 1974;15:529. [PubMed] [Google Scholar]
- 3.Rossman DJ, Strauss HW, Siegel ME, Pitt B. Accumulation of 99m Tc-glucoheptonate in acutely infarcted myocardium. J Nucl Med. 1975;16:875–8. [PubMed] [Google Scholar]
- 4.Rossman DJ, Rouleau J, Strauss HW, Pitt B. Detection and size estimation of acute myocardial infarction using 99m Tc-glucoheptonate. J Nucl Med. 1975;16:980–5. [PubMed] [Google Scholar]
- 5.Arnold RW, Subramanian G, McAfee JG, Blair RJ, Thomas FD. Comparison of 99m Tc complexes for renal imaging. J Nucl Med. 1975;16:357–67. [PubMed] [Google Scholar]
- 6.Tanasescu DE, Wolfstein RS, Waxman AD. Technetium-99m glucoheptonate as a brain-scanning agent. J Nucl Med. 1977;18:1037. [PubMed] [Google Scholar]
- 7.Léveillé J, Pison C, Karakand Y, Lemieux R, Vallières BJ. Technetium-99 m glucoheptonate in brain-tumour detection: An important advance in radiotracer detection. J Nucl Med. 1977;18:957–61. [PubMed] [Google Scholar]
- 8.Sty JR, Kun LE, Starshak RJ. Pediatric applications in nuclear oncology. Semin Nucl Med. 1985;15:171–200. doi: 10.1016/s0001-2998(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 9.Sodee DB, Ballistrea M. Usefulness of Tc-GH SPECT compared to CT with contrast and MRI. Nucl Med Biol. 1987;14:191–204. doi: 10.1016/0883-2897(87)90042-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan WD, Takvorian T, Morris JH, Rumbaugh CL, Connolly BT, Atkins HL. Thallium-201 brain tumor imaging: A comparative study with pathologic correlation. J Nucl Med. 1987;28:47–52. [PubMed] [Google Scholar]
- 11.Vorne M, Sakki S, Järvi K, Vähätalo S. Tc-99m glucohepatonate in detection of lung tumours. J Nucl Med. 1982;23:250–4. [PubMed] [Google Scholar]
- 12.Passamonte PM, Seger RM, Holmes RA, Hurst DJ. Technetium-99m glucohepatonate imaging in lung cancer and benign lung disease: Concise communication. J Nucl Med. 1983;24:997–1000. [PubMed] [Google Scholar]
- 13.Sauerland BA, Rosen PR, Weiland FL, Lasher JC, Myers DL, Hale AS. Uptake of Tc-99m glucoheptonate in cervical lymph node metastases from large-cell bronchogenic carcinoma. Clin Nucl Med. 1983;8:31–3. doi: 10.1097/00003072-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Crane JM, Nelson MJ, Ihde DC, Makuch RW, Glatstein E, Zabell A, et al. A comparison of computed tomography and radionuclide scanning for detection of brain metastases in small cell lung cancer. J Clin Oncol. 1984;2:1017–24. doi: 10.1200/JCO.1984.2.9.1017. [DOI] [PubMed] [Google Scholar]
- 15.de Kieviet W. Technetium radiopharmaceuticals: Chemical characterization and tissue distribution of Tc-glucoheptonate using Tc-99m and carrier Tc-99. J Nucl Med. 1981;22:703–9. [PubMed] [Google Scholar]
- 16.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, et al. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990;13:198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- 18.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–5. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 19.Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72:2979–85. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Kubota K, Kubota R, Yamada S. FDG accumulation in tumor tissue. J Nucl Med. 1993;34:419–21. [PubMed] [Google Scholar]
- 21.Conti PS, Lilien DL, Hawley K, Keppler J, Grafton ST, Bading JR. PET and [18F]-FDG in oncology: A clinical update. Nucl Med Biol. 1996;23:717–35. doi: 10.1016/0969-8051(96)00074-1. [DOI] [PubMed] [Google Scholar]
- 22.Aloj L, Caracó C, Jagoda E, Eckelman WC, Neumann RD. Glut-1 and hexokinase expression: Relationship with 2-fluoro-2-deoxy-D-glucose uptake in A431 and T47 D cells in culture. Cancer Res. 1999;59:4709–14. [PubMed] [Google Scholar]
- 23.Smith TA. FDG uptake, tumor characteristics and response to therapy: A review. Nucl Med Commun. 1998;19:97–105. doi: 10.1097/00006231-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–59. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 25.Taegtmeyer H. Energy metabolism of the heart: From basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 26.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–25. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myears DW, Sobel BE, Bergmann SR. Substrate use in ischaemic and reperfused canine myocardium: Quantitative considerations. Am J Physiol. 1987;253(Pt 2):H107–14. doi: 10.1152/ajpheart.1987.253.1.H107. [DOI] [PubMed] [Google Scholar]
- 28.Liedtke AJ. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981;23:321–36. doi: 10.1016/0033-0620(81)90019-0. [DOI] [PubMed] [Google Scholar]
- 29.Kalff V, Schwaiger M, Nguyen N, McClanahan TB, Gallagher KP. The relationship between myocardial blood flow and glucose uptake in ischemic canine myocardium determined with fluorine-18-deoxyglucose. J Nucl Med. 1992;33:1346–53. [PubMed] [Google Scholar]
- 30.Wheeler TJ. Translocation of glucose transporters in response to anoxia in heart. J Biol Chem. 1988;263:19447–54. [PubMed] [Google Scholar]
- 31.vom Dahl J, Eitzman DT, al-Aouar ZR, Kanter HL, Hicks RJ, Deeb GM, et al. Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation. 1994;90:2356–66. doi: 10.1161/01.cir.90.5.2356. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra R, Brosius FC., 3rd Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem. 1999;274:12567–75. doi: 10.1074/jbc.274.18.12567. [DOI] [PubMed] [Google Scholar]
- 33.Knuuti MJ, Nuutila P, Ruotsalainen U, Saraste M, Härkönen R, Ahonen A, et al. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med. 1992;33:1255–62. [PubMed] [Google Scholar]
- 34.Mobasheri A, Richardson S, Mobasheri R, Shakibaei M, Hoyland JA. Hypoxia inducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: Putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol Histopathol. 2005;20:1327–38. doi: 10.14670/HH-20.1327. [DOI] [PubMed] [Google Scholar]
- 35.Bolli R. Myocardial ‘stunning’ in man. Circulation. 1992;86:1671–91. doi: 10.1161/01.cir.86.6.1671. [DOI] [PubMed] [Google Scholar]
- 36.Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: An NHLBI workshop. Circulation. 1998;97:1848–67. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- 37.Gao WD, Liu Y, Mellgren R, Marban E. Intrinsic myofilament alterations underlying the decreased contractility of stunned myocardium. A consequence of Ca2+ -dependent proteolysis? Circ Res. 1996;78:455–65. doi: 10.1161/01.res.78.3.455. [DOI] [PubMed] [Google Scholar]
- 38.Schwaiger M, Neese RA, Araujo L, Wyns W, Wisneski JA, Sochor H, et al. Sustained non-oxidative glucose utilization and depletion of glycogen in reperfused canine myocardium. J Am Coll Cardiol. 1989;13:745–54. doi: 10.1016/0735-1097(89)90621-9. [DOI] [PubMed] [Google Scholar]
- 39.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, et al. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–8. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211–21. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Ma L, Linfert DR, Lai T, Fallon JT, Gillam LD, et al. Myocardial cell death and apoptosis in hibernating myocardium. J Am Coll Cardiol. 1997;30:1407–12. doi: 10.1016/s0735-1097(97)00309-4. [DOI] [PubMed] [Google Scholar]
- 42.Brosius FC, 3rd, Liu Y, Nguyen N, Sun D, Bartlett J, Schwaiger M. Persistent myocardial ishcemia increases GLUT1 glucose transporter expression in both ischemic and non-ischemic heart regions. J Mol Cell Cardiol. 1997;29:1675–85. doi: 10.1006/jmcc.1997.0405. [DOI] [PubMed] [Google Scholar]
- 43.Elsässer A, Schlepper M, Klövekorn WP, Cai WJ, Zimmermann R, Müller KD, et al. Hibernating myocardium: An incomplete adaption to ischemia. Circulation. 1997;96:2920–31. doi: 10.1161/01.cir.96.9.2920. [DOI] [PubMed] [Google Scholar]
- 44.Doenst T, Taegtmeyer H. Ischemia-stimulated glucose uptake does not require catecholamines in rat heart. J Mol Cell Cardiol. 1999;31:435–43. doi: 10.1006/jmcc.1998.0878. [DOI] [PubMed] [Google Scholar]
- 45.Lopaschuk G, Collins-Nakai R, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26:1172–80. doi: 10.1093/cvr/26.12.1172. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen N. Enhanced glucose utilization following pre-conditioning in the isolated rat heart. J Nucl Med. 1995;36:47. [Google Scholar]
- 47.Tong H, Chen W, London RE, Murphy E, Steenbergen C. Preconditioning enhanced glucose uptake is mediated by p38 MAP kinase, not by phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:11981–6. doi: 10.1074/jbc.275.16.11981. [DOI] [PubMed] [Google Scholar]
- 48.Egert S, Nguyen N, Schwaiger M. Myocardial glucose transporter GLUT1: Translocation induced by insulin and ischemia. J Mol Cell Cardiol. 1999;31:1337–44. doi: 10.1006/jmcc.1999.0965. [DOI] [PubMed] [Google Scholar]
- 49.Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, et al. Low flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–22. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- 50.Kocagozoglu G, Lambrecht FY, Gunduz C, Yucebaş M, Tabar EB, Ozgur A, et al. In vitro evaluation of apoptosis with 99m Tc-glucoheptonate. Appl Radiat Isot. 2011;69:955–9. doi: 10.1016/j.apradiso.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49:129S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 52.He ZX, Shi RF, Wu YJ, Tian YQ, Liu XJ, Wang SW, et al. Direct imaging of exercise-induced myocardial ischemia with fluorine-18-labeled deoxyglucose and Tc-99m-sestamibi in coronary artery disease. Circulation. 2003;108:1208–13. doi: 10.1161/01.CIR.0000088784.25089.D9. [DOI] [PubMed] [Google Scholar]
- 53.Jain D, He ZX. Direct imaging of myocardial ischemia: A potential new paradigm in nuclear cardiovascular imaging. J Nucl Cardiol. 2008;15:617–30. doi: 10.1016/j.nuclcard.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, et al. FDG uptake and glucose transporter subtype expressions in experimental tumour and inflammation models. J Nucl Med. 2001;42:1551–5. [PubMed] [Google Scholar]
- 55.Strauss LG. Fluorine-18 deoxyglucose and false-positive results: A major problem in the diagnostics of oncology patients. Eur J Nucl Med. 1996;23:1409–15. doi: 10.1007/BF01367602. [DOI] [PubMed] [Google Scholar]


