Abstract
Aim:
To determine whether F-18-fluorodeoxyglucose positron emission tomography (F-18-FDG PET) can be used to differentiate among common enhancing brain tumors such as gliomas, metastatic brain tumors, and lymphoma.
Materials and Methods:
We evaluated 20 patients with an enhancing brain tumor on magnetic resonance imaging (MRI). FDG PET scan was done in all patients pre operatively. For PET image analysis, regions of interest were placed over the tumor (T), contralateral cortex (C), and white matter (WM). Average and maximum pixel values were determined at each site. On the basis of these measurements, average and maximum standard uptake values (SUV avg and SUV max ) were calculated, and comparisons among lesions were then made.
Results:
SUVavg and SUVmax are significantly higher for central nervous system (CNS) lymphoma than for other tumors (P < 0.01). High-grade gliomas showed significantly higher SUVavg and SUVmax than the low grade gliomas (P < 0.05) and metastatic tumor showed higher SUVavg and SUVmax than all gliomas, both low and high grade (P < 0.05). When the lowest values of CNS lymphoma parameter were used as cutoff levels to distinguish CNS lymphomas from other tumors (i.e. 100% sensitivity), SUVmax was the most accurate parameter. Using a SUVmax of 15.0 as a cutoff for diagnosing CNS lymphoma, only one case of metastasis (SUV max , 16.3) was found to be false positive in this study.
Conclusion:
FDG PET appears to provide additional information for differentiating common enhancing malignant brain tumors, namely lymphoma versus high grade glioma and metastatic tumor, particularly when differential diagnoses are difficult to narrow using MRI alone.
Keywords: Brain tumor, nuclear medicine, PET, F-18 fluorodeoxyglucose
INTRODUCTION
Glioma,metastatic brain tumor and central nervous system(CNS) lymphoma are all examples of common, enhancing malignant brain tumors on magnetic resonance imaging (MRI).[1] Although radiological features of these tumors are well known, accurate diagnosis remains difficult in some cases on conventional MRI. As the therapeutic approaches for intra cerebral tumors differ considerably accordingly to tumor types, advanced MRI techniques such as perfusion weighted-MRI, diffusion-weighted MRI, and MR spectroscopy are being commonly used.[2–4] However, contribution of F-18-fluorodeoxyglucose positron emission tomography (F-18-FDG PET) to the differential diagnosis has not been evaluated yet.
Gliomas are the most common primary tumors of the CNS and represent approximately one-third of all intracranial tumors in adults. The therapeutic management and prognosis in patients with gliomas depend on the reliable distinction between high and low grade gliomas. Primary brain tumors frequently have a mixed histology of both high and low grade malignancy, and serious sampling errors have been reported with stereotactic biopsies. Necrosis is the microscopic hallmark of glioblastoma multiforme. The diagnosis is easily made when necrosis is present in the biopsy specimen, but a diagnosis of anaplastic astrocytoma will be difficult if limited sample does not contain evidence of necrosis.
PET is a powerful imaging technique that enables in vivo examination of brain functions. It allows non-invasive quantification of cerebral blood flow, metabolism, and receptor binding. In the past, PET has been employed mainly in the research setting due to the relatively high costs and complexity of the support infrastructure, such as cyclotrons, PET scanners, and radio chemistry laboratories. In recent years, because of advancements in technology and proliferation of PET scanners, PET is being increasingly used in clinical neurology to improve our understanding of disease pathogenesis, to aid in diagnosis, and to monitor disease progression and response to treatment.
For oncologic imaging, PET with fluorine 18-labeled FDG is an established diagnostic tool.[5,6] FDG is a glucose analog. It is transported and phosphorylated like glucose, but then trapped in the cells. Due to very slow dephosphorylation rate, PET imaging can be performed for certain time interval to evaluate regional metabolism.
F-18-FDG PET evaluates the lesions on the basis of glucose metabolic activity and has been successfully applied for brain tumor imaging in a wide variety of indications including diagnostic, prognosis, and assessment of response to therapy.[7–10] It has a prognostic significance, but whether FDG uptake value has an independent prognostic value above that of histology remains debated. Another fact is that it is still not in use to differentiate common enhancing brain tumors.
MRI has a very high soft tissue resolution with multiplaner capability. Although MRI imaging is the most commonly used radiological technique in the diagnostic and evaluation of common brain tumors, in many instances, it is difficult for differentiation of the common enhancing malignant brain tumors.[11] Depending on the MRI characters such as heterogeneity, cyst formation, necrosis, hemorrhage, tumor crossing the midline, edema and/or mass effect, definition of border, flow void, and degree and heterogeneity of contrast enhancement, gliomas could be classified as low grade astrocytoma, anaplastic astrocytoma, and glioblastoma multiforme on MRI.
While morphological methods such as computed tomography (CT) and MRI are commonly used to achieve a classification according to tumor-node-metastasis (TNM) system, PET can help improve the staging accuracy by limiting the number of false negative result.
MATERIALS AND METHODS
A total of 20 patients with MRI-enhancing suspected malignant lesions were prospectively included in this study.
Inclusion criteria
Patients having contrast enhanced brain tumors in MRI. Enhancing brain tumor is defined as nodular enhancement >1 cm in diameter or ring enhancement >1-cm-thick in marginal solid portion.
Patients who require surgical intervention or biopsy for the brain tumors for histopathological examinations.
Exclusion criteria
Random blood sugar > 200 mg/dl
Taking steroids
Previously irradiated tumor
Pregnancy
MRI
All tumors were imaged using 1.5T MRI scanner or a 3.0 T scanner. The scan included T1 SE (pre-contrast) and T2 TSE and FLAIR sequence, followed by intravascular (I.V) injection of 0.1 mmol/kg body weight of gadopentetate dimeglumine (magnevist); contrast enhanced axial T-1 weighted MRI was performed using spin echo sequence.
PET/CT
All patients with enhanced brain tumors underwent PET/CT scan of the brain. The patient were kept fasting for at least 6 h, FDG PET/CT images were obtained 60 min after the I.V injection of FDG (dose ranging from 370–555 MBq) and CT-based attenuation correction was also made.
Acquisition and reconstruction parameter of FDG PET are 2 min emission per bed position (total brain acquisition time is 5 min), 3D acquisition, 50-cm axial field of view, and an ordered-subsequent expectation maximization iterative reconstructions (subsets, 14; number of iterations, 2) with 3.5-mm slice thickness. The semiquantative parameter of standardized uptake value (SUV) was estimated using the following formula.
SUV = Activity at a pixel (kBq/CC)/injection dose (MBq)/weight (kg)
The SUV is a distribution parameter reflecting the accumulation of radiopharmaceutical in tissues. A value of 1.0 SUV represents a homogenous distribution, while value exceeding one reflect enhanced tracer uptake.
Image analysis
For FDG PET images analysis, single regions of interest (ROIs) as large as possible were placed over the tumor using information obtained from enhanced MRI. Slices display maximum tumor activity were selected. In the case of multiple tumors, the largest tumor were selected for analysis. ROI over the contralateral cortex and contralateral white matter were placed following the method described by Delbeke et al.[5]
Average SUV (SUVavg) and maximum SUV (SUVmax) of tumors were obtained on the basis of pixel values for every ROI. Average counts per pixel of tumor (T), white matter (WM), cortex (C), and counts of maximum pixel of tumor were used to generate activity ratios. On the basis of these measurements, the following parameters were generated: the SUVavg and SUVmax, the average tumor to cortex activity ratio (T/C avg), the average tumor to white matter activity ratio (T/WMavg), the ratio of the count of maximum pixels in the tumor to the average count per pixel in the cortex (T/C max) and the ratio of the count of maximum pixels in the tumor to the average count per pixel in the white matter (T/WMmax).
Histopathology
During the surgery, resected brain tumors samples were collected and fixed with formalin and submitted for routine hematoxylin-eosin staining in addition to immunohistochemical studies. After detection of tumors type, tumors were graded by using WHO criteria. For statistical purpose, tumors will be classified benign and malignant and later will be subdivided in to low grade (grade 2) and high grade (grades 3 and 4).
After collecting all data of MRI, FDG-PET, and histopathology report, they were correlated with each other and statistical analysis was done.
RESULTS
A total 20 patients were analysed. All patients had their PET scans prior to the histological diagnosis and any sort of therapeutic intervention. The mean age of the patients was 45 years (18-65 years) and with 13 males and 7 female in the study (M:F = 1.86:1). Seven patients had low-grade gliomas (astrocytoma grade 2, ODG grade 2), 8 patients had high grade gliomas (glioblastoma multiforme, GBM), 4 patients had metastatic lesion, and 1 case was proved as lymphoma.
Mean values and SDs of parameters in each group are summarized in Table 1 and scatter plots of maximum and average 18F-FDG uptake values (SUVavg and SUVmax) are shown in Figure 1. Contrast MRI images of diferrent brain tumors and their corresponding 18F-FDG PET scan are shown in Figures 2 and 3.
Table 1.
Parameter values for each group of lesions
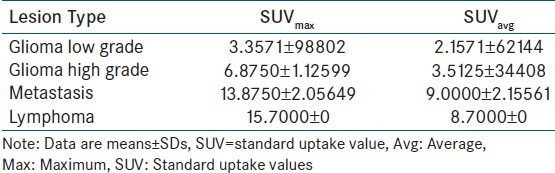
Figure 1.
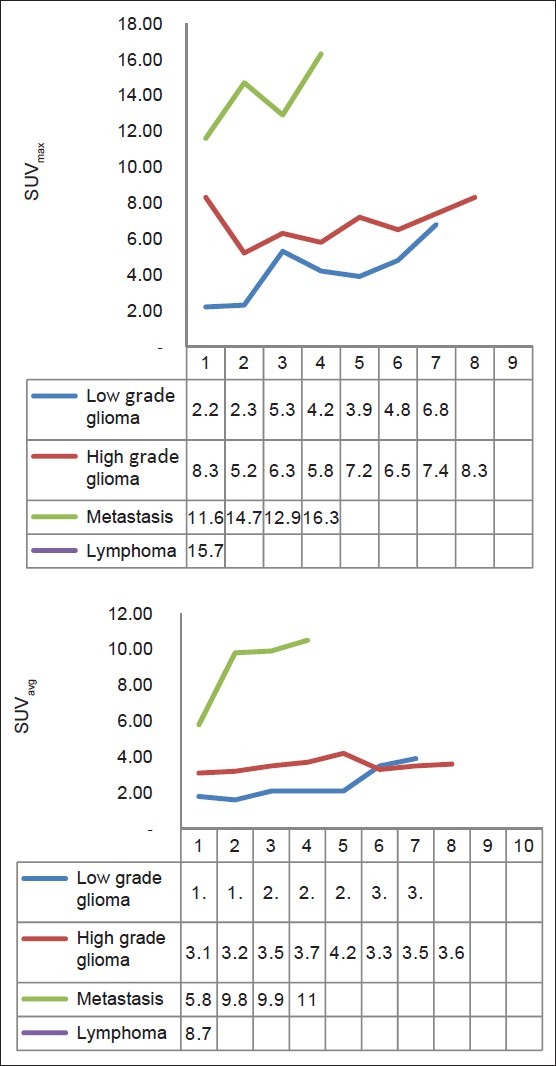
Scatter plots showing maximum and average 18-FDG uptake values (SUVavg and SUVmax)
Figure 2.
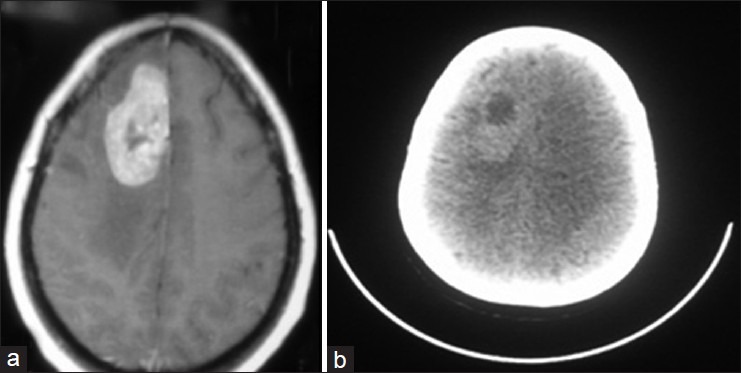
65-year-old woman with metastatic adenocarcinoma from lung cancer. (a) Axial contrast-enhanced T1 weighted MRI showing enhancing A brain tumor, (b) Corresponding axial 18F-FDG PET image showing high FDG accumulation.
Figure 3.
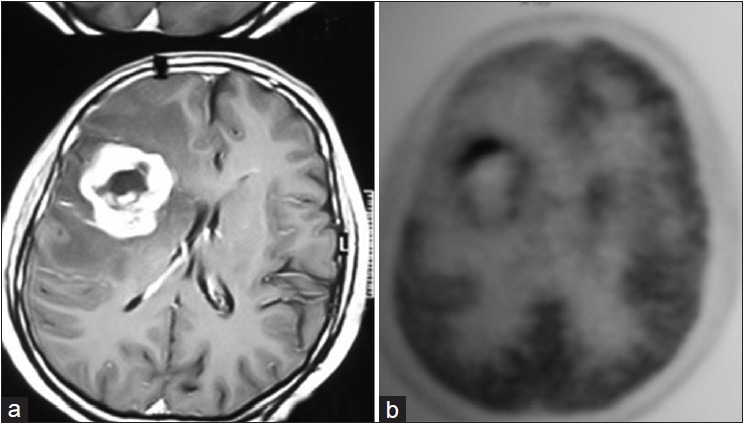
A 55-year-old man with glioblastoma multiforme. (a) Axial contrast-enhanced T1 weighted MR image shows enhancing brain tumor, (b) Corresponding axial 18F-FDG PET image shows moderate FDG accumulation
DISCUSSION
The above study revealed different FDG accumulation in common enhancing malignant brain tumors, suggesting that FDG PET may be useful in distinguishing among these brain tumors, particularly for distinguishing high grade and low grade gliomas and metastatic brain tumors and lymphoma.
It was also found that SUVmax was the most accurate parameter for distinguishing brain tumors in this study group. Several indications for FDG PET have been suggested in the literatures. Useful roles for FDG PET have been established in staging, evaluation of early response to chemotherapy, assessment of end response to therapy, planning of radiation therapy, and follow-up.[8]
Regarding CNS lymphoma, FDG PET is also reportedly useful for detecting tumors and distinguishing lymphoma from other lesions showing marked FDG accumulation, particularly in patients with AIDS.[6,9–12] The result of this study is in accordance with those of previous reports, suggesting that high FDG accumulation may indicate CNS lymphoma. However, Rosenfeld et al.,[10] reported different results that showed similar FDG accumulation between CNS lymphoma and high grade gliomas. They evaluated the activity ratios of FDG PET in 10 patients with CNS lymphoma and made comparisons with the ratios from 13 patients with high grade glioma. That study found no significant differences between ratios of CNS lymphoma and high grade glioma, whereas, in this study, all parameters were significantly higher for CNS lymphoma than for high grade glioma (P < 0.01).
Hustinx et al.,[13] previously evaluated SUVs and activity ratios in primary brain tumors on FDG PET and concluded that SUV measurements of brain tumor were influenced by a wide variety of factors, such as plasma glucose level, steroid treatment, tumor size and heterogeneity, time after injection, and previous irradiation; SUV measurements appeared to be of limited value in characterizing brain tumors as compared with the measurement of activity ratios and visual assessment. However, results of this study indicate that measurement of SUV is also useful in distinguishing newly diagnosed brain tumors.
In malignant brain tumors, areas of necrosis are often identified throughout the tumor, so apparent necrotic portions has been excluded from the ROI because necrotic portions show considerably less FDG accumulation and influence average counts inside the ROI. However, excluding small foci of necrosis from the ROI was impossible, and this may influence average counts in tumors. Furthermore, excluding all normal or oedematous brain tissue is virtually impossible when drawing an ROI on a tumor. Using counts of the maximum pixel can limit the importance of this factor. In addition, SUVmax may not be influenced by the variety of FDG accumulation in contralateral brain tissue, which may vary with age[14] or underlying diseases. These factors might explain why SUV max was the most accurate parameter for distinguishing CNS lymphomas from other tumors. This study also led to the conclusion that SUVmax may be easier to measure than activity ratio in clinical settings because the necrotic portion need not be excluded from the tumor in the ROI and the FDG accumulation in the contralateral brain tissue need not be measured.
Using the cutoff level, the possibility of lymphoma can be excluded and the differential diagnosis can be narrowed to high grade glioma and metastatic brain tumor. Between these two tumors, it was found in the present study that high grade gliomas show significantly lower SUVavg and SUVmax than does metastatic tumors. However, considerable overlap exists between these tumor types, which are, thus, unlikely to be distinguished by FDG accumulation alone in clinical settings. In this regard, Jeong et al.,[15] showed that whole body FDG PET is useful for detecting primary lesions in patients with suspected metastatic brain tumors and can be helpful in differentiating metastatic brain tumor from primary brain tumor. Such whole body screening is one of the advantages of FDG PET as compared with MRI.
Among men, 15-54 years old, primary CNS tumors are the third leading cause of cancer-related mortality.[16] FDG PET has the unique capability of non-invasively assessing glucose metabolism of tumors, although the injection of a radiotracer is considered by many to be invasive. Moreover, absolute quantification of the glucose metabolic rate of tumors and normal brain is possible through appropriate kinetic and mathematical modelling.[4] Such measurements have been found to strongly correlate with the histological grade of the tumors as well as to their outcome.[17] However, the required arterial blood sampling and the complexity of the approach have prevented its widespread use in either clinical or research applications. In addition, several authors have demonstrated that non-invasive methods, the simplest of all being visual analysis, may provide similar or even more accurate characterization of the lesion.[1,18] In 1995, Delbeke et al.,[8] proposed optimal cutoff levels of FDG uptake in the differentiation of low grade from high grade brain tumors. However, as shown by recent articles, the optimal method for assessing the degree of tumor activity with FDG PET remains to be determined. For instance, Kaschten et al.,[19] utilized numerous variable ROIs to generate count activity ratios. Normalization of the tumor uptake to the injected dose per body weight (SUV) was introduced as a reproducible and reliable semi-quantification of the metabolic activity.[20–22] It is widely used by most PET centres around the globe and is useful in several circumstances such as in differentiating malignant from benign breast lesions[23] or separating malignant mesotheliomas from benign pleural diseases.[9]
Recently, O’Doherty et al., characterized brain lesions in 19 HIV positive patients based on their metabolic activity, as measured by the SUV.[10] Non-hodgkin lymphomas consistently revealed higher SUVs than toxoplasmosis infections. There was no overlap between these two groups. The SUVs of the contralateral brain were highly variable (range 1-5.6) mainly because, according to the authors, the ROIs over the “normal” brain sometimes included parts of the basal ganglia that show a high FDG uptake.
Villringer et al.,[12] also demonstrated the usefulness of FDG PET for the same purpose, but they utilized the SUV ratio rather than the absolute SUV because of the great variability observed among these patients. It is usually recognized that visual grading of FDG uptake on PET images statistically separate hypometabolic LGGs from hypermetabolic HGGs.[6,9–11] High uptake of FDG is generally attributed to increased glycolytic rate in rapidly growing brain tumors cells.[3,11,12] Patronas et al., demonstrated an inverse statistical correlation between deoxyglucose trapping by the tumor and the length of survival.[4] They used optimal cutoff levels of FDG uptake to separate hypo and hyper metabolic HGGs and demonstrated that the level of FDG uptake is more important than histological grading as a prognostic factor. This finding was in accordance with other studies.[10,13] These PET studies, however, combined LGGs and HGGs, and were performed after the establishment of diagnosis and, in some cases, treatment application. The present study on PET studies performed before surgery in patients with anaplastic astrocytoma and glioblastoma. Median and range of age was not that found in the literature, the mean age of anaplastic astrocytoma was particularly low. Histologically, glioblastoma essentially differ from anaplastic astrocytoma by the presence of necrosis, since anaplasia is seen in both grades.[7]
This study also demonstrated that in glioblastoma, glucose uptake is higher than in anaplastic astrocytoma. This difference could be explained by the presence of necrosis. Necrosis in glioblastoma derives from the mismatch between rapid cell growth and insufficient endothelial development. Indeed, tumor blood flow is low in such tumors, and perfusion is inadequate to meet metabolic needs.[5] Therefore, important proportions of glioma cells are in a pre-necrotic status. Kubota et al., have shown that pre-necrotic cells are hypermetabolic[8,15] probably because anaerobic glycolytic pathways are activated with an increase of glucose uptake. The higher proliferation rate in glioblastoma may also participate in a higher energetic consumption in these tumors than in anaplastic astrocytoma, which would result in a hyper glycolytic rate. In tumor, where macroscopic necrosis largely predominates, metabolism may be reduced. It may be a relationship between proliferation index and metabolic grade in glioblastoma that converge to provide the predictive value to PET-FDG in these tumors. The fact that extremely necrotic tumors have a paradoxically low FDG uptake might slightly reduce this predictive value.
PET has the ability to detect cancer based on molecular and biochemical processes within the tumor tissues. The first attempt to visualize brain tumour with PET was published in 1953 by Bronell and Sweet.[24] PET uses radioisotopes of natural elements, oxygen-15, carbon-11, nitrogen-13, and fluorine-18. These radioisotopes retain their normal biological function and allow synthesis of numerous positron-emitting radiopharmaceuticals. PET imaging can provide quantitative information regarding blood flow, receptor status, and metabolic processes. The most widely used radiotracer in oncology at this time is the glucose analogue 18-FDG. Increased glycolysis is one of the most distinctive biochemical features of malignant cells because of the inefficient metabolism of glucose in malignant tumors. It results from the amplification of the glucose transporter proteins at the tumor cell surface as well as from increased activity of various key enzymes, including hexokinase. Like glucose, 18-FDG is transported into cells by a glucose transporter protein and rapidly converted into 18-FDG-6-phosphate. As the latter is not a substrate for glucose-6-phosphate isomerase, it is biochemically trapped in metabolizing tissues. 18-FDG has distinct advantages when compared with other positron-emitting radiopharmaceuticals. It can be efficiently radiolabeled by an automated method and its longer half-life (110 min) as compared with 20 min for carbon-11, for example, provides an opportunity for off-site preparation avoiding the need for an on-site cyclotron. 18-FDG PET has been used for cancer screening in asymptomatic patients, differential diagnosis of benign versus malignant lesion, cancer of unknown origin, detection of the primary site, staging at initial diagnosis, and end-of-treatment evaluation.
The following limitations were identified in this study. First, the number of patients was limited. Furthermore, all entities other than lymphoma, high and low grade glioma, and metastatic brain tumor were excluded. Second, ROI-based analysis was used in this study. ROIs were placed by the consensus of nuclear medicine physicians, but they were operator-dependent, which may influence the mean values in this study. Fusion of PET with MRI would provide the best view for drawing tumor regions, but that technique is not currently available at our institution. In conclusion, FDG PET appears to provide additional useful information for distinguishing lymphoma from other malignant enhancing brain tumors. FDG PET should be recommended when difficulty is encountered in narrowing the differential diagnosis on the basis of MRI alone. Oncogenic potential of PET scan is non-significant.
At last, it can be concluded that FDG PET appears to provide additional information for differentiating common enhancing malignant brain tumors, namely lymphoma versus high grade glioma and metastatic tumor. FDG PET can provide useful information for distinguishing between lymphoma and other malignant enhancing brain tumors, particularly, when differential diagnoses are difficult to narrow using MRI alone.
SUVavg and SUVmax are significantly higher for CNS lymphoma than for other tumors (P< 0.01). High grade gliomas showed significantly higher SUVavg and SUVmax than the low grade glioma (P < 0.05) and metastatic tumor showed higher SUVavg and SUV max than gliomas, both low and high grade (P < 0.05). When the lowest values of CNS lymphoma parameter were used as cutoff levels to distinguish CNS lymphomas from other tumors (i.e., 100% sensitivity), SUVmax was the most accurate parameter [Figure 3]. Using a SUVmax of 15.0 as a cutoff for diagnosing CNS lymphoma, only one case of metastasis (SUVmax, 16.3) was found to be false positive in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kosaka N, Tsuchida T, Uematsu H, Kimura H, Okazawa H, Itoh H. 18F-FDG PET common enhancing malignant brain tumors. AJR Am J Roentgenol. 2008;190:W365–9. doi: 10.2214/AJR.07.2660. [DOI] [PubMed] [Google Scholar]
- 2.Bulakbasi N, Kocaoglu M, Ors F, Tayfun C, Ucöz T. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol. 2003;24:225–33. [PMC free article] [PubMed] [Google Scholar]
- 3.Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumours. Eur J Radiol. 2006;58:394–403. doi: 10.1016/j.ejrad.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M. Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J Magn Reson Imaging. 2006;24:817–24. doi: 10.1002/jmri.20707. [DOI] [PubMed] [Google Scholar]
- 5.Wahl RL. Nuclear medicine in clinical diagnosis and treatment. Vol. 2. New York: Churchill Livingstone; 1994. Positron emission tomography: Application in oncology; pp. 801–20. [Google Scholar]
- 6.Delbeke D. Oncological applications of FDG PET imaging. J Nucl Med. 1999;40:1706–15. [PubMed] [Google Scholar]
- 7.Barker FG, 2nd, Chang SM, Valk PE, Pounds TR, Prados MD. 18-Flurodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer. 1997;79:115–26. [PubMed] [Google Scholar]
- 8.Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, et al. Optimal cutoff levels of F-18 flurodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995;195:47–52. doi: 10.1148/radiology.195.1.7892494. [DOI] [PubMed] [Google Scholar]
- 9.Palmedo H, Urbach H, Bender H, Schlegel U, Schmidt-Wolf IG, Matthies A, et al. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: Correlation with MRI and clinical follow up. Eur J Nucl Med Mol Imaging. 2006;33:164–8. doi: 10.1007/s00259-005-1917-6. [DOI] [PubMed] [Google Scholar]
- 10.Charnley N, West CM, Barnett CM, Brock C, Bydder GM, Glaser M, et al. Early change in glucose metabolic rate measured using FDG-PET in patients with high-grade glioma predicts response to temozolamide but not temozolomide plus radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:331–8. doi: 10.1016/j.ijrobp.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Bénard F, Romsa J, Hustinx R. Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med. 2003;33:148–62. doi: 10.1053/snuc.2003.127304. [DOI] [PubMed] [Google Scholar]
- 12.Levin VA, Leibel SA, Gutin PH. Neoplasm of the central nervous system. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer principles and practice of oncology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 13.Ricci PE. Imaging of adult brain tumors. Neuroimaging Clin N Am. 1999;9:651–69. [PubMed] [Google Scholar]
- 14.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48. [PubMed] [Google Scholar]
- 15.Wrenn FR, Jr, Good ML, Handler P. The use of positron-emitting radioisotopes for the localization of brain tumors. Science. 1951;113:525–7. doi: 10.1126/science.113.2940.525. [DOI] [PubMed] [Google Scholar]
- 16.Hustinx R, Smith RJ, Benard F, Bhatnagar A, Alavi A. Can the standardized uptake value characterize primary brain tumours on FDG-PET? Eur J Nucl Med. 1999;26:1501–9. doi: 10.1007/s002590050487. [DOI] [PubMed] [Google Scholar]
- 17.Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology. 1982;32:1323–9. doi: 10.1212/wnl.32.12.1323. [DOI] [PubMed] [Google Scholar]
- 18.Hoskin PJ. PET in lymphoma: What are the oncologist's needs? Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S37–41. doi: 10.1007/s00259-003-1158-5. [DOI] [PubMed] [Google Scholar]
- 19.O’Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38:1575–83. [PubMed] [Google Scholar]
- 20.Rosenfeld SS, Hoffman JM, Coleman RE, Glantz MJ, Hanson MW, Schold SC. Studies of primary central nervous system lymphoma with fluorine-18-fluorodeoxyglucose positron emission tomography. J Nucl Med. 1992;33:532–6. [PubMed] [Google Scholar]
- 21.Villringer K, Jäger H, Dichgans M, Ziegler S, Poppinger J, Herz M, et al. Differential diagnosis of CNS lesions in AIDS patients by FDG-PET. J Comput Assist Tomogr. 1995;19:532–6. doi: 10.1097/00004728-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman JM, Waskin HA, Schifter T, Hanson MW, Gray L, Rosenfeld S, et al. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med. 1993;34:567–75. [PubMed] [Google Scholar]
- 23.Kuwabara Y, Ichiya Y, Otsuka M, Miyake Y, Gunasekera R, Hasuo K, et al. High [18F] FDG uptake in primary cerebral lymphoma: A PET study. J Comput Assist Tomogr. 1988;12:47–8. doi: 10.1097/00004728-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Brownell G, Sweet WH. Localization of brain tumors with positron emitters. Nucleonics, 1953;11:40–5. [Google Scholar]


