Abstract
Objectives:
Data on the optimal head position for patients with acute ischemic stroke are unavailable. We evaluated the effects of mild head-down tilt (HDT) on cerebral blood flow (CBF) in mice during bilateral common carotid artery occlusion (BCCAO).
Materials and Methods:
We used mice with BCCAO (35 minutes) and divided these into 2 groups ( n=16): BCCAO at 0°-HDT and 5°-HDT. CBF was measured for both hemispheres with a non-invasive laser Doppler blood perfusion imager. Changes in CBF during BCCAO were examined in both groups.
Results:
A significantly greater increase in CBF in both hemispheres was observed in 5°-HDT mice than in 0°-HDT mice (126.1% (8.715)% vs. 102.1% (4.718)%; P=0.0294).
Conclusion:
HDT enhanced the increase in CBF in both hemispheres in the mouse BCCAO model. The potential mechanism underlying CBF increase enhanced by HDT during BCCAO warrants further investigation.
Keywords: Bilateral common carotid artery occlusion, brain infarction, cerebral blood flow, head down, mouse
Introduction
Intravenous recombinant tissue plasminogen activator (rt-PA) treatment for acute ischemic stroke works by achieving recanalization of intracranial occlusion resulting in restoration of flow and prevention of infarct expansion.[1,2] Besides the administration of rt-PA, measures that promote residual cerebral blood flow (CBF) during the acute phase of ischemic stroke may prevent the subsequent development of brain infarction and improve the clinical outcome. Although standardized studies have not provided any data, patients with acute ischemic stroke are routinely positioned with moderate head elevation by paramedics. However, some investigators reported that the 0°-head down position may be optimal for patients with acute ischemic stroke because it promotes residual CBF to the ischemic brain tissue[3,4] and the Trendelenburg position may further increase CBF in certain patients with acute ischemic stroke.[3] Therefore, we evaluated the effects of head-down tilt (HDT) on CBF in mice during bilateral common carotid artery occlusion (BCCAO).
Materials and Methods
The institutional animal care committees of The National Defense Medical College approved all experimental procedures. A total of 16 male C57BL/6J mice (age, 9-11 weeks; weight, 23-27 g) (CLEA Japan Inc., Tokyo, Japan) were divided into 2 groups: BCCAO at 0°-HDT and 5°-HDT. Rectal temperature was maintained at approximately 37.0°C with a heating pad. Indirect systolic blood pressures, heart rates, and respiratory rates were recorded. Mice were anesthetized with 20mg/kg sodium pentobarbital intraperitoneally. The skull was exposed after a midline scalp and periosteum incision [Figure 1a]. CBF in the cortex was measured for both hemispheres with a non-invasive and non-contact laser Doppler blood perfusion imager (PeriscanPIM II; PeriMed, Stockholm, Sweden). Pre-and post-values were recorded between 5 and 35 minutes after BCCAO. CBF changes after BCCAO were expressed as a percentage of the pre-value. BCCAO was performed as reported previously.[5] Briefly, both common carotid arteries were surgically exposed and occluded by applying non-traumatic small clips for 35 minutes. After 35 minutes of occlusion, mice were sacrificed by sodium pentobarbital overdose. In the 5°-HDT group, mice were tilted head-down to an angle of 5° between 5 and 35 minutes after BCCAO. Data are presented as the mean (SEM). Comparisons between the 2 groups for changes in CBF were made by the unpaired t test. P < 0.05 was considered statistically significant.
Figure 1.
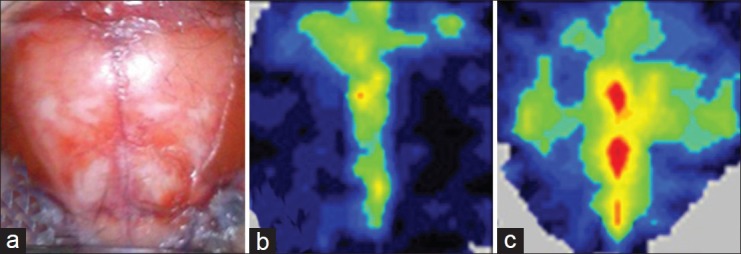
(a) Representative example of the exposure field. At the time of measurement, perfusion images were obtained. (b) In the 0°-head-down tilt (HDT) group, there was no remarkable change in cerebral blood flow (CBF) in both hemispheres at 35 minutes after bilateral common carotid artery occlusion (BCCAO). (c) However, an increase in CBF in both hemispheres was observed at 35 minutes after BCCAO in the 5°-HDT group
Results
All physiological parameters were similar and not significantly different between the 2 groups. In both groups, residual CBF in both hemispheres at 5 minutes after BCCAO was more than 40% of the pre-occlusion value; the values were not significantly different between the 2 groups. In the 0°-HDT group, there was no increase in CBF in both hemispheres during BCCAO [Figure 1b]. However, an increase in CBF in both hemispheres was observed at 35 minutes after BCCAO in the 5°-HDT group [Figure 1c]. Therefore, a significantly greater increase in CBF in both hemispheres was observed in 5°-HDT mice than in 0°-HDT mice (126.1% (8.715)% vs. 102.1% (4.718)%; P=0.0294) [Figure 2].
Figure 2.
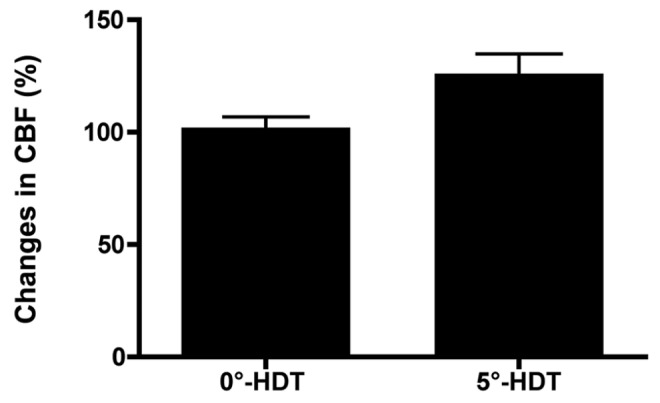
The changes in CBF (means±SEM) at 35 minutes after bilateral common cartoid artery occlusion at 0°-HDT and 5°-HDT mice. A significantly greater increase in cerebral blood flow in both hemispheres was observed in 5°-head down tilt mice than in 0°-HDT mice (126.1±8.715% vs. 102.1±4.718%; P=0.0294); HDT – Head-down tilt; CBF – Cerebral blood flow
Discussion
We examined for the first time the effect of 5°-HDT on CBF in a mouse BCCAO model. We found a significantly greater increase in CBF in both hemispheres in 5°-HDT mice than in 0°-HDT mice during BCCAO. Some previous reports concluded that lowering the head position to 0° may promote residual CBF to the ischemic brain tissue in the acute phase [3,4] and the Trendelenburg position may further increase CBF in certain patients with acute ischemic stroke.[3] Recently, Hunter et al. reported that 8 patients, who were divided into the recanalized (artery completely reopened) and incompletely recanalized groups on the basis of computed tomography angiography findings, underwent bilateral middle cerebral artery (MCA) transcranial Doppler monitoring during orthostatic variation from 30° to 0°at 24 hours after ischemic stroke.[6] For the stroke-affected MCA in the incompletely recanalized group, there was a median increase in the mean flow velocities (MFVs) (from that at 30° head-up tilt to 0° head-up tilt) of 26.0 cm/s (IQR=21.3 to 35.3), whereas in the recanalized group there was no observable change (median difference =−3.5 cm/s; IQR=−12.3 to 0.8) (P value for between-group difference for MFV change=0.021). They concluded that CBF in the incompletely recanalized group might be sensitive to orthostatic variation; however, the recanalized group may have retained CBF stability with orthostatic variation. These conclusions correspond with our result that 5°-HDT enhanced CBF increase in both hemispheres during BCCAO.
Cerebral autoregulation refers to the inherent ability of cerebral blood vessels to maintain a constant CBF over a wide range of systemic blood pressure.[7] However, it has been previously reported that this ability is diminished in stroke patients and can lead to a decrease in CBF velocity during orthostatic stress with head-up tilt.[8] Moreover, it has been previously suggested that the vasculature distal to the occlusion may undergo passive vasodilation by which blood flow is driven by local perfusion pressure gradients, and these gradients can be higher when the head position is lowered because of gravitational force.[3] We previously confirmed that mice with the posterior communicating artery (P-com A) showed high residual CBF (more than 40% of pre-occlusion values) in the same hemisphere during BCCAO.[5] From these reports, we consider that collateral blood flow from the posterior to anterior circulation via P-com A may be enhanced by 5°-HDT because of increasing local cerebral perfusion pressure, and believe that this assumption is consistent with that of a previous report by Hunter et al.,[6] who identified large increases in MFVs in patients with incompletely recanalized arteries when the head-up tilt was lowered horizontally. However, the precise mechanism of the increase in CBF enhanced by HDT during BCCAO is unknown. Further investigation into the relationship between HDT and CBF in the ischemic brain tissue may provide additional insights into the usefulness of HDT during an acute ischemic stroke.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Zangerle A, Kiechl S, Spiegel M, Furtner M, Knoflach M, Werner P, et al. Recanalization after thrombolysis in stroke patients: Predictors and prognostic implications. Neurology. 2007;68:39–44. doi: 10.1212/01.wnl.0000250341.38014.d2. [DOI] [PubMed] [Google Scholar]
- 3.Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: Flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64:1354–7. doi: 10.1212/01.WNL.0000158284.41705.A5. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke. 2002;33:497–501. doi: 10.1161/hs0202.102376. [DOI] [PubMed] [Google Scholar]
- 5.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010;42:566–76. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 6.Hunter AJ, Snodgrass SJ, Quain D, Parsons MW, Levi CR. HOBOE (Head-of-Bed Optimization of Elevation) Study: Association of higher angle with reduced cerebral blood flow velocity in acute ischemic stroke. PhysTher. 2011;91:1503–12. doi: 10.2522/ptj.20100271. [DOI] [PubMed] [Google Scholar]
- 7.Harper AM. Autoregulation of cerebral blood flow: Influence of the arterial blood pressure on the blood flow through the cerebral cortex. J NeurolNeurosurg Psychiatry. 1966;29:398–403. doi: 10.1136/jnnp.29.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak V, Chowdhary A, Farrar B, Nagaraja H, Braun J, Kanard R, et al. Altered cerebral vasoregulation in hypertension and stroke. Neurology. 2003;60:1657–63. doi: 10.1212/01.wnl.0000068023.14587.06. [DOI] [PubMed] [Google Scholar]


