Abstract
Background:
Anaplastic astrocytoma (AA; WHO grade-III) patients determination of prognostic factors helps generating multimodal therapy protocols. For this purpose, in the Baskent University, Adana Medical Research Center, specific characteristics of AA patients who have surgery were retrospectively investigated and factors which affect prognosis has been determined.
Patients and Methods:
Between January 2005 and 2009, 20 patients who have AA have been evaluated retrospectively. Totally, 20 patients had 31 operations. Sixteen patients had only adjuvant radiation therapy (RT). In the postoperative period, 8 patients received adjuvant RT. Nine of 10 patients with tumor recurrence received concomitant therapy with temozolomide (ConcT with TMZ) protocol. No adjuvant therapy protocol could be applied in three patients with poor general condition in the postoperative period.
Results:
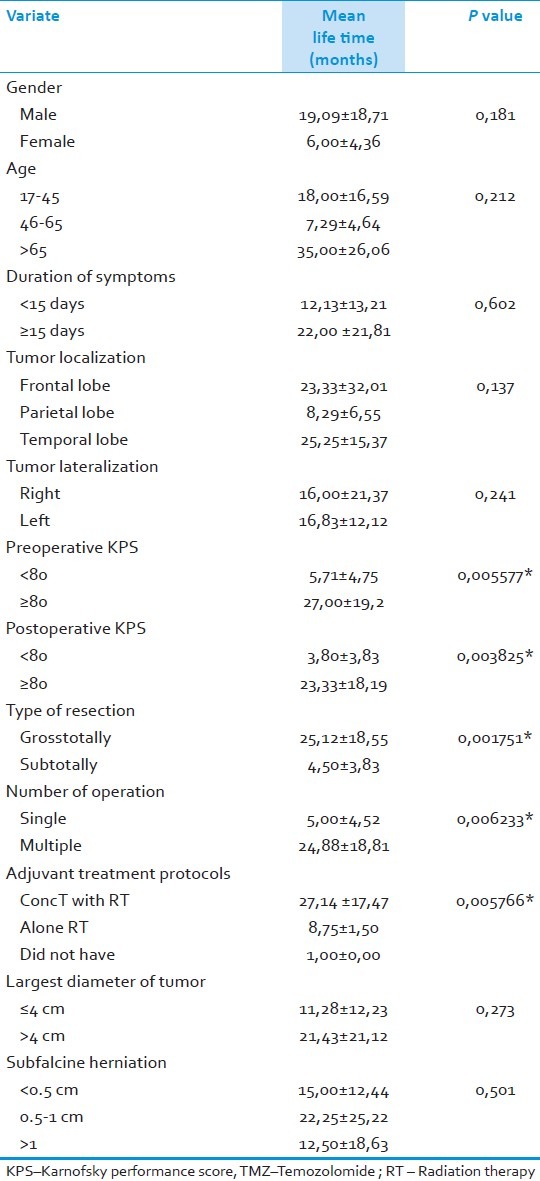
Median survival for patients died was 16±17 months; one year survival was 75% and five year survival 25%. After univariate analysis, preoperative Karnofsky performance score (KPS) was ≥80 (P=0.005577*), postoperative KPS was ≥80 (P=0.003825*), type of tumor resection (P=0.001751*), multiple operations (P=0.006233*), and ConcT with TMZ protocol (P=0,005766*) were all positive prognostic factors which extend the survival.
Conclusions:
The results of the multivariate analysis did not put forward an independent prognostic factor acting on the survival period (P>0.05).
Keywords: Anaplastic astrocytoma, concomitant therapy, prognostic factor, recurrence, temozolomide
Introduction
Primary brain tumors are 1-2% of all cancers. Supratentorial glial tumors are 40-50% of cerebral hemisphere tumors. The anaplastic astrocytomas (AAs) however form 30% of the glial tumors, but this differs from series to series. In Europe, about 60% of patients who are diagnosed of AA are between 45 and 69 years of age. In this age group, the annual incidence rate ranges from 0.5 to 0.7 per 100,000.[1,2] The treatment alternatives for the patients with AA (WHO grade-III) are; surgical treatment and adjuvant radiation therapy (RT). Surgical therapy aids both in the histological differentiation and definition of the tumor tissue and in the reduction of the tumor size. The adjuvant RT applied in the postoperative period has been demonstrated to extend the survival rates of the patients and postpone the recurrences.[3,4] Temozolomide (TMZ) which has been in the market for the last 10 years with a positive safety profile is now being considered to be a promising agent in the treatment of AA patients with recurrences.[5–7]
Patients and Methods
Between January 2005 and January 2009, at the Neurosurgery Clinics of Baskent University Medical School and Adana Medical Research Center, clinical information of 20 patients, who had supratentorially localized AA evaluated retrospectively from patient registries. Follow-up period was a minimum of 14 months and maximum of 60 months. Of the total 20 patients, 13 were male and 7 were female. Male:female ratio was 1.85. Age of the patients were between 23 and 72 years (mean, 49.3±13.6) [Figure 1].
Figure 1.
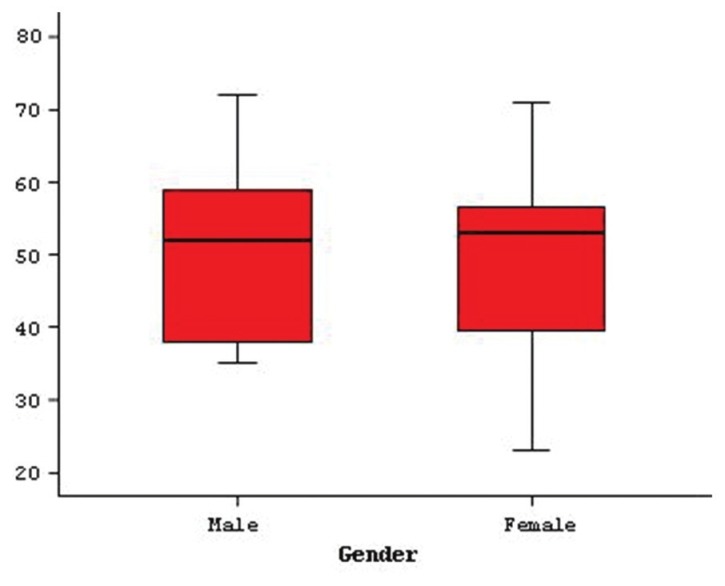
Gender and patients’ ages
Neurological examination
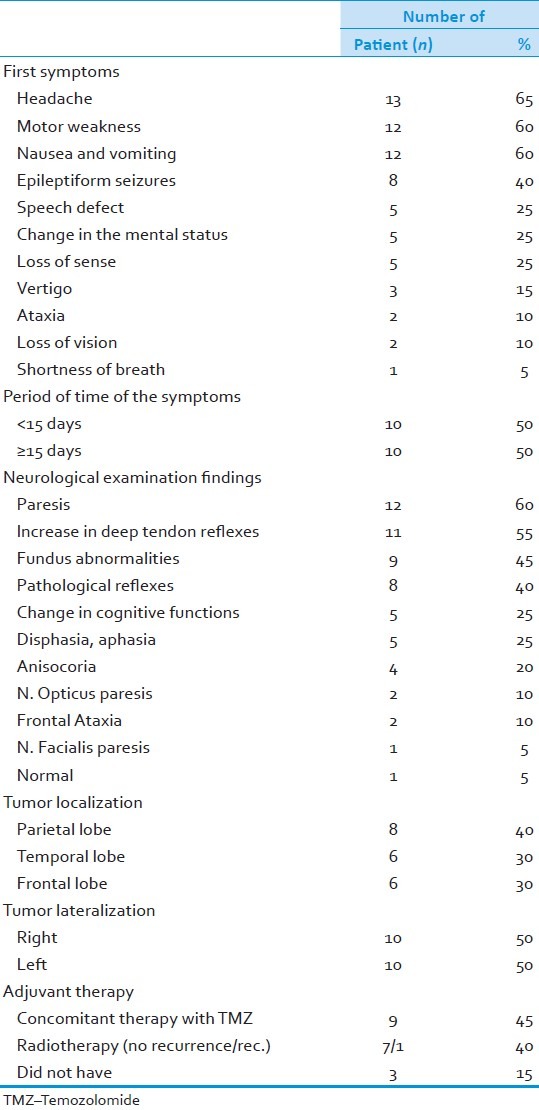
The most common symptom was headache (65%) followed by fatigue (60%), nausea and vomiting (60%), and epileptic seizures (40%). While in half of the patients the time elapsed until a diagnosis made was less than 15 days and in the other half this period lasted more than 15 days. Mostly seen neurologic findings were: Paresis (60%), increase in deep thendon reflexes (55%), fundus abnormalities (45%), and pathological reflexes (40%) [Table 1].
Table 1.
Spesific properties of tumors and the patients
Radiological techniques
Radiological diagnosis method: For 11 patients, both magnetic resonance imaging (MRI) and computed tomography (CT) has been used. For 9 patients, only MRI was used. Parietal lobe was the most affected part (40%). Temporal lobe (30%) and frontal lobe (30%) were less commonly less affected. Fifty percent of tumors were localized on the right hemisphere and 50% were localized on the left hemisphere [Table 1]. Diameter of tumor changes were between 1.5 cm and 7.5 cm (mean, 4.8±1.7 cm).
Surgical treatment
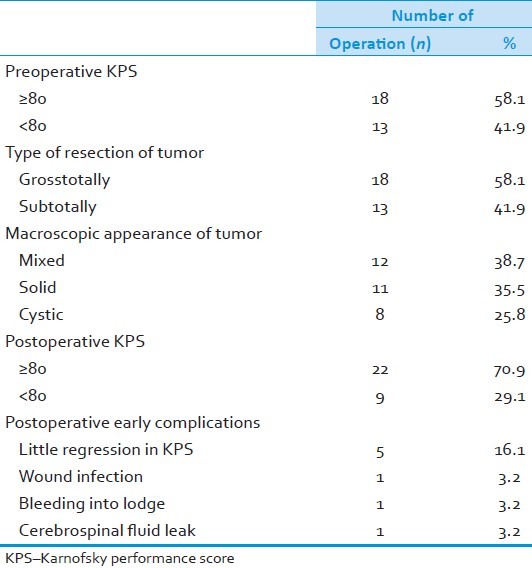
Totally, 20 clinically and radiologically diagnosed patients had 31 cranial surgeries. Preoperatively, 18 operations showed a Karnofsky performance score (KPS) of ≥80, and 13 of the operations showed a KPS of <80. When we looked at the surgical techniques, 18 grosstotal resection and 13 subtotal resection were performed. More agressive surgery was preferred for older patients with a bad neurologic status and whose radiologic images seems to be malignant. When macroscopic appearance of tumors were examined in the operation, 38.7% of them were mixed, 35.5% of them were solid, and 25.8% of them were cystic. Postoperative KPSs were ≥80 and <80 for 22 and 9 operations, respectively. When we look at the postoperative early complications, there were light regression of KPS after 5 (16.1%) operations, wound infection (3.2%), bleeding (3.2%), and cerebrospinal fluid leak (3.2%) [Table 2].
Table 2.
Specific properties of patients, tumor and treatment modality
Radiotherapy protocol
Eight patients were treated with 3D Conformal Radiotherapy (3DCRT) [Table 1]. All patients were immobilized with thermoplastic head mask, and a 2.5 cm slice thickness CT scans were taken in supine position after i.v. contrast injection. The CT images were transferred via DICOM to EclipseÒ treatment planning system. Two PTVs were generated: PTV1 was created by giving a 1.5-2 cm margin to tumor and edema seen on T2-weighted MR images, PTV2 was created by adding 2-2.5 cm margin to tumor without edema seen on T1-weighted MR images. The prescribed total dose was 60 Gy (40 Gy to PTV1 and 20 Gy to PTV2) with conventional fractionation with daily doses of 1.8-2.0 Gy treated from Monday through Friday. Besides target volumes, organs at risk (OAR) including eyes, lenses, and brain doses were also calculated using dose-volume histograms, and the doses of OARs were kept below the tolerance dose limits. Although edema was more prominent during RT, glucocorticoids were not given prophylactically, and were kept until the signs and symptoms of increased intracranial pressure. However, all patients had antiepileptic drugs prophylactically.
Follow-up of patients
First radiological imaging control was 1.5 months after treatment. Thereafter, monthly neurological and bimonthly radiologic controls have been done. Radiological correlation has been searched by MRI and MR-Spectroscopy in the patients that neurologic progression was observed. A total of 10 patients had tumor recurrence. Patients who had AA had 14.1 (±11.4) months of relaps time. Eight patients who had relapse were re-operated once, one patient was re-operated twice, and one patient was re-operated thrice.
Treatment protocol of recurrent tumor
Only one of the ten patients with tumor recurrence received adjuvant RT and nine patients received Concomitant Therapy with Temozolomide (ConcT with TMZ) protocol [Table 1]. In this protocol, TMZ 75 mg/m2/day given orally seven days of the week during the RT course. Adjuvant TMZ therapy was performed 150 mg/m2/day (5 days therapy for each 28 days), and if grade 3 hematologic toxicity did not occur, TMZ dose increased to 200 mg/m2/day (5 days therapy for each 28 days) for six cycles. No other chemotherapy regimens were given concurrently or adjuvant during treatment (ConcT with TMZ). Glucocorticoid therapy (4-16 mg) was given all patients in order to protect them from edema which may be seen during RT. Three cycles of adjuvant TMZ treatment was added for the management of the patients with recurrences; thus, a total of nine cycles of adjuvant TMZ treatment was administered.
Statistical methods
We used SPSS (version 15.0 Inc. Chicago, IL, USA) for statistical analyses. For all patients, median survival calculated by Kaplan-Meier method. Effect on survival of any variable calculated by log-rank test. Multivariate analyses done for factors which were significant with univariate analyses. For multivariate analyses, cox regression analyses were applied. We used analysis of variance (ANOVA) for numeric variables, and for nominal and ordinal variables, we used pearson X2 test. Variables which have P value less than 0.05 accepted as significant.
Results
Of the 20 patients, 6 were still alive. Totally, 31 operations were performed on 14 patients who had died during follow-up period. In this study, specific characteristics of patients were considered and prognostic factors which affect median survival of the patients were also considered. Median survival was 16 (±17) months. One-year survival rate was 75% and five-year survival rate was 25% [Figure 2]. Median survival time of eight patients with recurrence was 24.88 (±18.8) months, while the median survival time of six recurrence-free patients was 5.00 (±4.5) months.
Figure 2.
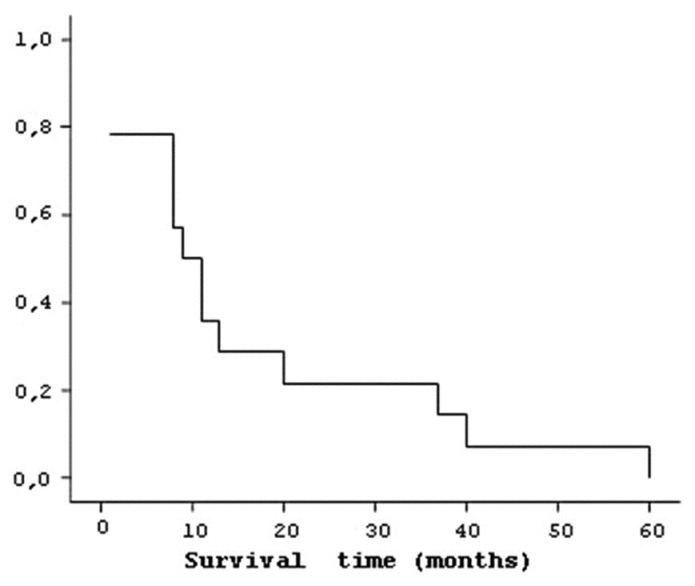
Logarithm of mean life time for patients who died
Patient disposition
Median survival of 11 male patients was 19.09 (±18.7) months and 6 (±4.3) months [Figure 3] for 3 female patients. When age was considered for four patients between 17-45 years, median survival was 18 (±16.5) months, for seven patients between 46-65 years, it was 7.29 (±4.6) months, and for three patients older than 65 years, it was 35 (±26.0) months [Figure 4]. Eight patients diagnosed of AA in less than 15 days had a median survival of 12.13 (±13.2) months and six patients diagnosed of AA after 15 days had median survival of 22 (±21.8) months.
Figure 3.
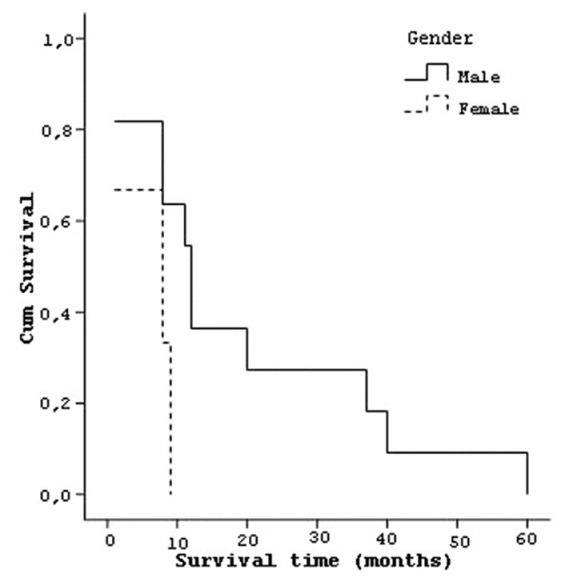
Curve of the survival and patients’ gender
Figure 4.

Curve of the survival and patients’ age
Seven patients with preoperative KPS of <80 had median life time of 5.71 (±4.7) months and seven patients with KPS of ≥80 had a median life time of 27 (±19.2) months [Figure 5]. Five patients who have postoperative KPS of <80 had a median life time of 3.8 (±3.8) months and nine patients with KPS of ≥80 had a median life of 23.33 (±18.1) months [Figure 6].
Figure 5.
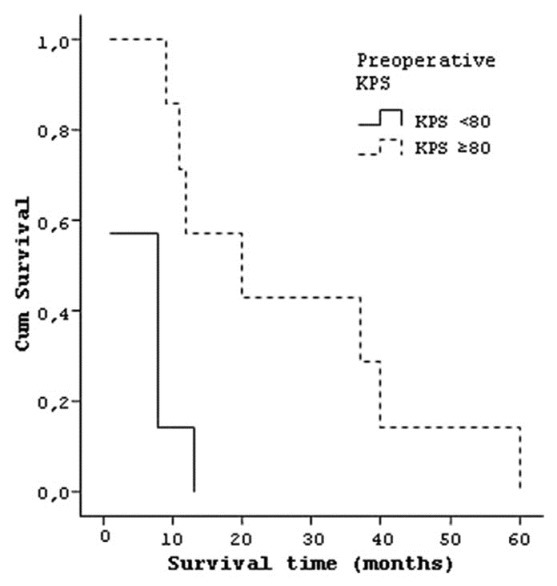
Curve of the survival and preoperative KPS of patients
Figure 6.
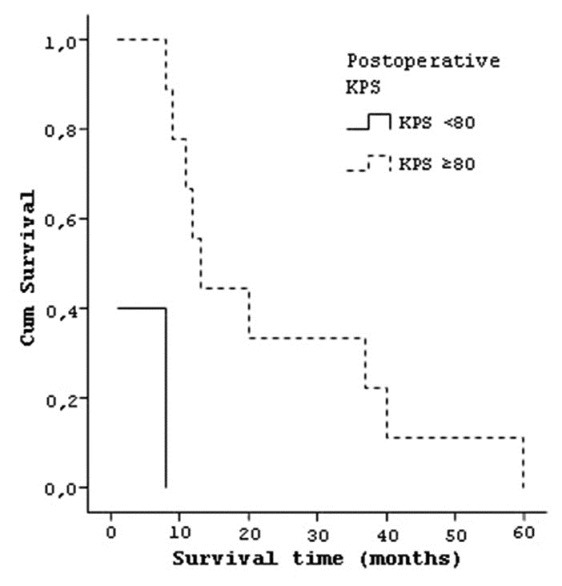
Curve of the survival and postoperative KPS of patients
Four patients who have tumor in temporal lobe had a median survival of 25.25 (±15.3) months, three patients with frontal lobe tumor lived 23.33 (±32.0) months and seven patients with parietal lobe tumor lived 8.29 (±6.5) months [Figure 7]. Six patients who had tumor on left hemisphere had a median survival 16.83 (±12.1) months and eight patients with right hemisphere localized tumors had median survival of 16 (±21.3) months. Seven patients whose tumor size was smaller than 4 cm had a median survival of 11.28 (±12.2) and seven patients who had a tumor size larger than 4 cm had a median survival of 21.43 (±21.1) months [Figure 8]. Similarly the effect of the degree of subfalcine herniation on prognosis studied and; six patients whose herniation less than 0.5 cm had a median survival 15 (±12.4) months, four patients with herniation between 0.5-1 cm had a mean survival of 22.25 (±25.2) months, and four patients with herniation larger than 1 cm had mean survival of 12.5 (±18.6) months [Figure 9].
Figure 7.
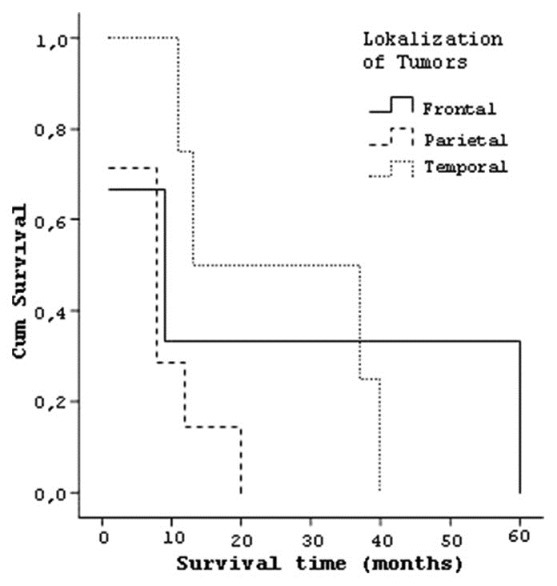
Curve of the survival and localization of tumors
Figure 8.
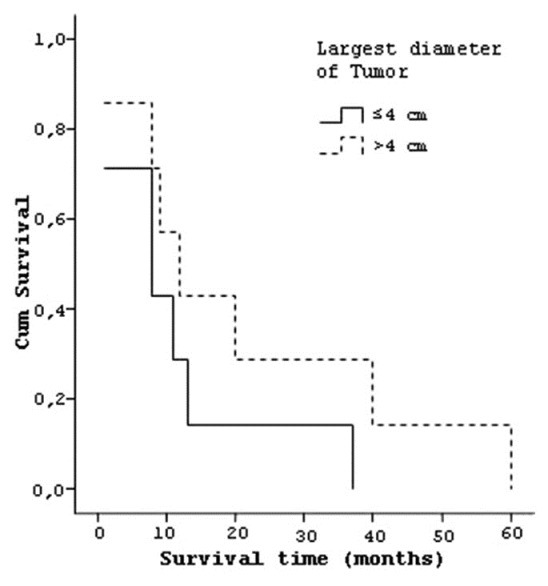
Curve of the survival and largest diameter of tumors
Figure 9.
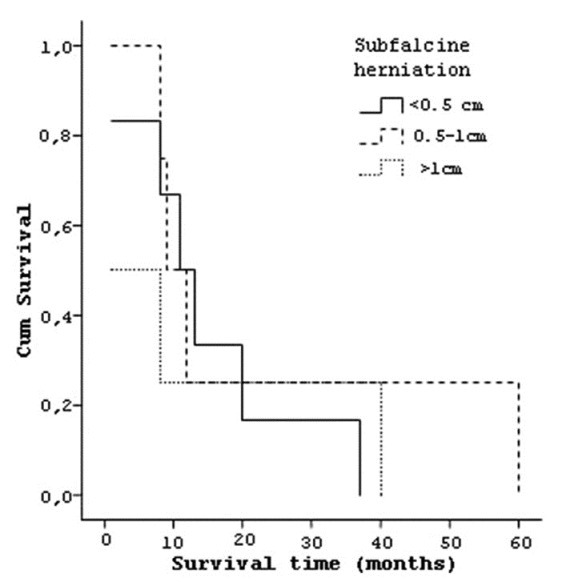
Curve of the survival and subfalcine herniation
Treatment outcomes
When median life time considered for the type of tumor resection, it was 4.5 (±3.8) months for six patients who had subtotal tumor resection, 25.12 (±18.5) months for eight patients who had grosstotal resection [Figure 10]. Six patients who had single operation had a median life time of 5 (±4.5) months, and eight patients who had multiple operations lived 24.88 (±18.8) months [Figure 11].
Figure 10.
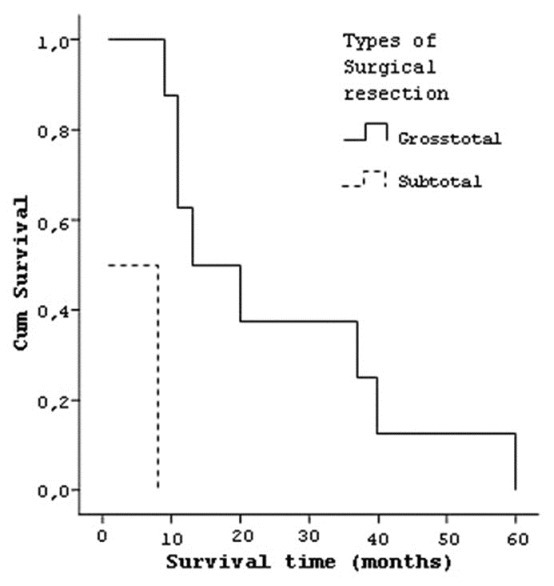
Curve of the survival and types of surgical resection of tumors
Figure 11.
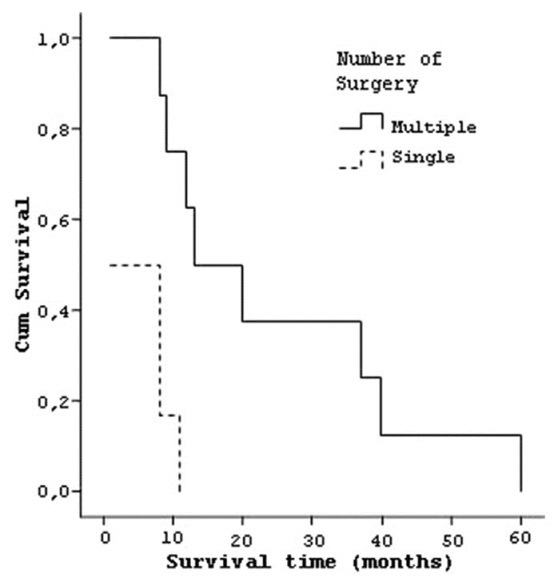
Curve of the survival and number of operation
When postoperative adjuvant treatment protocols were examined, it was found that of 14 patients who died, 7 had received ConcT with TMZ protocol and their median survival time was 27.14 (±17.47) months. The median survival time of four patients receiving adjuvant RT was calculated as 8.75 (±1.50) months. Three patients, with poor general conditions in the postoperative period and a median survival time of 1.00 (±0.00), did not receive any adjuvant therapy [Figure 12]. Two patients who had tumor recurrence and received ConcT with TMZ protocol were still alive for 5 and 17 months.
Figure 12.
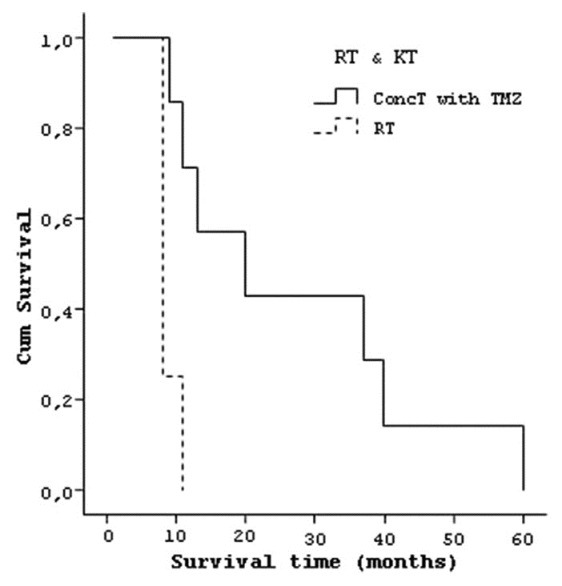
Curve of the survival and types of adjuvant therapy
Prognostic factors
According to univariate statistical analyses, gender (P=0.181), age (P=0.212), diagnosis time (P=0.602), localization of tumor (P=0.137), lateralization of tumor (P=0.241), diameter of tumor (P=0.273) ve subfalcine herniation (P=0.501) were not prognostic factors. Preoperatif KPS ≥80 (P=0.005577*), postoperatif KPS ≥80 (P=0.003825*), gross-total resection (P=0.001751*), multiple operations (P=0.006233*), and ConcT with TMZ protocol (P=0,005766*) were good prognostic factors [Table 3].
Table 3.
Factors which affect the survival of the patients; results of univariate analyses
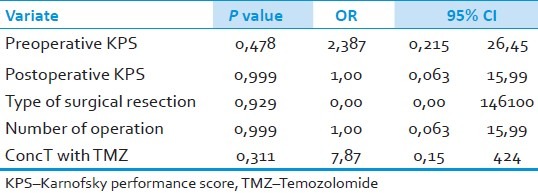
The factors that have finally turned out to be significant by univariate analyses were subjected to multivariate analyses which did not point out to any independent prognostic factor. We found out that none of the factors are effective on prolonging survival significantly: Preoperative KPS ≥80 (P=0.478, OR=2.387, 95% CI=0.215-26.45), postoperative KPS ≥80 (P=0.999, OR=1.00, 95% CI=0.063-15.99), type of surgical resection (P=0.929, OR=0.00, 95% CI=0.00-146100), multiple operations (P=0.999, OR=1.00, 95% CI=0.063-15.99), and ConcT with TMZ protocol (P=0.311, OR=7.87, 95% CI=0.15-424) [Table 4].
Table 4.
Factors which effect the median life time; results of multivariate analyses
Discussion
Although it may vary from series to series, AAs form approximately 30% of the glial tumors. The median age in AAs is 46 years. [1,3] Many studies point out to a shortened life expectancy as age increases. Six prognostic factors have been defined in the database containing the results of the clinical studies performed by the Radiation Therapy Oncology Group (RTOG). While in this study, we investigated the data related to the patients with the diagnosis of AA in Class I-III and determined for the prognostic factors, we also searched for the prognostic factors for the Class IV-VI patients with GBM. It has been reported that in patients younger than 50 years with normal mental status (Class I), the median survival was approximately five years (95% CI, 47 and 108 months), while this was found to be three years (95% CI, 26 and 46 months) in patients with symptoms that lasted more than three months (Class II) within the same age group. It has also been reported in this study that even in younger patients with the diagnosis of AA in whom alterations in mental status have occurred (Class III), the median survival period seemed to be similar to the patients who survived under the age of 50 years with the diagnosis of GBM. The median survival rate in this group has been reported to be 1.5 years (95% CI, 16 and 21 months).[8,9] In our study, age and gender had no prognostic value (P=0.212 and P=0.181).
Epileptic seizures drive attention as the initial symptom of the patients with AA. Symptoms of the increased intracranial pressure are seen in 40%, mental disorders in 15-20%, and focal deficits can be seen in 10-15% of the patients. They have a shorter duration of symptoms before a diagnosis is made.[3,10] Nevertheless, the longer the period of symptoms, that is to say, more than three months from the onset of the disease until a diagnosis is made have been defined as a good prognostic factor in the RTOG study indicating to a slow growing tumor.[8,9] Similarly, in our study, we also found out that the median survival of the patients with the duration of symptoms being longer than 15 days to be longer (22 months). However, univariate analysis did not prove the symptom duration to be valuable as a prognostic factor.
Mario Amminati et al. performed a retrospective analysis on 35 patients with GBM and 20 patients with AA, and reported that patients with better KPS have a better survival rate, with an average of 24 months in comparison with the others.[11] As in RTOG study, results of many studies with AAs also reported that the median survival rates of these patients are negatively affected by lower KPS values.[8,9] In our study, we have found out that the patients with preoperative KPS over 80 lived 21.3 months longer in comparison with the patients having preoperative KPS lower than 80. We also found out that patients with postoperative KPS equal to or over 80 lived 19.5 months longer. Univariate analysis put forward that preoperative KPS of ≥80 (P=0.005577*) and postoperative KPS of ≥80 (P=0.003825*) are favorable prognostic factors in terms of longer survival.
The prognostic factor investigation in the RTOG study, specifically for AA, have shown that the localization, lateralization and diameter of the tumor, its subfalcine herniation are invaluable.[8,9] The literature has little description concerning these factors and hence their prognostic values are controversial. The AAs commonly display a cerebral hemispheral localization. The distribution ratio in the cerebrum is 40% in the frontal lobe, 25% in the temporal lobe, and 25% in the parietal lobe, and this distribution shows similarities with the LGGs.[3] In our study, we observed longer survival periods in patients who had temporal lobe oriented tumors (25.2 months), a tumor with the diameter more than 4 cm (21.4 months) and in patients with subfalcine herniation between 0.5-1 cm (22.2 months) in comparison to the others. However, univariate analysis failed to demonstrate any prognostic value for the tumor localization and lateralization (P=0.137 and P=0.241 respectively), tumor diameter (P=0.273), and subfalcine herniation (P=0.501).
The literature has little information about the methods of treatment for the AA. It was just until recent times that the grade III and grade IV astrocytomas were investigated under malignant glioma in almost all of the studies. Hence this has been leading to a confusion during the evaluation of the results.[2] In a clinical study on 35 patients diagnosed with GBM conducted by Allahdini et al. in 2010, the contribution of surgical resection to prognosis has been investigated, and it has been reported that maximal resection of the tumor volume lengthens the average life span in patients.[12] The positive effect of the surgical resection on median survival rate, specifically for the AA patients, has been shown by the RTOG during the research of prognostic factors and has been recommended that extensive surgical resection is to be made whenever possible in order to increase the median survival rate. Number of surgical interventions was not found to be a prognostic factor in the same study.[8,9] Nevertheless as for the AAs, despite the radical interventions recurrences at the surgical locus is almost inevitable. Literally, a complete resection of this infiltrative disease seems to be virtually impossible. In our study, we found out that the median survival rates of the patients in whom gross total resections were performed was longer than the patients with subtotal resections by 20.6 months and univariate analysis pointed out that the type of surgery was a positive prognostic factor (P=0.001751*).
Studies concerning the effect of reoperations during the treatment of the AAs are not many and their actual role for the success of the treatment is controversial. There are some incorrect views denoting that reoperations would have nothing to offer to the patients with malignant glial tumors but still the patients are enrolled for reoperations.[13] In another study conducted by Harsh et al. with the patients having GBM and AA, it has been reported that when reoperations are to be combined with other adjuvant therapy methods they will potentially be beneficial and prolong the median survival rate of the patients.[14] In our study, the median survival rates of the patients who have undergone for repeated operations have been found to be longer than the patients who had a single intervention, by 19.9 months. Univariate analysis proved reoperations as to be a prognostic factor (P=0.006233*).
RT is the most effective adjuvant therapy for the HGG and prolongs the median survival rate up to 14-36 weeks. In two studies with both in the presence of grade III and IV tumors, reported by the Brain Tumor Study Group (BTSG) in 1978 and 1980, it has been pointed out that the survival of the patients has been prolonged by the application RT. The adjuvant RT application managed to prolong median survival from four months to eight months in patients in whom it was applied and also led to a rise in the 18 months survival rate from 10% to 15-20%.[15,16] Whole Brain Irradiation has been accepted as the standard adjuvant therapy method following these two studies.
Although some clinical studies in the literature denote that RT application is an independent prognostic factor[3,17–19] in prolonging the survival some other studies put forward that there is no difference in terms of survival in patients who receive more than 60 Gy or more of RT.[20,21] Moreover, it has also been reported that accelerated hyperfractioned RT has no advantage over whole brain irradiation in terms of survival; however it provides recurrences to be less. The application of 60 Gy RT in 30 day fractions in patients with adequate performance has been accepted as the standard adjuvant therapy level 1.[22,23] In our study, the prescribed total dose was 60 Gy (40 Gy to PTV1 and 20 Gy to PTV2) with conventional fractionation with daily doses of 1.8-2.0 Gy treated from Monday through Friday.
The contribution of chemotherapy to the survival period is controversial when combined with RT.[16,24] In 1990, Levin et al. reanalyzed their results of their randomized study with 148 patients having HGG, of whom, 73 had AA. In terms of survival rates of the patients, especially with AA, it has been reported that an adjuvant therapy consisting of Procarbasine, CCNU, and Vincristine is superior than the administration of BCNU protocol on its own. [25] On the other hand, the retrospective analysis performed by Prados et al. on 432 patients with AA (257 received adjuvant BCNU protocol and 175 received adjuvant PCV protocol) led to a debate by putting forward that both chemotherapy methods had no superiority over each other in prolonging the survival rates of the patients.[17] In the following years, a prospective, randomized, phase III study concerning 674 patients of whom 117 had AA was conducted by the United Kingdom Medical Research Council (MRC) in terms of survival time. Hence by the results of this study, it was denoted that AA is more chemosensitive in comparison to GBM, and although it does not lead to a significant increase in the survival rate, PCV should be the first adjuvant chemotherapy protocol in the management of AAs.[26] Levin et al. recently performed a phase III study on patients with anaplastic glioma to evaluate the effectiveness of adding an ornithine decarboxylase inhibitor (DFMO) to the adjuvant PCV protocol in comparison with the adjuvant PCV protocol. The majority of the patients who were enrolled in this study were AA patients. Although in this study, there was a significant difference between the groups during the first two years of the study in terms of survival (hazard ratio: 0.53, P=0.02) for the next two years, this difference in terms of survival and survival without progression disappeared (hazard ratio: 1.06, P=0.84).[27] As a result, the randomized data demonstrated that chemotherapy failed to improve the outcome of patients with AA.
TMZ which enrolled in the chemotherapy of glial tumors within the past 10 years is being accepted as a promising agent in the treatment of patients with recurring HGG. It has been reported that TMZ, for which higher doses are tolerated better when compared with the nitrosure group chemotherapy agents, has a more favorable safety profile. Moreover, the synergistic interaction between the TMZ and RT has been demonstrated in in-vitro studies. This instance have been the rationale for simultaneous and incessant use of TMZ together with RT in patients with HGG.[5–7]
Three very important phase II studies have been conducted to evaluate the effectivity of TMZ on patients diagnosed of recurrent HGG.[28–30] These studies analyzed the results of the patients with GBM and AA one by one and also the survival for six months without progression thus compared them with the historical database.[31] In a study conducted by Yung et al. that consisted of 162 patients with AA; TMZ therapy was administered (150-200 mg/m2/day on days 1-5 every 28 days) for the treatment of the first recurrence. The survival without progression was found out to be 46% for six months while an objective response obtained was 35% (full response was 8% and partial response was 27%). The patients were observed to have good tolerance to TMZ protocol with an acceptable safety profile (with moderate hematologic toxicity observed in less than 10% of the patients). This study demonstrated that TMZ has minimal toxicity with decent antitumoral activity in patients with recurrent AA.[30]
RTOG has commenced the randomized study 9813 on 454 patients in order to evaluate the efficiency of TMZ in adjuvant chemotherapy following RT in patients with AA. In this study, the efficiency of the TMZ (150-200 mg/m2/day on days 1-5 every 28 days) and the BCNU (80 mg/m2/day every 8 weeks) protocols administered following RT were being compared. Synchronous continuous concomitant TMZ protocol (concomitant application of RT and TMZ followed by six regimens of adjuvant TMZ administration; ConcT with TMZ) which is used in patients with GBM has lately become the preferred method of treatment in this study. It has been expressed that the role of TMZ in the treatment of AA has yet to be further clarified.[32]
In a NOA-04 randomized phase III study, published in 2009, a study was performed on 274 patients diagnosed of anaplastic glioma. In this study, positive prognostic factors were identified as patient being under 50 years of age (P=0.0004) and complete tumor resection (P=0.0006) by multivariate analysis. The 44 patients diagnosed of AA received radiotherapy as initial treatment and chemotherapy was applied when disease progression was detected (randomized 1:1 ratio, PCV or TMZ). The 44 patients diagnosed of AA received chemotherapy (PCV or TMZ) as initial treatment and radiotherapy was applied when disease progression was detected. Progression-free survival was 10.8 months (95% CI: 8.9 to 28.3) in group of patients who received radiotherapy as initial treatment and was 18.2 months (95% CI: 12.1 to 24.2) in group of patients who received chemotherapy as initial treatment. NOA-04 demonstrated that it is more likely that a patient will undergo adjuvant treatment when first treatment was radiotherapy compared to chemotherapy. These data, including AA diagnosed anaplastic glioma patients, can encourage us to recommend chemotherapy as first-line therapy.[33]
In our study, we found out that the median survival period in seven patients who had AAs and received ConcT with TMZ protocol for treatment to be 27.1 months, and in four patients who received solely RT, this has been observed to be 8.7 months. The synchronous use of ConcT with TMZ protocol that prolongs life for 18.4 months have proven to be a prognostic factor by using the univariate analysis (P=0.005766). Despite to this, a multivariate analysis did not confirm it as an independent prognostic factor. This brings up the necessity of conducting further randomized studies in the following years with large patient series specifically with AAs in order to unveil the efficiency of ConcT with TMZ protocol.
Conclusion
In recent years, a certain degree of progress has been gained in the treatment of patients with AA in parallel to the advances acquired in neurodiagnostic methods and surgical treatment methods. Although not yet at a desired level, the mortality rates of surgical interventions have been lowered and the survival periods have partially been improved. Nevertheless for the time being, the treatment outcomes in AA patients are still far from being satisfactory. In this study, the type of the surgical resection and repeated operations, preoperative KPS, postoperative KPS, and Concomittant Therapy with Temozolomide protocol have been found to be prognostic factors with univariate analysis for the patients having AA; however, evaluations with multivariate analysis did not confirm them to be independent prognostic factors.
As a result, our study put forward that six regimens of adjuvant Temozolomide administration following a Concomitant therapy with Temozolomide protocol is superior to the RT treatment alone. As this treatment method is reliable and well tolerated, it can constantly be administered to the patients with virtually no problems and it will help in prolonging the median survival rate. More randomized studies with large numbers of patients including specifically the patients with AAs are needed to be conducted in order to evaluate the efficiency of the Concomitant therapy performed by Temozolomide, particularly during the postoperative period in order to prolong the survival rate and quality of life in this patient group.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Giles GG, Gonzales MF. Epidemiology of brain tumors and factors in prognosis. In: Kaye AH, Laws ER Jr, editors. Brain Tumors. 2nd ed. New York: Churchill Livingstone Press; 2001. pp. 51–66. [Google Scholar]
- 2.Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007;63:72–80. doi: 10.1016/j.critrevonc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Oktar N. Supratentoriyal yuksek dereceli astrositomalar. In: Aksoy K, Palaoglu S, Pamir N, Tuncer R, editors. Temel Norosirurji. 1st ed. Ankara: Turk Norosirurji Dernegi yayinlari; 2005. pp. 653–60. [Google Scholar]
- 4.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–60. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Ostermann S, Calderoni A, Uhlmann C, Leyvraz S. Temozolomide and irinotecan (CPT-11) for primary brain tumors. A dose escalation study. Proc ASCO. 2003;22:104. [Google Scholar]
- 7.Van Rijn J, Heimans JJ, van den Berg J, van der Valk P, Slotman BJ. Survival of human glioma cells treated with various combination of temozolomide and x-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–84. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 9.Scott CB, Scarantino C, Urtasun R, Movsas B, Jones CU, Simpson JR, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: A report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51–5. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 10.McKernan RO, Thomas DG. The clinical study of gliomas. In: Thomas DG, Graham DI, editors. Brain Tumours; Scientific basis, clinical investiation, and current therapy. London: Butterworths; 1980. pp. 197–215. [Google Scholar]
- 11.Ammirati M, Galicich JH, Arbit E, Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607–14. doi: 10.1227/00006123-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Allahdini F, Amirjamshidi A, Reza-Zarei M, Abdollahi M. Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: Does maximal resection of the tumor lengthen the median survival? World Neurosurg. 2010;73:128–34. doi: 10.1016/j.wneu.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Stenning SP, Freedman LS, Bleehen NM. Prognostic factors for high-grade malignant glioma: Development of a prognostic index. A report of the Medical Research Council Brain Tumour Working Party. J Neurooncol. 1990;9:47–55. doi: 10.1007/BF00167068. [DOI] [PubMed] [Google Scholar]
- 14.Harsh GR, IV, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–21. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 16.Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. A randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–9. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 17.Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17:3389–95. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- 18.Kleinberg L, Wallner K, Malkin MG. Good performance status of long-term disease-free survivors of intracranial gliomas. Int J Radiat Oncol Biol Phys. 1993;26:129–33. doi: 10.1016/0360-3016(93)90183-v. [DOI] [PubMed] [Google Scholar]
- 19.Reni M, Cozzarini C, Ferreri AJ, Cereasoli GL, Galli L, Bianchi A, et al. A retrospective analysis of postradiation chemotherapy in 133 paients with glioblastoma multiforme. Cancer Invest. 2000;18:510–5. doi: 10.3109/07357900009012189. [DOI] [PubMed] [Google Scholar]
- 20.Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malign gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group Study. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Laperriere N, Zuraw L, Cairncross G Cancer Care Ontario Practice Guidelines Inititive Neuro-Oncology Disease Site Group. Radiotherapy for newly diagnosed malignant glioma in adults: A systematic review. Radioter Oncol. 2002;64:259–73. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 22.Bleehen NM, Stenning SP. A medical research council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769–74. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro WR, Green SB, Burger PC, Mahaley MS, Jr, Selker RG, VanGilder JC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma: Brain Tumor Cooperative Group trial 8001. J Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 24.Nelson DF, Diener-West M, Weintein AS, Schoenfeld D, Nelson JS, Sause WT, et al. A randomized comparison of misonidazole sensitized radiotherapy plus BCNU and radiotherapy plus BCNU for treatment of malignant glioma after surgery: Final report on RTOG study. Int J Radiat Oncol Biol Phys. 1986;12:1793–800. doi: 10.1016/0360-3016(86)90321-4. [DOI] [PubMed] [Google Scholar]
- 25.Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–4. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- 26.Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: A Medical Research Council trial. J Clin Oncol. 2001;19:509–18. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 27.Levin VA, Hess KR, Choucair A, Flynn PJ, Jaeckle KA, Kyritsis AP, et al. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–90. [PubMed] [Google Scholar]
- 28.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, MacDonald D, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–66. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 29.Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, et al. A phase II study of temozolomide vs.procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–93. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 31.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 32.Chang SM, Seiferheld W, Curran W, Share R, Atkins J, Choucair A, et al. Phase I study pilot arms of radiotherapy and carmustine with temozolomide for anaplastic astrocytoma (Radiation Therapy Oncology Group 9813): Implications for studies testing initial treatment of brain tumors. Int J Radiat Oncol Biol Phys. 2004;59:1122–6. doi: 10.1016/j.ijrobp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA-04 Randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–80. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]


