Abstract
Context:
Cirsoid aneurysms of scalp are rare lesions which are mainly treated by surgical excision. Endovascular embolization was described either alone or prior to surgery in order to minimize the risk of bleeding. However, the endovascular therapy also carries the risk of scalp necrosis, escape of embolization material to circulation, and recurrence of the lesion.
Aim:
To evaluate the results of well-planned classic surgical excision of cirsoid aneurysm.
Study Design:
This is a retrospective case series study.
Materials and Methods:
This is a retrospective case series study on nine patients with cirsoid aneurysms who were treated with surgical excision. Preoperative Planning for location, size, feeding arteries, and venous drainage of the lesions were done by plain and contrast enhanced CT, MRI, MR angiogram, and selective internal and external carotid angiograms. Complete surgical excision for the lesions was done. Postoperative evaluation of excision was done by cranial magnetic resonance angiography in all the patients. The mean follow up period was 34.1 (±7.62 STD) months.
Results:
The lesion was located in the occipital region in three (33.3%) cases, frontal region in two (22.2%) cases, temproparietal region in two (22.2%) cases, parietal region in one case (11.1%), and vertex in one case (11.1%). The superficial temporal artery was involved in seven (77.8%) cases, the occipital artery was involved in six (66.7%) cases, the posterior auricular artery was involved in five (55.6%) cases, the supraorbital artery was involved in two (22.2%) cases and the middle meningeal artery was involved in two (22.2%) cases. Total excision of the lesion was achieved in eight patients and en bloc resection and primary closure was done in one patient. Postoperative magnetic resonance angiogram showed no residual lesion in all patients. No postoperative complication related to the surgery had occurred. No recurrence had occurred during the follow-up period (mean 34.1 ± 7.62 STD months).
Conclusion:
Well-planned surgery of cirsoid aneurysm of the scalp without preoperative interventions could achieve complete excision of the lesion without any residual masses or recurrence and with a low incidence of complications.
Keywords: Arteriovenous fistula, cirsoid aneurysm, scalp, vascular malformation
Introduction
Arteriovenous fistula was first described by Hunter in 1757.[1] The term cirsoid aneurysm was applied to vascular malformations of the scalp in 1833 by Brecht[1] and is used to describe a fistulous connection between the arterial feeding vessels of the scalp and the draining veins without an intervening capillary bed.
The incidence of cirsoid aneurysms of scalp is rare and infrequently encountered by the neurosurgeon.[2] Most of the reports in the literature consist of individual case reports with very few studies consisting of a sufficient number of patients.[3–6] These lesions occur either following trauma or spontaneously.[2] Whatever the cause, the signs and symptoms are similar, including pulsatile scalp masses, headache, and hemorrhage.[7] The treatment of these lesions is difficult because of their complex vascular anatomy, high shunt flow, and cosmetic disfigurement. The treatment options of these lesions include endovascular treatment, direct intralesional injection of sclerosing agents, ligation of feeders, and surgical excision.[3,5,6,8–10] We hypothesize that a well-planned surgical excision is sufficient to achieve satisfactory outcome for management of cirsoid aneurysm. In this study, we evaluate the surgical results of nine patients with cirsoid aneurysms without any preoperative interventions for the lesion and our results are compared with literature results.
Materials and Methods
This is a retrospective case series study on nine patients with cirsoid aneurysms who were treated with surgical excision during the period from January 2004 and January 2010. Diagnosis was based mainly on the clinical picture of pulsatile swelling in the scalp with bruit and thrill [Figure 1]. The following investigations were done preoperatively: Plain and contrast-enhanced CT, MRI, MR angiogram, and selective internal and external carotid angiograms, to document the location, size, feeding arteries, and venous drainage of the lesions and if there are associated intracranial lesions. In CT skull, the lesion appeared as soft tissue scalp swelling while in MRI as numerous flow voids underlying the subcutaneous tissue [Figure 2]. Although, angiography is the gold-standard investigation to delineate the lesion and to exclude an intracranial component, MRA was a good non-invasive alternative [Figure 3]. No patient had undergone prior interventions for the lesion. Postoperative cranial magnetic resonance angiography was done for all the patients.
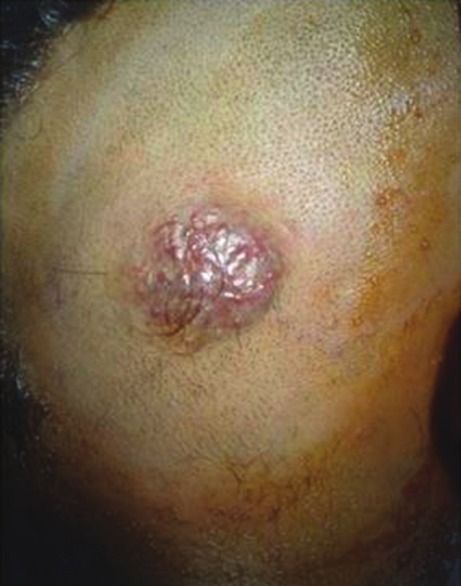
Figure 1.
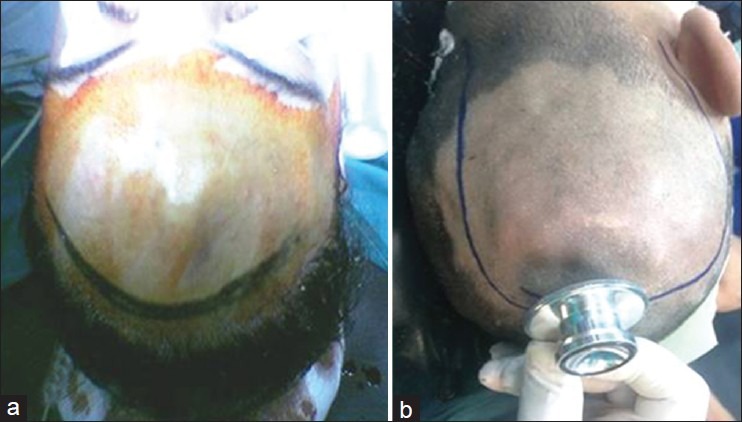
Two patients with cirsoid aneurysm (a) in the middle of forehead and (b) in the left occipital region presented as pulsatile swelling with bruit and thrill
Figure 2.
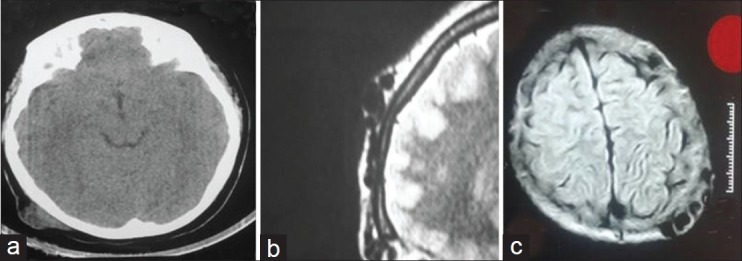
(a) CT and (b and c) MRI pictures of scalp cirsoid aneurysm in different patients
Figure 3.
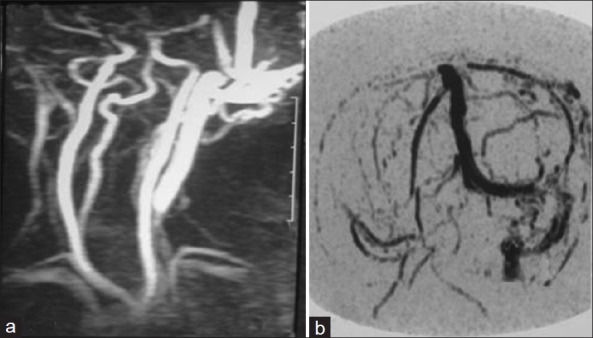
MR angiogram of left occipital cirsoid aneurysm showing (a) feeding from the occipital and posterior auricular arteries and (b) draining into sigmoid and internal jagular veins
Surgical procedure
All patients were operated under general anesthesia. Horse-shoe shaped or bicoronal skin flaps (in the case of frontal lesions) were raised. Local anesthetic solution with adrenaline was injected at the site of skin incision, avoiding direct intravenous injection [Figure 4a]. In making this incision, care was taken to include the galea in the flap, in other words, the incision was carried down to the pericranium and all tissues superficial to that were reflected with the lesion. At the circumference, the large vessels entering the aneurysm were individually ligated and divided as they were encountered in the incision prior to raising the scalp flap [Figure 4b].
Figure 4.
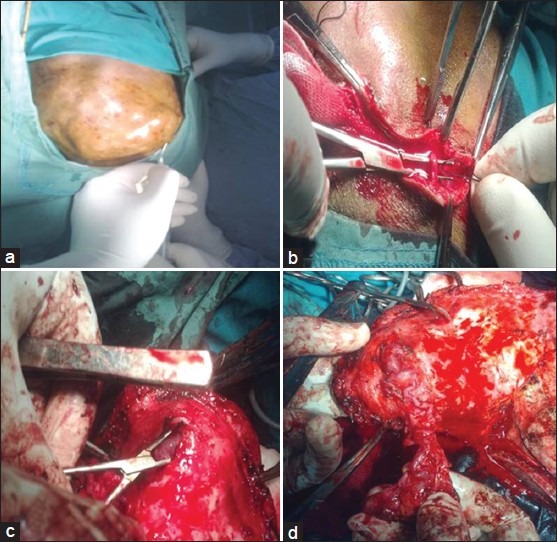
Surgical excision of scalp cirsoid aneurysm. (a) Injection of local anesthetic solution with adrenaline at the site of skin incision. (b) The large vessels entering the aneurysm are individually ligated and divided as they were encountered in the incision. (c) The galea around the lesion is incised and the lesion is separated from the underlying skin. (d) The lesion is progressively separated from the underlying skin and totally excised
Once the scalp flap was raised, the lesion could be seen and palpated through the galea. The pericranium underlying the lesion was thicker than normal in all patients and there were large feeding vessels. During elevation of the flap, it was of great importance not to damage these large feeding vessels in order to avoid excessive bleeding and they were cauterized or ligated.
The galea around the lesion was incised and the lesion was separated from the underlying skin using a combination of bipolar diathermy and sharp dissection [Figure 4c]. Particular care was taken not to button-hole the skin also to avoid excessive cauterization that may cause postoperative scalp necrosis. The lesion was progressively separated from the underlying skin and totally excised [Figure 4d]. The pericranium underlying the lesion was also incised and resected.
After excision of the lesion, the flap was replaced with interrupted stitches of silk in the galea and the skin.
In case (No. 3), the lesion was small (nidus was about 2 cm × 2 cm) and the overlying skin was so thin to be preserved and for that reason an en bloc resection was done (the lesion was excised into). The scalp defect after that was repaired using primary closure after gentle dissection of the galea aponeurotica [Figure 5].
Figure 5.
Small scalp cirsoid aneurysm with thin overlying skin was treated with en bloc resection and primary closure of the scalp
Results
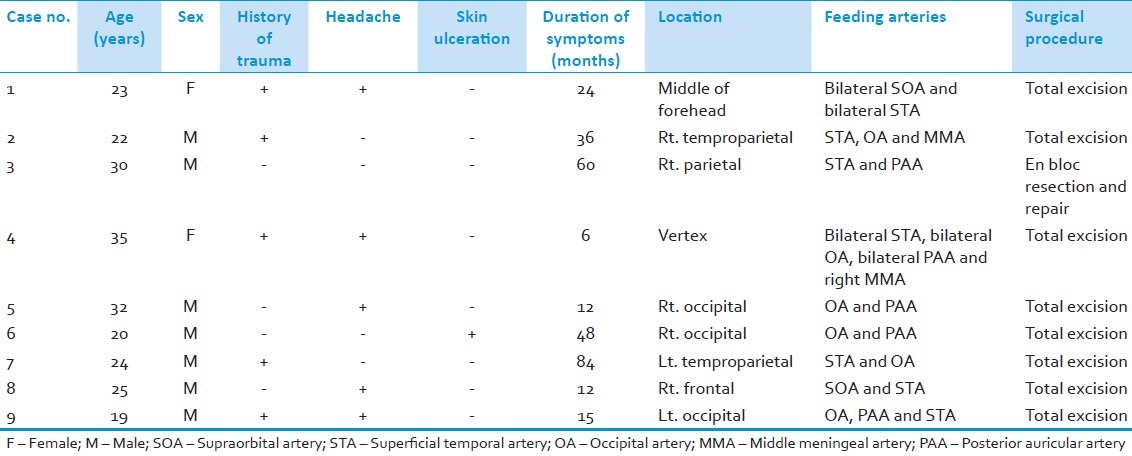
The study was conducted on nine patients. There were 7 males (77.8%) and 2 females (22.2%). The mean age of patients was 25.6 (±5.54 STD) years (range 19-35 years). History of trauma was present in five patients (55.6%), many years before the appearance of the lesion. All the patients (100%) had a pulsatile swelling with bruit and thrill. Five patients (55.6%) had headache and only one patient (11.1%) presented with ulceration of the skin. None of our patients presented with hemorrhage; also, none had neurological deficits. The duration of the symptoms varied from 6 months to 7 years with a mean period of 33 (±26.4 STD) months. The lesion was located in the occipital region in three (33.3%) cases, frontal region in two (22.2%) cases, temproparietal region in two (22.2%) cases, parietal region in one case (11.1%), and vertex in one case (11.1%). The superficial temporal artery (STA) was involved in seven (77.8%) cases, the occipital artery (OA) was involved in six (66.7%) cases, the posterior auricular artery (PAA) was involved in five (55.6%) cases, the supraorbital artery (SOA) was involved in two (22.2%) cases and the middle meningeal artery (MMA) was involved in two (22.2%) cases. The lesion in midfrontal region (case NO. 1) was receiving feeding arteries from SOA and STA bilaterally also the lesion in the vertex (case No. 4) was receiving feeding arteries from STA, OA, and PAA bilaterally. No intracranial lesion was demonstrated in any patient. The preoperative data are listed in Table 1.
Table 1.
Preoperative data
All patients were treated surgically (total excision of the lesion was achieved in eight patients and en bloc resection and primary closure was done in one patient). The operating time ranged from 60 to 190 min (mean 117.8±42.36 min). Blood loss ranged from 75 to 1000 ml (mean 397.2±302.19 ml). All the preoperative symptoms and signs were cured. All the patients had good postoperative cosmetic appearance [Figure 6]. All the patients had postoperative magnetic resonance angiogram without residual lesion. No postoperative complication related to the surgery had occurred. The patients were followed up for a period ranged from 24 to 48 months (mean 34.1±7.62 STD months). No recurrence had occurred during the follow-up period.
Figure 6.
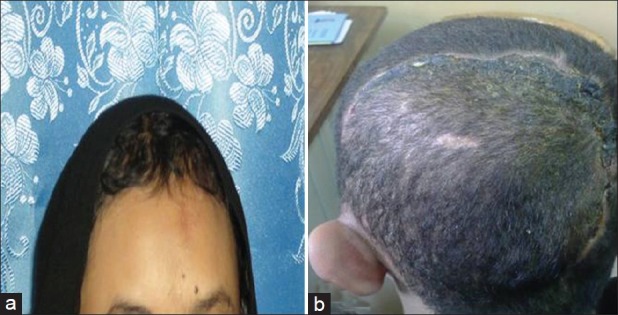
Postoperative photographs of patients with (a) frontal and (b) occipital cirsoid aneurysms that excised surgically with good postoperative cosmetic result
Discussion
The etiology of cirsoid aneurysms is still controversial. However, it is generally accepted that it may be either of congenital or traumatic origin.[5,6,11,12] Many such lesions have been reported after interventions such as hair transplantation, craniotomy, infusions through scalp veins[13–17] In approximately half the cases reported in the literature, there is no history of trauma; iatrogenic, or otherwise.[2] Khodadad (1971)[12] reported three siblings with occipital cirsoid aneurysms which were present since birth. Nagasaka et al.[6] believed that at least in some of the cases, there might have been functionally nonpatent fistulae which were clinically unrecognized and already existed before trauma. They believed that trauma just activated the fistulae to become patent. Once the shunts were established, they received more blood through the abundant collaterals in the scalp. In our study, history of trauma was present in five (55.6%) patients many years before the appearance of the lesion.
The superficial temporal artery is particularly vulnerable to trauma due to its long and relatively exposed course in the scalp.[18,19] For this reason, the superficial temporal artery is involved in 75% of the cases in Barnwell et al.[3] study and in 90.5% of the cases in Gurkanlar et al.[4] study. In our study, the STA was involved in 77.85 of cases.
The lesion usually begins as a small, subcutaneous lump in the head which over a period of time evolves into a large deforming mass. A bruit or a throbbing headache might be the chief complaint in some patients. Other presenting symptoms include pulsatile tinnitus or rarely, hemorrhage from the mass.[8] Occasionally, large lesions may be associated with scalp necrosis.[20] Congestive heart failure has occurred with large fistulas.[3] Also large lesions are rarely associated with cerebral steal phenomenon that resulted in neurological symptoms including seizure.[21] All the patients in our serie shad a pulsatile swelling with bruit and thrill. Five (55.6%) patients had headache and only one patient presented with ulceration of the skin. None of our patients had history of bleeding from the lesions or any neurological deficits.
The highest incidence of these lesions was seen during the third decade of life.[4] In our study, the mean age of patients was 25.6 (±5.54 STD) years (range 19-35 years).
Diagnosis of cirsoid aneurysm of the scalp can be made easily on clinical examination. Angiography is the gold standard investigation to delineate the lesion and to exclude anintracranial component.[22] Other supplementary investigations are CT, MRI, and MRA. MRA is non-invasive and may become the best alternative to the conventional contrast angiography in evaluation of the cirsoid aneurysm. In our study, all the patients had preoperative plain and contrast enhanced CT, MRI, MRA, and selective internal and external carotid angiograms and postoperative MRA. None of our patients had any associated intracranial lesion.
As the use of endovascular techniques has been improved, authors offer embolization of these lesions based upon the suggestion that these lesions are very difficult to manage surgically due to severe intraoperative blood loss, and they may have vital risk.[3,8,10] Endovascular interventions, both transarterial and transvenous routes, have been used. Endovascular methods involve the placement of glues or thrombogenic coils or radio-opaque gel foam either in the fistula or the feeding arteries.[3,8,23]
Intralesional injection of sclerosing agents like sodium tetradecyl sulfate, absolute alcohol, thrombogenic coils also has been used.[8–10] In selected cases, this therapy has completely obliterated the lesions without need for further intervention.[8,9,24]
However, these techniques are not without pitfalls. Tenderness and hyperemia over the skin, necrosis over the lesion, permanent patchy hair loss, pain at the fistula site and escape of embolization material into the general circulation are the complications that can be seen after both endovascular and percutaneous embolization procedures.[3,8,10,25] Surgery was also performed after these procedures especially for tenderness and necrosis following embolization.[8,10] In addition, there have been reports of recurrences following endovascular intervention.[9]
Ligation of the feeding arteries was one of the earlier treatments performed for this condition. However, this is almost always followed by recurrence because of the development of collateral vessels.[11,12,26] The collateral vessels which develop following ligation of the feeding arteries might parasitize blood flow from the brain leading to ischemic complications.[27]
In one of the largest series of these lesions reported to date, surgery alone gave excellent results.[5] Also many authors concluded that conventional excision of the lesion is possible in the vast majority of cases and surgery is neither difficult nor dangerous.[2,4,5,25,28] However, in many cases where the lesion cannot be totally excised either because of the excessive vascularity or because the overlying skin is exceedingly thin, en bloc resection of the scalp with subsequent reconstruction is a feasible option.[2,6,27] Some recommended preliminary step in the operation by independently ligating the principal artery or arteries, leading to the lesion followed by excision of the lesion.[29] Also some recommend preoperative endovascular intervention either transarterial or transvenous to reduce blood loss followed by excision of the lesion.[6]
In our study all patients were treated surgically without any prior interventions for the lesion. Total excision of the lesion was achieved in eight patients and en bloc resection and primary closure was done in one patient because of the small size of the lesion and the very thin overlying skin. All the patients had postoperative magnetic resonance angiogram without residual lesion. No postoperative complication related to the surgery had occurred. We think that, injection of local anesthetic solution with adrenaline at the site of skin incision, individually ligating and dividing the large vessels as they were encountered in the incision prior to raising the scalp flap, gentle dissection of the scalp from the cirsoid aneurysm, ligation of the feeding and draining vessels, followed by the resection of the lesion together with underlying pericranium, are important steps to achieve the operation without massive hemorrhage. While ligating the feeding arteries, the scalp AVM spontaneously gets smaller and at the end of the procedure, one observes only a small amount of AVM [Figure 4d].
The main advantage of the surgical resection of cirsoid aneurysms is the prevention of very often seen recurrences because of a development of collateral vessels following embolization of cirsoid aneurysms.[2,4–6,25,28] In our study, no recurrence have been occurred during a mean follow up period of 34.1 (±7.62 STD) months. There have been reports of cirsoid aneurysms recurring as long as 18 years after surgical excision.[30] Hence, we believe that these patients should be closely followed for possible recurrences.
Conclusion
Well-planned surgery of cirsoid aneurysm of the scalp without preoperative interventions could achieve complete excision of the lesion without any residual masses or recurrence and with a low incidence of complications. Preoperative interventions should not be used as a routine management for these lesions but it should be preserved for advanced and complex lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Elkin DC. Cirsoid aneurysm of the scalp with the report of an advanced case. Ann Surg. 1924;80:332–40. doi: 10.1097/00000658-192409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthukumar N, Rajagopal V, Manoharan AV, Durairaj N. Surgical Management of Cirsoid Aneurysms. ActaNeurochir. 2002;144:349–56. doi: 10.1007/s007010200048. [DOI] [PubMed] [Google Scholar]
- 3.Barnwell SL, Halbach VV, Dowd CF, Higshida RT, Hieshima GB. Endovascular treatment of scalp arteriovenous fistulas associated with a large varix. Radiology. 1989;173:533–9. doi: 10.1148/radiology.173.2.2798886. [DOI] [PubMed] [Google Scholar]
- 4.Gurkanlar D, Gonul M, Solmaz I, Gonul E. Cirsoid aneurysms of the scalp. Neurosurg Rev. 2006;29:208–12. doi: 10.1007/s10143-006-0023-y. [DOI] [PubMed] [Google Scholar]
- 5.Fisher-Jeffes ND, Domingo Z, Madden M, De Villiers JC. Arteriovenous malformations of the scalp. Neurosurgery. 1995;36:656–60. doi: 10.1227/00006123-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Nagasaka S, Fukushima T, Goto K, Ohjimi H, Iwabuchi S, Maehara F. Treatment of scalp arteriovenous malformations. Neurosurgery. 1996;38:671–7. [PubMed] [Google Scholar]
- 7.Baysefer A, Gönül E, ErdoĢan E, Akay K M, Bedük A, Sehe N. Cirsoid aneurysms of the scalp. Eur J PlastSurg. 1998;21:48–50. [Google Scholar]
- 8.Heilman CB, Kwan ES, Klucznik RP, Cohen AR. Elimination of a cirsoid aneurysm of the scalp by direct percutaneous embolization with thrombogenic coils. J Neurosurg. 1990;73:296–300. doi: 10.3171/jns.1990.73.2.0296. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix LE, Meyer GA, Erickson SJ. Cirsoid aneurysm treatment by percuataneous injection of sodium tetradecyl sulfate. SurgNeurol. 1996;46:557–61. doi: 10.1016/s0090-3019(96)00225-x. [DOI] [PubMed] [Google Scholar]
- 10.Mourao GS, Hodes JE, Gobibn YP, Casasco A, Aymard A, Merland JJ. Curative treatment of scalp arteriovenous fistula by direct puncture and embolization with absolute alcohol. J Neurosurg. 1991;75:634–7. doi: 10.3171/jns.1991.75.4.0634. [DOI] [PubMed] [Google Scholar]
- 11.Khodadad G. Arteriovenous fistula of the scalp. Ann Surg. 1973;177:79–85. doi: 10.1097/00000658-197301000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodadad G. Familial cirsoid aneurysm of the scalp. J NeurolNeurosurg Psychiatry. 1971;34:664–7. doi: 10.1136/jnnp.34.6.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis AJ, Nelson PK. Arteriovenous fistula of the scalp secondary to punch autograft hair transplantation: Angioarchitecture, histopathologoy, endovascular and surgical therapy. PlastReconstrSurg. 1997;100:242–9. doi: 10.1097/00006534-199707000-00036. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert MS, Capozzi JD, Forster AM. Acquired arteriovenous fistula of the scalp in hemophiliacs. Pediatrics. 1986;77:722–4. [PubMed] [Google Scholar]
- 15.Morioka T, Nishio S, Hikita T. Traumatic arteriovenous fistula of the scalp at the site of previous craniotomy. SurgNeurol. 1988;30:406–7. doi: 10.1016/0090-3019(88)90206-6. [DOI] [PubMed] [Google Scholar]
- 16.Tornambe R, Antell D, Castellano M. Arteriovenous fistulae following hair transplantation: Collective review and report of a case. Ann PlastSurg. 1994;33:214–5. doi: 10.1097/00000637-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura S, Hashimoto N, Kazekawa K, Obata A, Yutani C, Ogata J. Arteriovenous fistula around a ventriculoperitoneal shunt system in a patient with a duralarteriovenous malformation of the posterior fossa. Case report. J Neurosurg. 1995;82:288–90. doi: 10.3171/jns.1995.82.2.0288. [DOI] [PubMed] [Google Scholar]
- 18.Hyshaw C, Di Tullio M, Renaudin J. Superficial temporal arteriovenous fistula. SurgNeurol. 1979;12:46–8. [PubMed] [Google Scholar]
- 19.Li F, Zhu S, Liu Y, Chen Y, Chi L, Chen G, et al. Traumatic arteriovenous fistula of the superficial temporal artery. J ClinNeurosci. 2007;14:595–600. doi: 10.1016/j.jocn.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Coleman CC, Hoopes JE. Congenital arteriovenousanomalies of the head and neck. PlastReconstrSurg. 1971;47:354–64. doi: 10.1097/00006534-197104000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kelly K, Trites JR, Taylor SM, Bullock M, Hart RD. Arteriovenous malformation of the scalp with cerebral steal. Head Neck. 2009;31:1520–3. doi: 10.1002/hed.21032. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal A. cirsoid aneurysm with impending rupture. Pak J NeurolSci. 2009;4:74–6. [Google Scholar]
- 23.Kadson DL, Altemus LR, Stein BM. Embolization of atraumatic arteriovenous fistula of the scalp with radio-opaquegel foam pledgets. Case report and technical note. J Neurosurg. 1976;44:753–6. doi: 10.3171/jns.1976.44.6.0753. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Purkayastha S, Bodhey NK, Kapilamoorthy TR, Krishnamoorthy T, Kesavadas C, et al. Endovascular treatment of scalp cirsoid aneurysms. Neurol India. 2008;56:167–72. doi: 10.4103/0028-3886.41995. [DOI] [PubMed] [Google Scholar]
- 25.Luessenhop AJ. Cirsoid aneurysms of the scalp. J Neurosurg. 1991;75:167. doi: 10.3171/jns.1991.75.1.0167. [DOI] [PubMed] [Google Scholar]
- 26.Dandy WE. Arteriovenous aneurysms of the scalp and face. Arch Surg. 1946;52:1–32. doi: 10.1001/archsurg.1946.01230050003001. [DOI] [PubMed] [Google Scholar]
- 27.Malan E, Azzolini A. Congenital arteriovenous malformationsof the face and scalp. J Cardiovasc Surg. 1986;8:109–40. [PubMed] [Google Scholar]
- 28.Maehara F. Treatment of scalp arteriovenous malformations. Neurosurgery. 1996;38:671–7. [PubMed] [Google Scholar]
- 29.Elkin DC. Cirsoid aneurysm of the scalp; Report of four cases. Trans South Surg Assoc. 1946;57:122–31. [PubMed] [Google Scholar]
- 30.Wilkinson HA. Recurrence of vascular malformation of the scalp 18 years following excision. Case repot. J Neurosurg. 1971;34:435–7. doi: 10.3171/jns.1971.34.3.0435. [DOI] [PubMed] [Google Scholar]


