Abstract
Background:
Primary intracranial germ cell tumors are rare (ICGCTs) and usually localized in the pineal and suprasellar regions of the brain. They are divided into histologic types: Germinoma, teratoma choriocarcinoma, embryonal carcinoma, yolk sac tumor, and malignant mixed germ cell tumors (MMGCTs). Neuroimaging evaluation is useful to distinguish between the types of ICGCTs. Germinoma is highly sensitive to radiotherapy or/and chemotherapy, and is potentially curable without surgery. MMGCTs are treated with the combination of chemotherapy and radiation, with a poorer prognosis. ICGCTs warrant correct pre-operative diagnosis in order to decide on appropriate management.
Aim:
To report the clinicopathological and immunohistochemical findings in three cases of primary intracranial germ-cell tumor.
Materials and Methods:
Three cases of intracranial germ cell tumors inclusive of both genders and all brain regions were retrieved from the archives of the Anatomical Pathology Department at King Abdul Aziz University Hospital, Jeddah between the years, 1995-2011, through a computerized search.
Results:
Based on histological examination, they were respectively diagnosed as MMGCTs (two cases) and germinoma. Clinical, radiological, pathological characteristics and immunohistochemical profile of the three ICGCTs are presented along with the review of recent literature.
Conclusion:
ICGCTs are rare tumors affecting males more than females, and at the end of three years follow-up in the present study, treatment morbidity appears to be low with no recurrence observed among surviving patients, indicating that suprasellar and basal ganglia ICGCTs may have a favorable prognosis regardless of histological type. Pineal MMGCTs may have an aggressive course.
Keywords: Germinoma, immunohistochemical, intracranial, mixed, pineal
Introduction
Intracranial germ cell tumors (ICGCT) are rare, biologically diversified, and diagnostically challenging tumors.[1,2] Extragonadal germ cell tumors (GCTs) should always be included in the differential diagnosis of pineal and suprasellar intracranial tumors.[3] GCTs constitute nearly 2[4,5] to 3% of pediatric brain tumors, and germinomas’ alone account for approximately two thirds of all GCTs.[6]
Rarity of ICGCTs limits our knowledge about them, making preoperative diagnosis difficult. To the knowledge of authors, only one case of ICGCT has been reported so far from Saudi Arabia.[7] As such, the present study was performed to enhance our understanding regarding these tumors in our population.
Objective
The purpose of this retrospective study was to present the clinicopathological and immunohistochemical findings in three cases of primary intracranial germ-cell tumor along with the review of literature.
Materials and Methods
A computerized search through the archives of the Anatomical Pathology Department at King Abdul Aziz University Hospital, Jeddah between the years, 1995-2011, was performed to identify all cases of intracranial germ cell tumors inclusive of all regions of the brain. The data was filtered using appropriate morphology Systematized Nomenclature of Medicine (SNOMED) codes indicating the following parameters: Date of receiving biopsy, personal identity (medical record number, age, sex etc), clinical diagnosis, morphology, and topography. The data was rechecked manually to delete duplications. Computerized search was then exported to Microsoft Excel format and used for analysis. Specific target group of intracranial germ cell tumors was identified. Three cases of ICGCTs were retrieved and classified using World Health Organization (WHO) classification system.[8] Clinical and radiological correlation was evaluated. Sufficient blocks were submitted from the maximum tumor diameter in order to ensure sampling adequacy. Microscopic examination was done to determine the type of different components of the tumor. Immunohistochemical staining using an automated stainer with the avidin-biotin-peroxidase complex method was performed using the antibodies alpha-feto protein (AFP:dilution 1:100; Dako, Carpintena, CA, USA), pan cytokeratin (CK-PAN:dilution 1:50; Dako, Carpintena, CA, USA), placental alkaline phosphatase (PLAP:dilution 1:50; Dako, Carpintena, CA, USA), CD117 (c-kit:dilution 1:50; Dako, Carpintena, CA, USA). Antibodies such as neuron specific enolase (NSE), and synaptophysin (SYN) were all available as ready to use kits (Ventana, Rocklin, CA, USA). Antibodies were used to highlight various histological components, and as the case scenario necessitated. Results were scored as follows: -, not seen; +/-, rare/focal positivity; and +/, diffuse positivity. Positive and negative controls were performed for the stains. Clinical data were obtained from the patients’ hospital records and follow-up was obtained on personal basis from the concerned neurosurgical and medical teams. Clinical parameters at the time of initial diagnosis were classified in terms of tumor, nodes and metastasis classification.[9]
Results
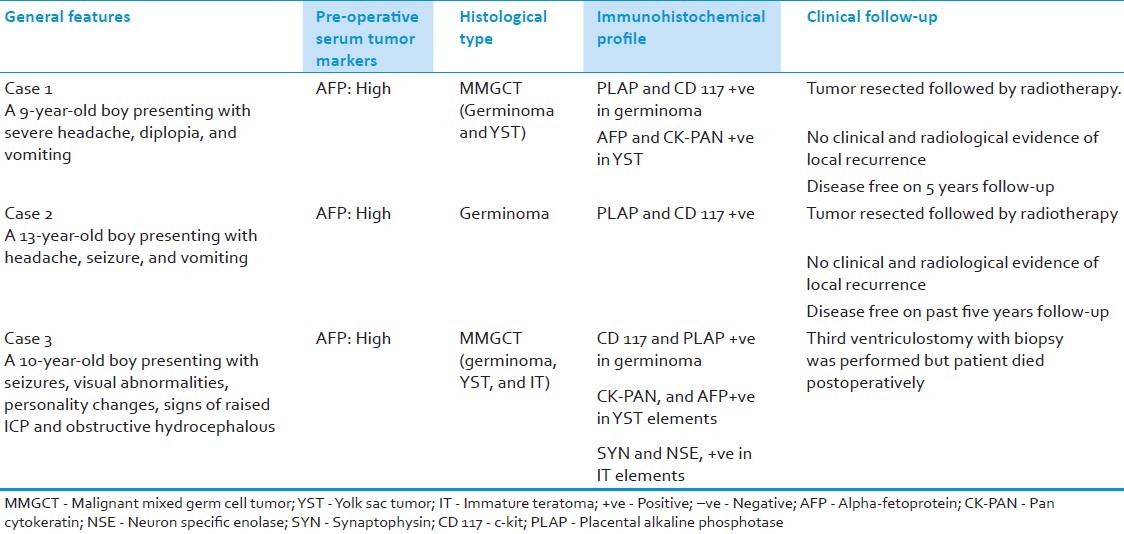
All three cases in the present study were boys in pediatric age group with a mean age of 10 years. The presenting features were mainly visual abnormalities, seizures, headache, and vomiting. The levels of serum markers, gradually, returned to normal, except in the case of malignant mixed germ cell tumors (MMGCTs) of the pineal body. The radiological features are presented in Table 1. Clinicopathological characteristics and immunohistochemical profile of the three ICGCTs are summarized in Table 2.
Table 1.
Radiological features in each case of ICGCTs
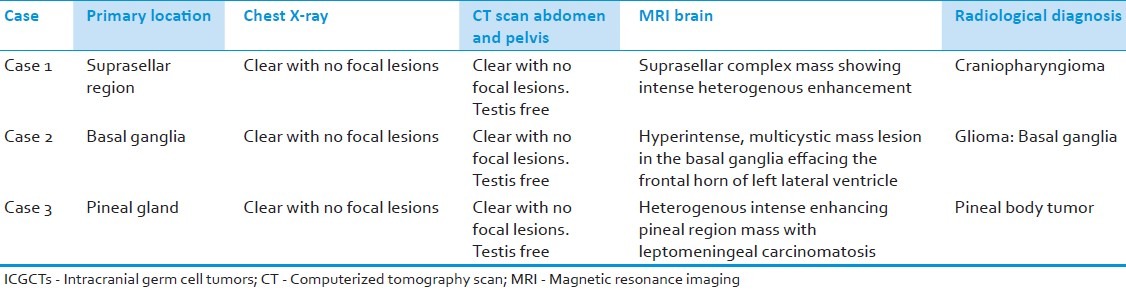
Table 2.
Clinicopathological and immunohistochemical features of primary intracranial germ cell tumors
Discussion
GCTs arise in the central nervous system as primary tumors. Primary ICGCTs are rare and encompasses a wide pathological spectrum and share histologic, genetic, and therapeutic similarities to extracranial GCTs. Two theories could explain the pathogenesis of ICGCTs. The germ cell theory hypothesizes malignant transformation of mis-migrational primordial germ cells.[3,10,11] The embryonic theory, in contrast, suggests that these tumors arise from mis-migrational pluripotent germ cells.[3,12] GCT constitutes nearly 2[4,5] to 3% of pediatric brain tumors, with 85% of them occurring before the second decade of life.[13,14] The reported incidence of primary ICGCTs in children is significantly higher in Asian countries compared with Western countries. In an epidemiological study from Japan, germ cell tumors were the second-most common tumors (14.3%, annual incidence 5.0 per million) affecting mainly 10-14 year-old boys.[15]
The comparative incidences are 14.3% in Japan, 14.0% in Taiwan, 11.2% in Korea, 2.3% in USA, and 2.5% in Germany, in various reported series. There is still no explanation for this extreme geographic and ethnic difference between the three Asian series and the two Western series (P<0.0001).[15–19] In Western countries, they account for 0.4%–3.4% of all pediatric CNS tumors, while series from Japan and other Asian countries have reported that CNS GCTs account for up to 11% of all pediatric brain tumors.[6] Epidemiological study presenting meta analysis data on ICGCT diagnosed between 2000 and 2004 from the Central Brain Tumor Registry of the United States (CBTRUS) report an incidence rate of 0.051/100,000 for nonpineal GCT and significantly lower incidence of pineal GCT 0.028/100,000.[20] The reported rate of pineal region ICGCT is higher in Asia, where it exceeds 9% of all intracranial masses.[2] Lee and Suh reported a frequency of 27.4% for primary MMGCTs of the CNS from Korea.[21] To the best of our knowledge, only one case of midbrain germinoma has been reported so far from Saudi Arabia.[7] Reasonably, then genomic differences exist and need to be considered. Asian-Americans have higher rates than other races.[13] Also, these findings suggest a robust, but poorly understood influence of sex, either genetic or hormonal, and race on the occurrence of central nervous system (CNS) GCTs.[13]
The most peculiar feature of ICGCTs is that they arise in specific loci in the brain: The suprasellar and pineal regions[11] and should, therefore, always be included in the differential diagnosis of a pineal and suprasellar intracranial tumors.[3] Some GCTs develop in unusual loci in the CNS, including the basal ganglia, thalamus, fourth ventricle, and spinal cord.[11] Few recent reports have described ICGCT in the corpus callosum.[4,22] Among the 363 malignant and non-malignant GCTs identified in the CBTRUS by Villano et al.[20] 129 (35.5%) were located in the pineal region. Of the remaining nonpineal GCTs, the most common site was the brain, NOS (not otherwise specified) (31.6%), followed by the ventricles (17.1%), the pituitary (14.1%), and the cerebrum (9.8%). Sites comprising the suprasellar region accounted for 64.5% of the nonpineal GCT.[20]
Males have higher incidence of CNS GCTs, primarily germinomas’, than females, starting in the second decade.[13] Ninety percent of ICGCTs occur in patients before the age of 20 years.[6] The peak incidence for ICGCTs is 10–12 years of age.[6] This peak in incidence can be separated by histology, with most non-germinomatous germ cell tumors (NGGCTs) occurring in younger children, whereas pure germinomas are more commonly seen in older patients.[6] Incidences by location also have specific trends based on gender. In males, 70% of tumors occur in the pineal region, and in females, 75% of tumors are suprasellar. There is an overall male predominance in ICGCTs.[6,13] Occasionally, MMGCT has been reported in older adults around 59 years of age.[23]
Clinically, they present with ocular signs or signs of obstructive hydrocephalus. Major symptoms are headache, nausea, vomiting, lethargy, and visual disturbances. Ocular signs predominate and include upward gaze, pupillary abnormalities, and papilledema.[2] Ataxia, hemiperesis, and brain stem hemiatrophy may also be present.[24,25] Other symptoms at diagnosis may include weight loss, diabetes insipidus, amenorrhea, retardation, and precocious puberty. The following post-therapeutic complications are reported in literature; the requirement for ventriculo-peritoneal shunt, cerebral hemorrhage, impaired vision, facial nerve paresis, reduced hearing/tinnitus, hypothyroidism, low growth hormone levels/impaired growth, adrenal insufficiency, diabetes insipidus, and low testosterone levels were reported by CE Hoei-Hansen et al.[26]
Based on the histologic components and the variable degree of differentiation, the classification of GCTs has classically been divided into germinomas’ and NGGCTs. Germinomas account for approximately 50%–70% of cases and NGGCTs make up the remaining third. NGGCTs include choriocarcinomas, endodermal sinus tumors (yolksac tumors), embryonal carcinomas, and mixed tumors.[6] Among all primary ICGCTs, approximately 65% are germinomas’, 18% are teratomas’, 5% are embryonal carcinomas’, 7% are yolk sac tumors’ and 5% are choriocarcinomas’. The most common histological subtypes reported by Lee and Suh[21] were germinomas’ (48.4%), followed by MMGCTs (27.4%), and teratomas’ (19.4%). In a series of 176 primary pediatric CNS GCTs studied by Wong et al., at Taiwan, 58.5% were germinomas’ and 41.5% were non-germinomas’.[19] In the CBTRUS data analysis on ICGCT, Villano et al. reported that germinoma (61.5%) was the most frequent diagnosis, followed by teratoma (27.8%) and mixed GCT (8.5%).[20] Histologically, two tumors in the present study were MMGCTs, showing variable proportions of germinoma, YST, and IT ([Table 2], case 1 and 3). One was a pure germinoma ([Table 2], case 2). Photomicrographs of Case 1 suprasellar MMGCT are presented as [Figure 1] (a, b, Hematoxylin and eosin staining) and [Figure 1] (c, d, e Immunohistochemical stains). Photomicrographs of Case 2 basal ganglia germinoma are presented as [Figure 2] (a, b Hematoxylin and eosin staining). Photomicrographs of Case 3 pineal MMGCT are presented as [Figure 3] (a, b Hematoxylin and eosin staining). Germinoma and teratoma components are most frequently seen in the MMGCTs of the brain.[3] In the whole group of intracranial MMGCTs, Sano K[3] reported that germinoma components were found in 79%, teratoma components in 63%, yolk sac tumor components in 33.3%, and embryonal carcinoma components in 15.8%.[3] At the molecular level, frequent imbalances of chromosomes have been described in intracranial GCTs, including chromosomes 1, 8, 12, 13, 18, and X. Recently, p14 and c-kit gene alterations have been reported, particularly in some intracranial germinomas; however, their importance remains unclear.[1]
Figure 1.
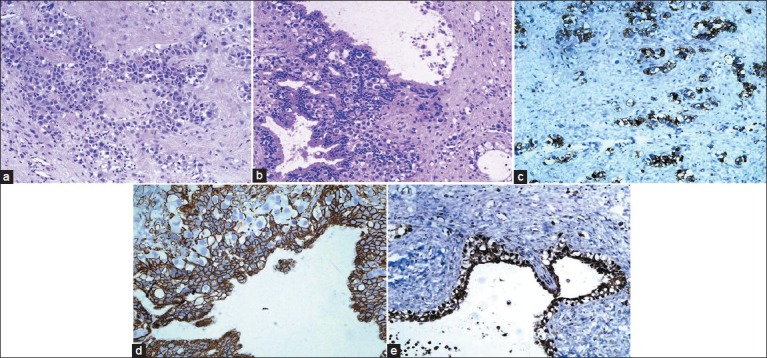
(a) Suprasellar MMGCT, ×40; showing germinoma component. Cells with high N C ratio with defined cell borders and sprinkling of lymphocytes; (b) Suprasellar MMGCT, ×40; showing germinoma component admixed with glandular structures of yolk sac origin; (c) Suprasellar MMGCT, ×40; showing CD-117 positivity in germinoma component; (d) Suprasellar MMGCT, ×40; showing CK-PAN positivity in glandular structures of yolk sac origin. Note the negative germinoma cells in between; (e) Suprasellar MMGCT, ×40; showing AFP positivity in yolk sac component
Figure 2.
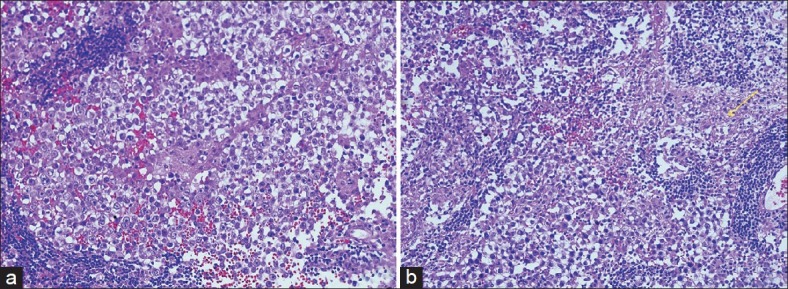
(a) Basal ganglia germinoma, ×40; cells with high N C ratio with defined cell borders and surrounded by lymphocytes (b) note the glial tissue on the left side
Figure 3.
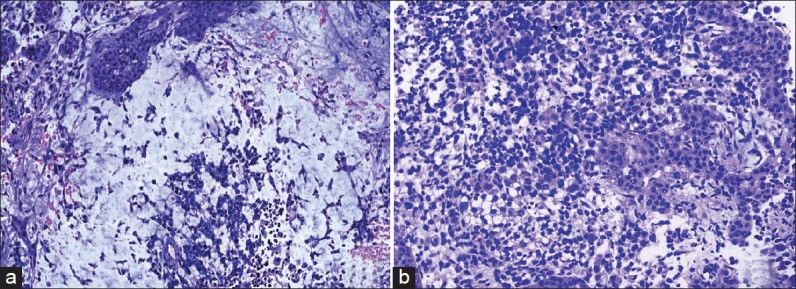
(a) Pineal MMGCT, ×40; showing reticular pattern of yolk sac elements (b) showing immature epithelial elements
Immunohistochemical markers such as AFP, PLAP, HCG, and —c-kit/CD117 are known markers for GCTs, regardless of location. However, recent advances in the use of immunohistochemical markers are providing new depth not only in diagnosis, but also in predicting the biological behavior of these tumors. Intracranial germinomas express OCT4 variably[27] and is even reported to be superior to PLAP.[28] Variation in expression is likely to be predictive of a five-year progression-free survival rate.[27] In addition to being a diagnostic marker, OCT4 may also probably be a prognostic marker for intracranial germinoma. A recent study, examining both the biology and the origin of ICGCTs, analyzed the expression of a wide panel of stem cell-related proteins (C-KIT, OCT-3/4, AP-2γ [TFAP2C and NANOG a homeobox gene]) and developmentally regulated germ cell-specific proteins (including MAGE-A4 melanoma antigen-encoding gene-A4), NY-ESO-1 (cancer testis gene), and TSPY (testis-specific proteinY-encoded gene).[26] Markers of gonadal supportive cells (Sertoli or granulosa cells) including AMH, MIC2, and NSE were also used. Stem cell related proteins were highly expressed in intracranial germ cell tumors, and many similarities were detected with their gonadal equivalents, including a close similarity with primordial germ cells. A notable difference was the sex-specific expression of TSPY, a gene previously implicated in the origin of gonadoblastoma. TSPY was only detected in germ cell tumors in the CNS from males, suggesting that it is not required for the initiation of malignant germ cell transformation. The expression of genes associated with embryonic stem cell pluripotency in CNS GCTs strongly suggests that these tumors are derived from cells that retain, at least partially, an embryonic stem cell-like phenotype, which is a hallmark of primordial germ cell.[26]
Correlation with the serum tumor markers, beta- HCG (human chorionic gonadotropin) and AFP (alpha feto protein), is an essential consideration in the diagnosis of ICGCTs.[29,30] Elevated levels of markers such as AFP, HCG, and PLAP warrant extensive sampling and thorough search for different components.[29,30] In the present study, all three cases showed expression of serum AFP, regardless of the histological type and location, and returned back to normal, in the two cases that survived. Post surgical persistent raised serum AFP in case of pineal body tumor correlated with the presence of a residual tumor. Elevated serum levels of AFP are usually seen in tumors containing yolk sac elements.[29,30] Teratomatous glands may also cause a serum elevation of AFP.[29,30] Detection of raised AFP and beta HCG in the cerebrospinal fluid also serves as an accepted method for initial diagnosis of these tumors without tissue biopsy.[31] The soluble isoform of c-kit (s-kit) is reportedly detectable in cerebral spinal fluid of patients with germinomas’ and germinomas’ with syncytiotrophoblastic giant cells. C-kit and s-kit may be powerful tumor markers for germinomas’ with or without syncytiotrophoblastic giant cells.[32] Some studies indicate that patients with elevated serum and or cerebrospinal fluid markers have a poorer overall survival.[33]
The MRI features can facilitate correct diagnosis of primary ICGCT. The solid components of germinomas’ may show homogeneous and heterogeneously enhancement, while all non-germinomatous GCTs showed heterogeneous enhancement, including histological subtypes.[5,22] However, in general, the neuroimaging characteristics of germinomas’ and NGGCTs are similar enough to limit diagnostic certainty, and either tissue confirmation or the measurement of specific tumor markers are needed for the diagnosis.[22,34]
Over 90% of localized primary intracranial germinomas’ can be effectively treated with radiation therapy and exhibit a relatively good prognosis.[2,34,35]
Chemotherapy is reserved for disseminated germinomas’. Mature teratomas’ are treated with surgery. The rest of germ cell tumors are managed with various combinations of surgery, chemotherapy, and radiotherapy, depending on the tumor type. If the tumors secrete beta-human chorionic gonadotrophin (HCG) or AFP, these tumor markers can be used to accurately monitor the response to treatment.[2] The outcome for patients with NGGCTs is less favorable. Radiation therapy alone will result in disease control in 40%-60% of patients.[30]
Wong et al.[19] have identified global microRNA expression patterns (the miRNome), mRNA signatures, and chromosome copy number variation regions associated with the two pediatric GCT histological entities (germinoma and non-germinomatous GCTs) and two prognostic groups (GPG: Good prognostic group including mainly germinomas and IPG/PPG: Intermediate prognostic group including mainly MMGCTs and immature teratomas’/poor prognostic group including mainly pure yolk sac and MMGCTs with predominant yolk sac). The clinical discrepancies between the two histological entities (germinomas of GPG and non-germinomatous GCTs of IPG/PPG) were mirrored by their differences in global transcriptome patterns and their unique stem cell traits.[19] The literature seems deficient on whether pediatric GCTs from other ethnic background also express similar transcriptome traits and chromosome copy number variation regions.
In conclusion, ICGCTs are rare tumors affecting males more than females; and at the end of three years follow-up, no recurrence was observed among the surviving patients in the present study indicating that suprasellar and basal ganglia ICGCTs may have a favorable prognosis, regardless of histological type. Pineal MMGCTs may have an aggressive course. Although much progress in the understanding of ICGCTs has transpired in the last few years, only continued meticulous study of these neoplasms will reveal possible differences in their biological behavior in both genders and in different locations. This is important, since they are potentially curable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sato K, Takeuchi H, Kubota T. Pathology of intracranial germ cell tumors. Prog Neurol Surg. 2009;23:59–75. doi: 10.1159/000210053. [DOI] [PubMed] [Google Scholar]
- 2.Kyritsis AP. Management of primary intracranial germ cell tumors. J Neurooncol. 2010;96:143–9. doi: 10.1007/s11060-009-9951-z. [DOI] [PubMed] [Google Scholar]
- 3.Sano K. Pathogenesis of intracranial germ cell tumors reconsidered. J Neurosurg. 1999;90:258–64. doi: 10.3171/jns.1999.90.2.0258. [DOI] [PubMed] [Google Scholar]
- 4.Voirin J, Klein O, Chastagner P, Moret C, Vignaud JM, Auque J, et al. Germ-cell tumors of the central nervous system in childhood: Retrospective study of 13 patients. Neurochirurgie. 2008;54:55–62. doi: 10.1016/j.neuchi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zou L, Gao B. Intracranial germinoma: Clinical and MRI findings in 56 patients. Childs Nerv Syst. 2010;26:1773–7. doi: 10.1007/s00381-010-1247-2. [DOI] [PubMed] [Google Scholar]
- 6.Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors. A review. Oncologist. 2008;13:690–9. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 7.Ben Amor S, Siddiqui K, Baessa S. Primary midbrain germinoma. Br J Neurosurg. 2004;18:310–3. doi: 10.1080/02688690410001732832. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Review. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th ed. Oxford: Blackwell Publishing; 2009. p. 22. 222,249. [Google Scholar]
- 10.Agrawal M, Uppin MS, Patibandla MR, Bhattacharjee S, Panigrahi MK, Saradhi V, et al. Teratomas in central nervous system: A clinico-morphological study with review of literature. Neurol India. 2010;58:841–6. doi: 10.4103/0028-3886.73740. [DOI] [PubMed] [Google Scholar]
- 11.Kalra SK, Vaid VK, Jaiswal AK, Behari S, Jain VK, Kumari N. Primary midbrain germinoma. J Clin Neurosci. 2008;15:1282–4. doi: 10.1016/j.jocn.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 12.Wang KC, Kim SK, Park SH, Kim IO, Phi JH, Cho BK. Intracranial Germ Cell Tumors, Chapter 43. In: Tonn JorgChristain, Westphal Manfred, Rutka JT., editors. Oncology of CNS Tumors. 2nd ed. Berlin Heidelberg: Springer-Verlag; 2010. pp. 571–85. [Google Scholar]
- 13.Goodwin TL, Sainani K, Fisher PG. Incidence Patterns of Central Nervous System Germ Cell Tumors: A SEER Study. J Pediatr Hematol Oncol. 2009;31:541–4. doi: 10.1097/MPH.0b013e3181983af5. [DOI] [PubMed] [Google Scholar]
- 14.Khatua S, Phillips A, Fangusaro J, Bovan S, Dhall G, Finlay JL. Recurrent pure CNS germinoma with markedly elevated serum and cerebrospinal fluid human chorionic gonadotropin-beta (HCGβ) Pediatr Blood Cancer. 2010;56:863–4. doi: 10.1002/pbc.22789. [DOI] [PubMed] [Google Scholar]
- 15.Makino K, Nakamura H, Yano S, Kuratsu J Kumamoto Brain Tumor Group. Population-based epidemiological study of primary intracranial tumors in childhood. Childs Nerv Syst. 2010;26:1029–34. doi: 10.1007/s00381-010-1126-x. [DOI] [PubMed] [Google Scholar]
- 16.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–11. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 17.Cho KT, Wang KC, Kim SK, Shin SH, Chi JG, Cho BK. Pediatric brain tumors: Statistics of SNUH, Korea (1959-2000) Childs Nerv Syst. 2002;18:30–7. doi: 10.1007/s00381-001-0547-y. [DOI] [PubMed] [Google Scholar]
- 18.Committee of Brain Tumor Registry of Japan: Report of Brain Tumor Registry of Japan (1969-1996) Neurol Med Chir (Tokyo) 2003;43(Suppl:i-vii):1–111. [PubMed] [Google Scholar]
- 19.Wong TT, Ho DM, Chang KP, Yen SH, Guo WY, Chang FC, et al. Primary pediatric brain tumors: Statistics of Taipei VGH, Taiwan (1975-2004) Cancer. 2005;104:2156–67. doi: 10.1002/cncr.21430. [DOI] [PubMed] [Google Scholar]
- 20.Villano JL, Virk IY, Ramirez V, Propp JM, Engelhard HH, McCarthy BJ. Descriptive epidemiology of central nervous system germ cell tumors: Nonpineal analysis. Neuro-Oncology. 2010;12:257–64. doi: 10.1093/neuonc/nop029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee D, Suh YL. Histologically confirmed intracranial germ cell tumors; an analysis of 62 patients in a single institute. Virchows Arch. 2010;457:347–57. doi: 10.1007/s00428-010-0951-3. [DOI] [PubMed] [Google Scholar]
- 22.Liang L, Korogi Y, Sugahara T, Ikushima I, Shigematsu Y, Okuda T, et al. MRI of intracranial germ-cell tumours. Neuroradiology. 2002;44:382–8. doi: 10.1007/s00234-001-0752-0. [DOI] [PubMed] [Google Scholar]
- 23.Bohara M, Hirano H, Tokimura H, Hanaya R, Yonezawa H, Campos F, et al. Pineal mixed germ cell tumor with a synchronous sellar lesion in the sixth decade. Case Report. Brain Tumor Pathol. 2011;28:163–6. doi: 10.1007/s10014-011-0018-4. [DOI] [PubMed] [Google Scholar]
- 24.Ozelame RV, Shroff M, Wood B, Bouffet E, Bartels U, Drake JM, et al. Basal ganglia germinoma in children with associated ipsilateral cerebral and brain stem hemiatrophy. Pediatr Radiol. 2006;36:325–30. doi: 10.1007/s00247-005-0063-4. [DOI] [PubMed] [Google Scholar]
- 25.Crawford JR, Santi MR, Vezina G, Myseros JS, Keating RF, LaFond DA, et al. CNS germ cell tumor (CNSGCT) of childhood: Presentation and delayed diagnosis. Neurology. 2007;68:1668–73. doi: 10.1212/01.wnl.0000261908.36803.ac. [DOI] [PubMed] [Google Scholar]
- 26.Hoei-Hansen CE, Sehested A, Juhler M, Lau YF, Skakkebaek NE, Laursen H, et al. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: Expression of pluripotency and cell differentiation markers. J Pathol. 2006;209:25–33. doi: 10.1002/path.1948. [DOI] [PubMed] [Google Scholar]
- 27.Wanggou S, Jiang X, Yuan X, Ren C, Zeng Y, Li G, et al. Prognostic value of OCT4 in primary intracranial germinoma: A single institute analysis of 31 cases. Br J Neurosurg. 2011;25 doi: 10.3109/02688697.2011.605483. in press. [DOI] [PubMed] [Google Scholar]
- 28.Hattab EM, Tu PH, Wilson JD, Cheng L. OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol. 2005;29:368–71. doi: 10.1097/01.pas.0000149709.19958.a7. [DOI] [PubMed] [Google Scholar]
- 29.Barlow LJ, Badalato GM, McKiernan JM. Serum tumor markers in the evaluation of male germ cell tumors. Nat Rev Urol. 2010;7:610–7. doi: 10.1038/nrurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 30.Calaminus G, Bamberg M, Harms D, Jürgens H, Kortmann RD, Sörensen N, et al. AFP/beta–HCG secreting CNS germ cell tumors: Long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics. 2005;36:71–7. doi: 10.1055/s-2005-837582. [DOI] [PubMed] [Google Scholar]
- 31.Luther N, Edgar MA, Dunkel IJ, Souweidane MM. Correlation of endoscopic biopsy with tumor marker status in primary intracranial germ cell tumors. J Neurooncol. 2005;79:45–50. doi: 10.1007/s11060-005-9110-0. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Takeshima H, Makino K, Kuratsu J. C-kit expression in germinoma: An immunohistochemistry-based study. J Neurooncol. 2005;75:163–7. doi: 10.1007/s11060-005-1593-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim A, Ji L, Balmaceda C, Diez B, Kellie SJ, Dunkel IJ, et al. The Prognostic Value of Tumor Markers in Newly Diagnosed Patients With Primary Central Nervous System Germ Cell Tumors. Pediatr Blood Cancer. 2008;51:768–73. doi: 10.1002/pbc.21741. [DOI] [PubMed] [Google Scholar]
- 34.Packer RJ, Cohen BH, Coney K. Intracranial Germ Cell Tumors. Oncologist. 2000;5:312–20. [PubMed] [Google Scholar]
- 35.Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: Time to sever historical ties? Lancet Oncol. 2005;6:509–19. doi: 10.1016/S1470-2045(05)70245-X. [DOI] [PubMed] [Google Scholar]


