Abstract
Background and Objectives:
A global distribution has been shown for hepatitis E virus (HEV) infection. Although the fecal-oral was considered as the primary infection route, there is controversial evidence for increased risk of the infection and consequent problems in patients on maintenance hemodialysis (HD) with suppressed immunity. The aim is to find if the prevalence of anti-HEV IgG, in patients with maintenance HD is higher than normal population in southwest of Iran.
Materials and Methods:
During November and December 2010, in a cross-sectional study we compared the seroprevalence of HEV among 80 patients with maintenance HD and 276 healthy individuals from Jahrom and Shiraz, Southwest of Iran. In addition to the clinical and laboratory records, serum samples were tested for the presence of IgG anti-HEV antibody by enzyme immunoassay (ELISA) test. The Chi-square, the Student's ‘t’ and Fisher's exact tests were used for the statistical analysis.
Results:
ELISA tests detected anti-HEV antibody in five males of the patients (6.3%) and in eight of the healthy controls (2.9%, 6 males and 2 female) which statistically were not different. The mean levels of the aspartate aminotransferase and the alanine aminotransferase in the sera of the patients were 19.96±11.08U/L and 23.93±14.26 IU/L, respectively. However, no one of the individuals with positive anti-HEV antibody showed elevated liver enzymes. Moreover, there was not a significant association between positive anti-HEV antibody result, age and the history of the hemodialysis.
Conclusions:
We did not observe statistically significant higher anti-HEV prevalence among patients with chronic HD; however, more safety precaution is needed to keep HD patients from the risk of possible exposure to HEV infection.
Keywords: ELISA, hemodialysis, Hepatitis E virus, iran, seroprevalence
Introduction
Hepatitis E virus (HEV) is an icosahedral; nonenveloped single-stranded RNA virus that has been classified as the only single member of the genus hepevirus in the family Hepeviridae.[1] The infection is self-limited and primarily is transmitted by the fecal-oral route through contaminated water, with mucosal replication and shedding of the virus.[2] Some recent evidence also demonstrated zoonotic transmission of HEV to human. Other probable modes of transmission in endemic areas include vertical transmission, person-to-person contact and zoonotic transmission.[3] As transient viremic stage occurs during the infection, the parenteral transmission can also be a possible route of infection.[4] Genotyping of the virus was used to track the infection; however, attempts to find HEV genome in different groups of patients has not been very successful in many studies; due to short-time persistence of the circulating viruses, during or short after acute phase of infection.[5] An association of HEV infection with hepatitis C virus (HCV) infection has been reported, which may point to similar or overlapping routes of transmission.[6,7] In this regard a substantial proportion of blood donors (1.5%) were positive for HEV RNA, and, viremic blood donors were considered as potential source of transfusion associated hepatitis E infection in areas of high endemicity.[8]
Results from international studies indicate that HEV is spreading globally, not just in developing countries but also in more industrialized countries with more developed health programs.[9] Even there have been reports of nontravel-related HEV infections in western countries such as the Netherland.[10–12] The highest incidence of HEV infection has been reported from Asia, Africa, Middle East and Central America.[13] HEV is the second most common cause of sporadic hepatitis in North Africa and the Middle East.[14] Iran is also among the countries with few suspected outbreaks of HEV in the past.[13] The seroprevalence of HEV in different cities of Iran and between different age groups has been varied (3.8% (Esfahan),[15] 7.4% (Tabriz),[16] 9.3% (Nahavand),[17] 11.5% (Khuzestan),[18] 1.2% in children < 10 years and 7.3% in individuals aged 20-25 years (Sari)[19]).
Several studies have been conducted to explain the possible association of parenteral type of HEV infection in specific groups of patients, such as patients with chronic hemodialysis (CHD). Patients undergoing CHD are potentially at higher risks of exposure to parenterally transmitted microorganisms. Therefore, our study aimed to determine the prevalence of HEV infection based on serological findings, among CHD patients, and to evaluate whether chronic hemodialysis could be associated with an increased risk of infection in patients located in Fars province; southwest of Iran.
Materials and Methods
Patients and sampling
This cross-sectional study was carried out in November and December 2010 on all enrolled 80 patients on maintenance hemodialysis at two centers of Shiraz and Jahrom, cities in southwest of Iran, with an extra 276 healthy individuals from same cities. Sampling was done, after fully informed consent was obtained from the patients or their guardians. This study conforms to the declaration of Helsinki and approved by the ethics committee of Shiraz University of Medical Sciences.
Neither of the patients nor healthy controls had symptomatic active infection or suffered from immunological disorders or consumed immunosuppressive drugs and had history of intravenous drug abuse at the time of sampling for this study. Routine hemodialysis techniques were performed within 3 hours procedure, three times a week for the patients. Blood samples were obtained from the patients before the start of hemodialysis sessions. Sera were separated, coded and stored in aliquots at -70°C until further tests. Demographic and clinical information (such as sex, age, history of hemodialysis or evidence of HBV and HIV infections) were obtained from the medical records. Serum levels of the aspartate aminotransferase (AST), alanine aminotranferease (ALT), the alkaline phosphatase (ALP), prothrombin time (PT), direct bilirubin, indirect bilirubin, serum protein and albumin were also measured for CHD patients.
Anti-HEV immunoglobulin G (IgG) measurement
The anti-HEV immunoglobulin G (IgG) was detected and measured by enzyme-linked immunosorbent assay (ELISA, DIA-PRO, Italy) according to the manufacturer's instruction.
Based on that, the cutoff value of the tests was defined by the positive and the negative control sera that were included in the kit. Duplicated samples were tested and were considered positive if the optical density (OD) value was above the cutoff value and retested again to confirm the initial results.
Statistical analysis
Descriptive statistics were reported. Quantitative variables were expressed as mean ± standard deviation (SD). The Chi-square test, the Student's t test and Fisher's exact test were used to compare the findings between groups. A P-value <0.05 was considered to be statistically significant. Statistical analysis was performed with SPSS software (version15).
Results
Eighty patients on maintenance hemodialysis (51 males and 29 females) and 276 healthy controls (183 males and 93 females) were enrolled in this study. The mean age (± SD) of the patients and healthy control groups were 55.69 ± 14.70 and 51.73 ± 15.10 years, (Age range 26-80 years in patients and 24-77 in healthy volunteers), respectively. The mean duration (± SD) of the HD treatment was 15.60 ± 13.70 months, ranged from 6 to 96 months. Participants in this study were from two cities in southwest of Iran. Of the 80 patients, 48 patients were recruited from Shiraz (60%) and the rest were all registered patients from Jahrom. Almost 80% (N = 220) of the tested healthy volunteers were from Shiraz as well.
Patients with chronic hemodialysis with a minimum history of treatment were the main target of this study therefore we only measured anti-HEV IgG antibodies in those patients; although measurement of IgM could produce additional results. Among the studied individuals, ELISA results demonstrated the existence of anti-HEV IgG antibody in five (all male) of the patients (6.25%) and in eight (six males and two females) of the healthy controls (2.9%). Statistical analysis showed the prevalence rates were not significantly different (P = 0.160). Neither of the patients with positive anti-HEV results showed any clinical symptoms for the acute hepatitis at the time of this study. Results did not show a significant association between positive anti-HEV antibody result and the duration of hemodialysis in seropositive individuals. Moreover, there was no relation between these results with patients’ age and sex. The prevalence of HEV was more in men of both hemodialysis group and healthy participants (P < 0.001). The prevalence rate of HBV infection in the studied patients was 1.25% (N = 1). No significant association was found between HEV positivity and hepatitis B infection in CHD patients (P = 0.097). All the studied patients were seronegative for anti-HIV antibody.
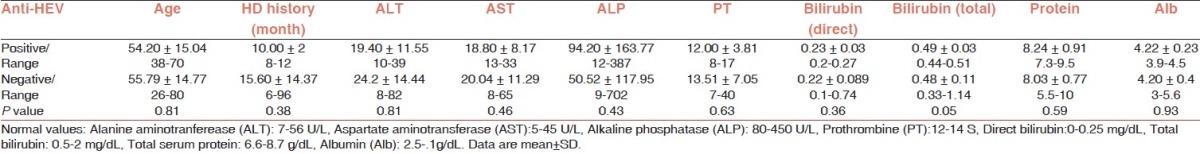
The mean levels of AST (SGOT) and ALT (SGPT) enzymes in the studied patients were 19.96 ± 11.08 U/L (ranged from 8 to 65 U/L) and 23.93 ± 14.26 U/L (ranged from 8 to 82 U/L), respectively. Measuring alkaline phosphatase (ALP), AST and ALT enzymes and bilirubin did not show extensive increase in those parameters among patients with positive anti-HEV antibody result. Moreover, it did not cause abnormal change in albumin and total protein concentrations, except for one patient. Direct and total bilirubin were abnormal in only two of five patients with positive anti-HEV results (Direct bilirubin equal 0.27 and 0.26 and total bilirubin equal 0.47 and 0.44, respectively). Neither of healthy persons with positive anti-HEV results showed abnormal bilirubin in their lab data. Statistical analysis showed that neither of these variations significant. Table 1 summarizes the statistics and clinical laboratory findings for patients with and without anti-HEV antibody.
Table 1.
The ages, the durations of hemodialysis and the clinical laboratory data from HD patients with positive and negative anti-HEV antibody result
Discussion
Patients with chronic hemodialysis are always at risk of infectious diseases due to their compromised immune system.[20] Moreover, these patients are at the frequent exposure to infectious agents during their visits to HD centers. Among viral infections, the association of HEV infection with the parenteral transmission of the virus remains highly controversial. In the present study we investigated the occurrence of anti-HEV IgG antibodies in a selected population of CHD patients with history of chronic hemodialysis from two centers in southwest of Iran and compared the findings with healthy individuals of the same geographical regions. Although anti-HEV seropositivity was more frequent among the studied patients (6.3%), it was not statistically different from the control group (2.9%). Those findings were in the range of anti-HEV IgG seroprevalance in previous reports from Iran.[13,16,17,19,21] The HEV seroprevalence rates reported for different countries and CHD patients have been highly variable, ranged as low as 0.9% in France, 2.6% in Italy, and as high as 7.3% or more among adult population in Spain or in Brazil.[22–25] Higher prevalence of anti-HEV antibodies (IgG or IgM) than normal population was found in HD patients from Greece (9.7% vs 0.23%), Japan (9.4% vs 3.7%) or Taiwan (31% vs 8.9%).[26–29] Therefore, high variations in the results have delayed a comprehensive conclusion for the existence of parenteral HEV infection. This conflict should be resolved with more standardized global studies on different groups of people. Yet, some findings could explain (at least in part) some of these disagreements. As mentioned, patients with chronic hemodialysis and renal diseases suffer from immunocompromised condition.[20] Such condition may cause weakening the immune response against infections, like HEV, and may cause the production of varied amounts of antibodies in the affected patient. In addition to that, studies have shown a wide range of the persistence of anti-HEV IgG antibodies in the circulation.[30] Therefore, it is possible that some of the studies screened the cases after the clearance of the antibody and in this way designated them as seronegative. Assessing IgM in addition to IgG and considering certain phases of the disease may better normalize data. Moreover, genetic variation of the virus can cause massive diversion of the immune responses in the infected patients. Only one single serotype has been reported for the HEV samples isolated from patients with hepatitis; however, there has been considerable genetic diversity among the isolates enough to subgroup the virus into four genotypes with different features and invasiveness.[28,31] Genetic variations in HEV can cause more antigenic diversification in the isolates from different geographical locations. Therefore, it could be speculated that not all immune responses against the altered determinants could be detected by the available commercial diagnostic kits. Effect of genetic variation could be shown by cellular immune response. As an example HEV antigens from genotype 1 were analyzed by overlapping peptides from the open reading frame (ORF) 2 and ORF3. Results of this study could show the presence of at least two sequences (Amino acid 181-249 and 301-489 of ORF2) as immunodominant regions.[32] Moreover, some of the anti-HEV antibody responses detected in patients or healthy donors could be resulted from other infections, cross-reacting with HEV antigens in serological tests as false positive results. More reasons can contribute for the variation in the results of serological tests of anti-HEV antibody which needs to be found by further investigation.
There are other areas of controversy for HEV infection in human. Although elevated ALT and AST have been reported in some HEV-seropositive patients during acute hepatitis phase,[33,34] our biochemical studies similar to others showed no significant differences for these two liver enzymes between patients with positive anti-HEV antibody and seronegative patients. In fact, the level of these enzymes could reduce to the normal levels after a period of 1 month post-acute hepatitis peak.[35] Such fast conversion can explain the observation of normal liver enzymes even in the patients with HEV seropositive test.
Copresence of HEV infection with viruses with definite parenteral route of infection can be considered as an evidence for similar infection route for HEV. Several lines of evidence well demonstrated that patients with maintenance hemodialysis have a higher prevalence of HCV and/or HBV infections.[36] However, studies could not establish a strong link between these two viral infections and HEV infection in CHD patients although some evidence supports such connection.[25] We also could not demonstrate a considerable correlation between the rate of HBV infection and seropositivity for HEV in the studied patients.
In overall, it seems after all studies done on HEV infection, particularly in patients with chronic hemodialysis, still results are not conclusive to answer all questions about different aspects of HEV infection. This could be due to high variation in the studies and the nature of the virus. However, even with controversial results, serious safety measurements should be considered to prevent dissemination of the HEV infection during blood and blood products transfusion mainly in endemic regions.[37] Such precaution could help to limit the risk of viral dissemination in the community and particular in patients with maintenance hemodialysis to avoid worsening their health condition. Standardized and comprehensive studies with more contributors should examine both the immune response and the virus simultaneously to find the truth.
Acknowledgments
This work partially was supported by grants from Shiraz University of Medical Sciences (grant No. 4409) and Jahrom University of Medical Sciences. We also would like to express our sincere gratitude to all participants in the study and all the students from research committees of both Universities.
Footnotes
Source of Support: This work was financially supported by grant 4409 from Shiraz University of Medical Sciences and Jahrom University of Medical Sciences
Conflicting Interest: No.
References
- 1.Yamashita T, Mori Y, Miyazaki N. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci USA. 2009;106:129–86. doi: 10.1073/pnas.0903699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci. 2008;33:451–64. doi: 10.1007/s12038-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 3.Geng J, Wang L, Wang X, Fu H, Bu Q, Liu P, et al. Potential risk of zoonotic transmission from young swine to human: seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J Viral Hepat. 2011;18:e583–90. doi: 10.1111/j.1365-2893.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlauder GG, Dawson GJ, Mushahwar IK, Ritter A, Sutherland R, Moaness A, et al. Viraemia in Egyptian children with hepatitis E virus infection. Lancet. 1993;341:378. doi: 10.1016/0140-6736(93)90187-l. [DOI] [PubMed] [Google Scholar]
- 5.Cacciola I, Messineo F, Cacopardo B, Di Marco V, Galli C, Squadrito G, et al. Hepatitis E virus infection as a cause of acute hepatitis in Southern Italy. Dig Liver Dis. 2011;43:996–1000. doi: 10.1016/j.dld.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DL, Mahley RW, Badur S, Palaoglu KE, Quinn TC. Epidemiology of hepatitis E virus infection in Turkey. Lancet. 1993;341:1561–2. doi: 10.1016/0140-6736(93)90698-g. [DOI] [PubMed] [Google Scholar]
- 7.Pisanti FA, Coppola A, Galli C. Association between hepatitis C and hepatitis E viruses in Southern Italy. Lancet. 1994;344:746–7. doi: 10.1016/s0140-6736(94)92233-0. [DOI] [PubMed] [Google Scholar]
- 8.Arankalle VA, Chobe LP. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang. 2000;79:72–4. doi: 10.1159/000031215. [DOI] [PubMed] [Google Scholar]
- 9.Abe K, Li TC, Ding X, Win KM, Shrestha PK, Quang VX, et al. International collaborative survey on epidemiology of hepatitis E virus in 11 countries. Southeast Asian J Trop Med Public Health. 2006;37:90–5. [PubMed] [Google Scholar]
- 10.Widdowson MA, Jaspers WJ, van der Poel WH, Verschoor F, de Roda Husman AM, Winter HL, et al. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in the Netherlands. Clin Infect Dis. 2003;36:29–33. doi: 10.1086/345439. [DOI] [PubMed] [Google Scholar]
- 11.Herremans M, Vennema H, Bakker J, van der Veer B, Duizer E, Benne CA, et al. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in the Netherlands. J Viral Hepat. 2007;14:140–6. doi: 10.1111/j.1365-2893.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Borgen K, Herremans T, Duizer E, Vennema H, Rutjes S, Bosman A, et al. Non-travel related Hepatitis E virus genotype 3 infections in the Netherlands; A case series 2004–2006. BMC Infect Dis. 2008;8:61. doi: 10.1186/1471-2334-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini-Moghaddam SM, Zarei A, Alavian SM, Mansouri M. Hepatitis E virus infection: A general review with a focus on hemodialysis and kidney transplant patients. Am J Nephrol. 2010;31:398–407. doi: 10.1159/000294505. [DOI] [PubMed] [Google Scholar]
- 14.Fabrizi F, Lunghi G, Bacchini G, Corti M, Pagano A, Locatelli F. Hepatitis E virus infection in haemodialysis patients: A seroepidemiological survey. Nephrol Dial Transplant. 1997;12:133–6. doi: 10.1093/ndt/12.1.133. [DOI] [PubMed] [Google Scholar]
- 15.Ataei B, Nokhodian Z, Javadi AA, Kassaian N, Shoaei P, Farajzadegan Z, et al. Hepatitis E virus in Isfahan Province: A population-based study. Int J Infect Dis. 2009;13:67–71. doi: 10.1016/j.ijid.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Taremi M, Khoshbaten M, Gachkar L, EhsaniArdakani M, Zali M. Hepatitis E virus infection in hemodialysis patients: A seroepidemiological survey in Iran. BMC Infect Dis. 2005;5:36. doi: 10.1186/1471-2334-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taremi M, Mohammad Alizadeh AH, Ardalan A, Ansari S, Zali MR. Seroprevalence of hepatitis E in Nahavand, Islamic Republic of Iran: A population-based study. East Mediterr Health J. 2008;14:157–62. [PubMed] [Google Scholar]
- 18.Assarehzadegan MA, Shakerinejad G, Amini A, Rezaee SA. Seroprevalence of hepatitis E virus in blood donors in Khuzestan Province, southwest Ira. Int J Infect Dis. 2008;12:387–90. doi: 10.1016/j.ijid.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Saffar MJ, Farhadi R, Ajami A, Khalilian AR, Babamahmodi F, Saffar H. Seroepidemiology of hepatitis E virus infection in 2-25-year-olds in Sari district, Islamic Republic of Iran. East Mediterr Health J. 2009;15:136–42. [PubMed] [Google Scholar]
- 20.Eleftheriadis T, Liakopoulos V, Leivaditis K, Antoniadi G, Stefanidis I. Infections in hemodialysis: A concise review. Part II: Blood transmitted viral infections. Hippokratia. 2011;15:120–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Ansar MM, Kooloobandi A. Prevalence of hepatitis C virus infection in thalassemia and haemodialysis patients in north Iran-Rasht. J Viral Hepat. 2002;9:390–2. doi: 10.1046/j.1365-2893.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- 22.Halfon P, Ouzan D, Chanas M, Khiri H, Feryn JM, Mangin L, et al. High prevalence of hepatitis E virus antibody in haemodialysis patients. Lancet. 1994;344:746. doi: 10.1016/s0140-6736(94)92232-2. [DOI] [PubMed] [Google Scholar]
- 23.Buti M, Dominguez A, Plans P, Jardi R, Schaper M, Espunes J, et al. Community-based seroepidemiological survey of hepatitis E virus infection in Catalonia, Spain. Clin Vaccine Immunol. 2006;13:1328–32. doi: 10.1128/CVI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parana R, Cotrim HP, Cortey-Boennec ML, Trepo C, Lyra L. Prevalence of hepatitis E virus IgG antibodies in patients from a referral unit of liver diseases in Salvador, Bahia, Brazil. Am J Trop Med Hyg. 1997;57:60–1. doi: 10.4269/ajtmh.1997.57.60. [DOI] [PubMed] [Google Scholar]
- 25.Bayram A, Eksi F, Mehli M, Sozen E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50:281–6. doi: 10.1159/000103916. [DOI] [PubMed] [Google Scholar]
- 26.Dalekos GN, Zervou E, Elisaf M, Germanos N, Galanakis E, Bourantas K, et al. Antibodies to hepatitis E virus among several populations in Greece: Increased prevalence in an hemodialysis unit. Transfusion. 1998;38:589–95. doi: 10.1046/j.1537-2995.1998.38698326339.x. [DOI] [PubMed] [Google Scholar]
- 27.Mitsui T, Tsukamoto Y, Yamazaki C, Masuko K, Tsuda F, Takahashi M, et al. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: Evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–72. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda H, Honda T, Hayashi K, Katano Y, Goto H, Kumada T, et al. Prevalence of hepatitis E virus IgG antibody in Japanese patients with hemophilia. Intervirology. 2008;5:121–5. doi: 10.1159/000118792. [DOI] [PubMed] [Google Scholar]
- 29.Lee CC, Shih YL, Laio CS, Lin SM, Huang MM, Chen CJ, et al. Prevalence of antibody to hepatitis E virus among haemodialysis patients in Taiwan: Possible infection by blood transfusion. Nephron Clin Pract. 2005;99:c122–7. doi: 10.1159/000083978. [DOI] [PubMed] [Google Scholar]
- 30.Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859–60. doi: 10.1056/NEJMc0708687. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 32.Husain MM, Aggarwal R, Kumar D, Jameel S, Naik S. Effector T cells immune reactivity among patients with acute hepatitis E. J Viral Hepat. 2011;18:e603–8. doi: 10.1111/j.1365-2893.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 33.Turner J, Godkin A, Neville P, Kingham J, Ch’ng CL. Clinical characteristics of hepatitis E in a “Non-Endemic” population. J Med Virol. 2010;82:1899–902. doi: 10.1002/jmv.21905. [DOI] [PubMed] [Google Scholar]
- 34.Gad YZ, Mousa N, Shams M, Elewa A. Seroprevalence of subclinical HEV infection in asymptomatic, apparently healthy, pregnant women in Dakahlya Governorate, Egypt. Asian J Transfus Sci. 2011;5:136–9. doi: 10.4103/0973-6247.83238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuo H, Suzuki K, Takikawa Y, Sugai Y, Tokita H, Akahane Y, et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol. 2002;40:3209–18. doi: 10.1128/JCM.40.9.3209-3218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mederacke I, Meier M, Luth JB, Schmidt-Gurtler H, Raupach R, Horn-Wichmann R, et al. Different kinetics of HBV and HCV during haemodialysis and absence of seronegative viral hepatitis in patients with end-stage renal disease. Nephrol Dial Transplant. 2011;26:2648–56. doi: 10.1093/ndt/gfq757. [DOI] [PubMed] [Google Scholar]
- 37.Bajpai M, Gupta E. Transfusion-transmitted hepatitis E: Is screening warranted? Indian J Med Microbiol. 2011;29:353–8. doi: 10.4103/0255-0857.90158. [DOI] [PubMed] [Google Scholar]


