Abstract
Background:
Although red cell transfusions are lifesavers for patients with thalassemia, they are responsible for a series of complications and expose the patients to a variety of risks.
Material and Methods:
This cross-sectional study included 464 Egyptian beta(β) thalassemia major patients whose age ranged between 10 months and 31 years (mean 10.2 ± 6.6 years). All patients were subjected to thorough history taking with special emphasis on blood transfusions regarding rate of blood transfusion, type of received blood, and history of previous transfusion reactions in addition to type of chelation and compliance to iron chelation therapy and history of diabetes. Serum ferritin and pretransfusion hemoglobin assessment were done for all patients.
Results:
The mean pretransfusion hemoglobin level was 5.7 ± 1.16 g/dl. Allergic reactions were observed in 3.9% of the patients during the period of the study, while the history of previous allergic reaction was given by 72% of the patients. Deferiprone showed better compliance (58.6%) than deferoxamine (26.3%). The prevalence of diabetes was 10.1% among the studied group. On comparing diabetics to nondiabetics, serum ferritin, transfusion intervals, and age were statistically higher among diabetics (P<0.001).
Conclusion:
Lower pretransfusion hemoglobin and high rate of prevalence of diabetes, in addition to better compliance to deferiprone than deferoxamine, were detected among the patients.
Keywords: β-thalassemia, blood transfusion, allergic transfusion reactions, Iron chelators
Introduction
The thalassemias are the most common genetic disorder on a worldwide basis.[1] In Egypt, beta(β) thalassemia major represents a major public health problem. The carrier rate varies between 5.5 and ≥ 9%; it was estimated that 1000/1.5 million per year live births have β-thalassemia.[2]
Blood transfusion therapy and iron chelation are the cornerstones in management of thalassemia. Early and regular blood transfusion decreases the complications of severe anemia and prolongs survival. Yet, transfusion carries the risk of complications. Therefore knowing different adverse effects of blood transfusion represents a great issue in managing thalassemic patients. Adverse events which occur in association with the transfusion of blood products are commonly called transfusion reactions.[3]
Blood transfusions are associated with hazards of iron overload leading to endocrinal dysfunction and risk of acquiring transfusion transmitted infection as hepatitis.[4]
Iron chelators in current clinical use include subcutaneous or intravenous, oral deferiprone, and oral deferasirox. In a substantial proportion of patients in routine clinical practice, adequate control of tissue iron levels is not achieved, despite clinical trials demonstrating this to be possible.[5]
The aim of the present work was to study the prevalence of blood transfusion complications among β-thalassemia major patients in the hematology clinic of Children Hospital, Cairo University, during a period of 6 months.
Materials and Methods
Four hundred and sixty four β-thalassemia major patients coming for regular follow-up and blood transfusion at the hematology clinic of the New Children's Hospital, Cairo University during a period of 6 months (from the beginning of March 2009 to the end of August 2009) were randomly recruited to participate in this study. Their age ranged between 10 months and 31 years (mean = 10.2 ± 6.6 years); 57.1% were males and 42.9% females with a male to female ratio of 1.3:1.
All patients were subjected to thorough history taking with special emphasis on blood transfusions regarding rate of blood transfusion, type of received blood, and history of previous transfusion reactions in addition to compliance to iron chelation therapy and history of diabetes mellitus. Clinical assessment was done for each patient during the transfusion session in order to detect any transfusion reaction.
After explaining the procedure to the patients and acquiring informed consent from the patients or guardians, 4 ml of venous blood was withdrawn under complete aseptic conditions and divided into 1 ml on EDTA to perform ABO and Rhesus (Rh) blood grouping and hemoglobin level before transfusion. The other 3 ml was left to clot and centrifuged to separate the serum to perform the cross matching and estimation of serum ferritin.
Pretransfusion hemoglobin was determined using a Sysmex KX-21N hematology analyzer which utilizes the noncyanide sodium lauryl sulfate (SLS) method in order to determine the hemoglobin level.
Serum was separated within 4-5 hours of collection, and each sample was transferred into four cryovials and stored at -70°C. All samples were tested for serum ferritin using microparticle enzyme immunoassay (MEIA).
Statistical methodology
Data were statistically described in terms of range, mean ± SD (standard deviation), frequencies, and percentages when appropriate. Comparison of quantitative variables between the study groups was done using the Student t-test for independent samples in comparing two groups and one-way analysis of variance (ANOVA) test in comparing more than two groups.
For comparing categorical data, a Chi-square (χ2) test was performed. The exact test was used instead when the expected frequency was less than 5. A probability value (P value) less than 0.05 was considered statistically significant.
All statistical calculations were done using Microsoft Excel 2003 (Microsoft Corporation, NY, USA) and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
The age distribution among the group of patients showed a great variation; 310/464 (66.8%) were less than 12 years old (194/310 (62.6%) males and 116/310 (37.4%) females), where 85/464 (18.3%) were between 12 and 18 years (37/85 (43.5%) males and 48/85 (56.5%) females) while 69/464 (14.9%) patients were above 18 years (34/69 (49.3%) males and 35/69 (50.7%) females).
The pretransfusion hemoglobin level varied markedly with a range of 2.8-9.3 g/dl (mean = 5.7 ± 1.16 g/dl). The intervals between blood transfusions ranged from 1 to 6 weeks (mean = 4.41 ± 2.1 weeks) in addition to one patient being transfused for the first time and another one for the second time.
Transfusion reactions that developed to the patients during or immediately after blood transfusion during the period of study were allergic reactions in the form of urticaria, skin flushing, and itching among 18/464 (3.9%) patients; 12/18 (66.7%) males and 6/18 (33.3%) females. The transfusion was stopped immediately to evaluate the clinical state of the patient and intravenous antihistamine and corticosteroid were given to the affected patients and the blood transfusion was continued slowly. One of the patients gave history of low-grade fever few hours after the transfusion that was relieved by oral paracetamol.
Regarding the history of transfusion reactions that developed to the patients throughout their previous blood transfusion sessions, 334/464 (72%) patients gave history of acute transfusion reactions. The majority of symptoms reported were mild and transient, 286/334 (85.8%) of the patients developed these reactions during transfusion; 254/334 (76%) patients developed rash and urticaria, 72/334 (21.6%) developed fever and rigors, while 5/334 (1.5%) patients developed acute hemolytic reactions (in the form of fever, chills, vomiting, urticaria, and shortness of breath, and 3/334 (0.9%) patients developed anaphylactic shock, those patients were immediately hospitalized and received antishock treatment.
Ten out of the 464 patients (2.2%) were receiving prophylactic antihistaminic and corticosteroids. While 49/464 (10.6%) used filters during transfusion or were transfused with washed blood due to previous development of transfusion reactions.
The serum ferritin level of the patients ranged from 96 to 9849 ng/ml (mean 1928.66 ±1543.732 ng/ml). We classified the patients into three groups according to their serum ferritin level. Those who had serum ferritin below 1000 ng/ml were 139 (30%) patients (mean age 5.8 ± 4.3 years) while 215 patients (46.3%) had ferritin level between 1000 and 2500 ng/ml (mean age was 11.5 ± 6.8 years). Seventy percent of those patients were receiving deferiprone as a chelating agent, 23.5% were receiving deferoxamine, and 6.5% were receiving deferasirox. There were 110 (23.7%) patients whose serum ferritin level was above 2500 ng/ml. (mean age 13.1 ± 5.9 years) with 52.7% of them receiving deferiprone, 46.4% were on deferoxamine, and one (0.9%) patient was on deferasirox.
Three hundred twenty one patients of the group (69.2%) were receiving different forms of chelation therapy. Compliance rate among patients on deferiprone was 58.6% and 26.3% for patients on deferoxamine, while all patients on deferasirox were adherent to the chelation regimen.
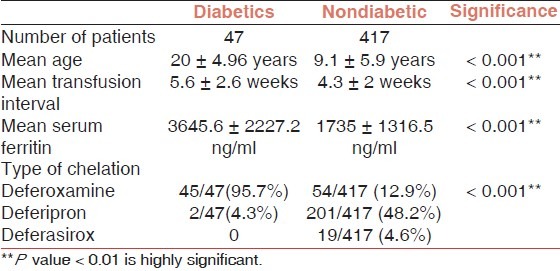
History of diabetes was given by 47/464 (10.1%) of the patients (30 males and 17 females) and they were on regular insulin therapy. Diabetics were compared to the rest of the patients [Table 1].
Table 1.
Comparison between the diabetic and nondiabetic patients
Discussion
The treatment of thalassemia major is blood transfusions to maintain the hemoglobin level.[6] The present study was designed to explore the prevalence of complications of blood transfusion among 464 β- thalassemic patients who receive regular blood transfusion in the hematology clinic of Children Hospital, Cairo University during a period of 6 months.
Although life expectancy of thalassemic patients has increased by the progress achieved in the lines of management of thalassemia, but still the majority of our patients are young, as 66.8% were younger than 12 years. Noor Haslina and colleagues reported that 47.7% of their studied groups were younger than 12 years. They concluded that this could be related to the negligence of the follow-up by the patients or bad compliance to prescribed therapy that leads to frequent complications and affects the patients’ quality of life.
The mean pretransfusion hemoglobin level was 5.7±1.16 g/dl which is lower than similar studies in which median baseline hemoglobin concentration of 10.0 g/dl was observed.[7] The low hemoglobin concentration level among the patients compared to similar studies may be related to the low availability of blood due to low rate of blood donation as this blood was given to the patients free of charge.
Allergic reactions developed in 18 patients (3.9%) during the period of the study in agreement with previous results reporting acute transfusion reactions in 16% of patients in which chills, fever, and urticaria accounted for more than 80% of symptoms.[8]
It should be taken into consideration that 2.2% of the patients used prophylactic antihistaminics and corticosteroids before transfusion. In addition, 10.6% of the patients used a filter during transfusion or transfused washed blood due to previous high incidence of development of transfusion reactions.
In a study from Puerto Rico, the overall incidence of immediate reactions was 0.2%. This low incidence was suggested to be due to underreporting of the transfusion reactions and the use of leukoreduced components.[9,10]
The history of transfusion reactions that developed to the patients throughout their disease history revealed that 72% of them developed acute transfusion reactions which were mainly allergic and 1.5% were acute hemolytic in origin.
This prevalence is higher than that reported in the United States where allergic transfusion reactions accounted for 17% of the transfusion reactions and severe forms were observed in 7.7% of allergic reactions.[11] This difference could be attributed to the use of leukodepleted blood components which reduce the incidence of acute nonhemolytic febrile reactions.[12]
The current study demonstrated that adherence to chelation therapy was a major issue. Compliance rates among the patients were different from those of other cohorts on thalassemia as compliance was 26.3% among those who received deferoxamine and 58.6% among those who received deferiprone.
In several studies, compliance to deferoxamine ranged from 59% to 78%, while studies of deferiprone reported better compliance ranging from 79 to 98%.[3,13]
In these studies the difficulty of access to iron chelating medications was not an issue in contrast to our population as this was a significant reason for noncompliance. Poorer compliance to deferoxamine may be due to the hazards of application in relation to the easier oral chelation.
Under conditions of ideal chelation, it is expected that serum ferritin levels be maintained within normal limits irrespective of the total number of transfusions. However such a uniform maintainance of serum ferritin levels was not found. Thus, indicating irregular and inadequate chelation practices or variable response to chelation therapy.[4] Setting the cut-off limit for serum ferritin of below 1000 ng/ml between adequately chelated and poorly chelated patients according to the Thalassemia International Federation's guidelines,[14,15] we found that 30% of the patients had a ferritin level below 1000 ng/ml while Shah and coworkers reported a prevalence rate of 6.3% among their studied group.
In our study, the prevalence of diabetes mellitus among the patients was 10.1% which was higher than that in previous studies reporting incidence of 9.4%, 8%, and 3.2%.[16–18]
The mean serum ferritin of the diabetic patients was 3645.6 ± 2227.2 ng/ml and 95.7% of those patients were on deferoxamine for iron chelation.
The main risk factors associated with endocrine complications including diabetes mellitus are high serum ferritin levels and poor compliance to chelation therapy. Serum ferritin levels of approximately 3000 ng/ml were found to correlate with diabetes.[19,20]
Conclusion
Lower pretransfusion hemoglobin and high rate of prevalence of diabetes, in addition to better compliance to deferiprone than deferoxamine, were detected among the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–7. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]
- 2.El-Beshlawy A, Kaddah N, Moustafa A, Mouktar G, Youssry I. Screening for β-thalassemia carriers in Egypt: Significance of the osmotic fragility test. East Mediterr Health J. 2007;13:780–6. [PubMed] [Google Scholar]
- 3.Katz EA. Blood transfusion: Friend or foe. AACN Adv Crit Care. 2009;20:155–63. doi: 10.1097/NCI.0b013e3181a0d688. [DOI] [PubMed] [Google Scholar]
- 4.Shah N, Mishra A, Chauhan D, Vora C. Study on effectiveness of transfusion program in thalassemia major patients receiving multiple blood transfusions at a transfusion centre in Western India. Asian J Transfus Sci. 2010;4:94–8. doi: 10.4103/0973-6247.67029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidson-Gerber GL, Francis S, Lindeman R. Management and clinical outcomes of transfusion-dependent thalassaemia major in an Australian tertiary referral clinic. Med J Aust. 2008;188:68–9. doi: 10.5694/j.1326-5377.2008.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 6.Hazirolan T, Eldem G, Unal S, Akpinar B, Gümrük F, Alibek S, et al. Dual-echo TFE MRI for the assessment of myocardial iron overload in beta-thalassemia major patients. Diagn Interv Radiol. 2010;16:59–62. doi: 10.4261/1305-3825.DIR.2555-09.1. [DOI] [PubMed] [Google Scholar]
- 7.Cario H, Stahnke K, Kohne E. Beta-thalassemia in Germany. Results of cooperative beta-thalassemia study. Klin Padiatr. 1999;211:431–7. doi: 10.1055/s-2008-1043828. [DOI] [PubMed] [Google Scholar]
- 8.Rebulla P. Transfusion reactions in thalassemia. A survey from the Cooley care. The Cooley care Cooperative Group. Haematologica. 1990;75:122–7. [PubMed] [Google Scholar]
- 9.Climent-Peris C, Vélez-Rosario R. Immediate transfusion reactions. P R Health Sci J. 2001;20:229–35. [PubMed] [Google Scholar]
- 10.Martínez-Díaz H, Frye-Maldonado AC, Climent-Perís C, Vélez-Rosario R. Evaluation of serologic markers for transfusion transmitted infectious diseases for allogeneic blood donors in Puerto Rico. P R Health Sci J. 1997;16:255–8. [PubMed] [Google Scholar]
- 11.Domen RE, Hoeltge GA. Allergic transfusion reactions: An evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–20. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 12.Pruss A, Kalus U, Radtke H, Koscielny J, Baumann-Baretti B, Balzer D, et al. Universal leukodepletion of blood components results in a significant reduction of febrile non-hemolytic but not allergic transfusion reactions. Transfus Apher Sci. 2004;30:41–6. doi: 10.1016/j.transci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Delea TE, Edelsberg J, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion- dependent thalassemia. Transfusion. 2007;47:1919–29. doi: 10.1111/j.1537-2995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 14.Thalassemia International Federation: Guidelines for the clinical management of thalassemia. [Last accessed on 2-9-2011]. Available from: http://www.thalassaemia.org.cy/Publications.htm .
- 15.Gattermann N. Guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusion iron overload. Leuk Res. 2007;31S:10–5. doi: 10.1016/S0145-2126(07)70461-7. [DOI] [PubMed] [Google Scholar]
- 16.Toumba M, Sergis A, Kanaris C, Skordis N. Endocrine complications in patients with Thalassaemia Major. Pediatr Endocrinol Rev. 2007;5:642–8. [PubMed] [Google Scholar]
- 17.Ong CK, Lim SL, Tan WC, Ong EE, Goh AS. Endocrine complications in transfusion dependent thalassaemia in Penang Hospital. Med J Malaysia. 2008;63:109–12. [PubMed] [Google Scholar]
- 18.De Sanctis V, Eleftheriou A, Malaventura C. Thalassaemia International Federation Study Group on Growth and Endocrine Complications in Thalassaemia. Prevalence of endocrine complications and short stature in patients with thalassaemia major: A multicenter study by the Thalassaemia International Federation (TIF) Pediatr Endocrinol Rev. 2004;2:249–55. [PubMed] [Google Scholar]
- 19.Gamberini MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: Incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol Rev. 2008;1:158–69. [PubMed] [Google Scholar]
- 20.Suvarna J, Ingle H, Deshmukh CT. Insulin resistance and beta cell function in chronically transfused patients of thalassemia major. Indian J Pediatr. 2006;43:393–400. [PubMed] [Google Scholar]


