Abstract
Introduction:
Apheresis procedures [Plateletpheresis, Plasmapheresis/ Therapeutic Plasma Exchange (TPE), & Peripheral Blood Stem Cell Collection (PBSC)] are usually well tolerated. Occasionally, Adverse Events (AEs) of variable severity may occur during or after the procedure. AEs that occur in Donors/Patients are divided into local reactions and systemic reactions.
Materials and Methods:
A total of 3,367 apheresis procedures were performed, out of which 3,120 were plateletpheresis procedures, and out of which 1,401 were on Baxter CS 3000 & 1,719 were on Haemonetics MCS+ cell separators. Rest of 247 TPE & PBSC procedures were done on Haemonetics MCS+ cell separators.
Results:
90 AEs were reported in relation to the 3,367 procedures. Out of 90 AEs, 85 AEs (94%) were associated with plateletpheresis (n = 3,120) and 05 AEs (06%) with TPE & PBSC (n = 247). The rate of vascular injury (VI), Citrate reaction (CR), and Presyncopal/Syncopal (PS/S) in plateletpheresis was 1.6% (52/3,120), 0.96% (30/3,120), and 0.096% (03/3,120), respectively. The rate of CR in TPE and PBSC was 1.23% (02/162) and 2.3% (02/85), respectively. The rate of PS/S in PBSC was 1.17% (01/85). AEs for Plateletpheresis, TPE & PBSC were 2.7% (85/3,120), 1.23% (02/162), and 3.5% (03/85), respectively. VI, CR, and PS/S were mostly of mild intensity. Both cell separators were equally safe, when AEs associated with plateletpheresis were compared with each other; 2.8% on CS 3000 & 2.6% on MCS+.
Conclusion:
Apheresis procedures performed on cell separators are safe, with a low incidence of significant AEs. No significant difference was noted in AEs among the two cell separators studied.
Keywords: Adverse events, citrate reaction, peripheral blood stem cell, presyncopal/syncopal, therapeutic plasma exchange, vascular injury
Introduction
Apheresis procedures are usually well tolerated. Adverse Events (AEs) of variable severity may occur during or after the procedure. AEs that occur in donors can be divided into local reactions and systemic reactions.[1,2]
Local reactions are usually haematomas due to extravasation from the veins, caused by incorrect placement of the needle during the venipuncture. Pain, hyperaemia and swelling may develop at the site of the extravasation. Local phlebitis and thrombophlebitis are very rare.[2,3]
Systemic reactions are mainly vasovagal reactions that can be triggered by the pain of the venipuncture, or by the anxiety and state of tension of undergoing the donation, etc. These are characterised by the pallor, sweating, dizziness, nausea, hypotension, bradycardia, and syncope. Citrate toxicity occurs because of the use of acid-citrate-dextrose (ACD) in apheresis.[3–5]
Materials and Methods
A total of 3,367 apheresis procedures were performed, out of which 3,120 were plateletpheresis procedures, 1,401 were on Baxter CS 3000 and 1,719 were on Haemonetics MCS+ cell separators. Rest of 247 TPE and PBSC procedures were done on Haemonetics MCS+ cell separators.
Baxter CS 3000 (Fenwal) is double-needle continuous type of cell separator, while MCS+ (Haemonetics) is single-needle intermittent type of cell separator. Plateletpheresis was performed on both the cell separators and all donations were collected using a 16-gauge needle inserted into a vein in the antecubital fossa, with all aseptic precautions. Plasmapheresis/Therapeutic plasma Exchange (TPE) and Peripheral Blood Stem Cell Collection (PBSC) were performed using a Haemonetics MCS+ machine with central venous line on the patient.
Donors were selected as per the set criteria for single donor platelet (SDP) preparation according to AABB guidelines:
-
(i)
Weight > 50 kg
-
(ii)
Age - 18 to 60 years
-
(iii)
At least three months from last donation/three days from last Plateletpheresis
-
(iv)
Haemoglobin >12.5 gm/dl
-
(v)
Platelet count > 150 × 103/μl
-
(vi)
Absence of any illness
-
(vii)
No consumption of non-steroidal anti-inflammatory drugs for last seven days
-
(viii)
Negative test for HIV, Hepatitis B, Hepatitis C, Syphilis and Malaria.
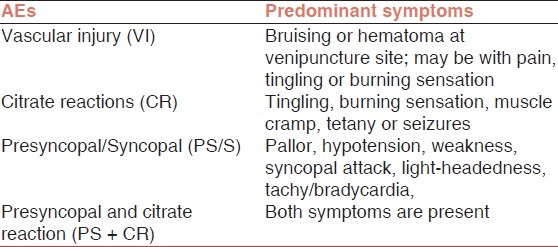
All adverse events were recorded by the staff. The adverse events occurring during or after the procedures were classified as vascular injuries (VIs), citrate reaction (CR), presyncopal/syncopal (PS/S), and PS + CR both [Table 1].
Table 1.
Definition and predominant symptoms of types of donor AEs
Results
We analysed a total of 3,367 procedures during the study periods including 3,120 (92.6%) plateletpheresis, 162 (4.8%) TPE, and 85 (2.5%) PBSC, [Figure 1]. All AEs were divided into mild/moderate to severe reactions [Table 2].
Figure 1.
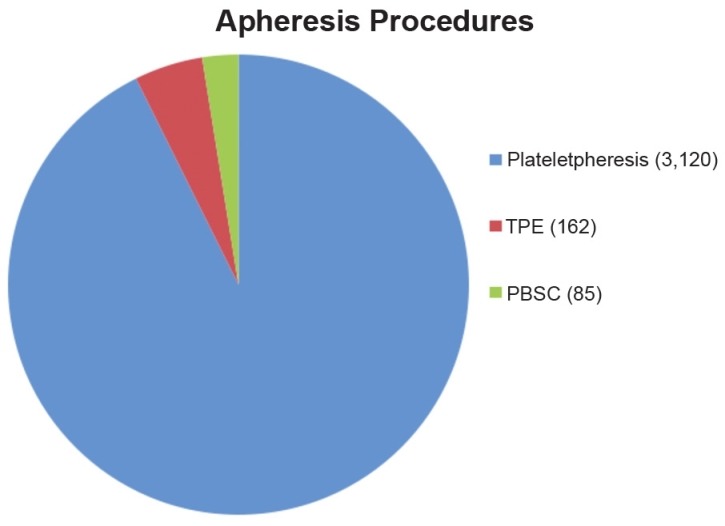
Types of apheresis procedures during study periods (n = 3,367)
Table 2.
Adverse events associated with apheresis procedures
90 AEs were reported in relation to the 3,367 procedures [Figure 2]. Out of 90 AEs, 85 AEs (94%) were associated with plateletpheresis (n = 3,120) and 05 AEs (06%) with TPE and PBSC (n = 247). The rate of vascular injury (VI), Citrate reaction (CR), and Presyncopal/Syncopal (PS/S) in plateletpheresis was 1.6% (52/3,120), 0.96% (30/3,120), and 0.096% (03/3,120), respectively [Table 3]. The rate of CR in TPE and PBSC was 1.23% (02/162) and 2.3% (02/85), respectively. The rate of PS/S in PBSC was 1.17% (01/85) [Table 4]. AEs for Plateletpheresis, TPE and PBSC were 2.7% (85/3,120), 1.23% (02/162), and 3.5% (03/85), respectively.
Figure 2.
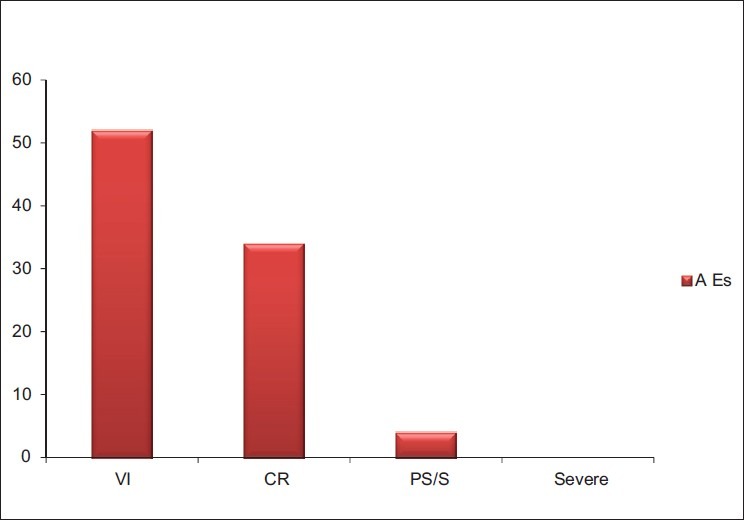
Types of adverse events during apheresis procedures
Table 3.
Adverse events occurring during and after plateletpheresis procedures
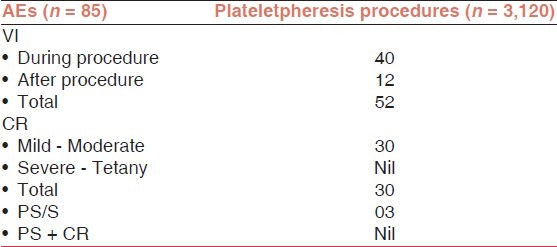
Table 4.
Adverse events occurring during and after TPE and PBSC procedures
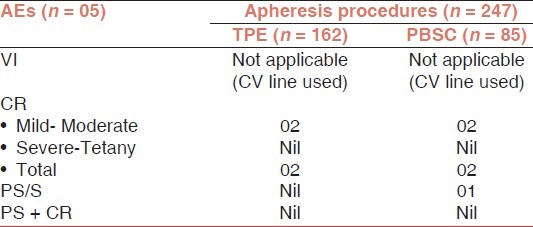
VI, CR, and PS/S were mostly of mild intensity. No severe adverse reactions were observed during the study period.
VI is not applicable for TPE and PBSC because in these procedures central venous line was used. Out of 52 VI (bruising/hematoma or pain), 40 was during procedure and 12 was after the plateletpheresis procedure.
Out of 34 CR, 30 was mild/moderate (circumoral paraesthesia/tingling and numbness) type and no severe reaction (tetany) was reported in plateletpheresis. In TPE and PBSC, 02 and 02 CR of mild type was reported, respectively.
A total of 04 PS/S reactions were reported during the study period, out of 04 reactions, 03 in plateletpheresis and 01 in PBSC. No case was reported as a combination of PS + CR during the study periods.
AEs for Plateletpheresis on healthy donors were 2.7% (85/3,120) [Figure 3], and for TPE and PBSC on patients were 1.23% (02/162), and 3.5% (03/85), respectively [Figure 4].
Figure 3.
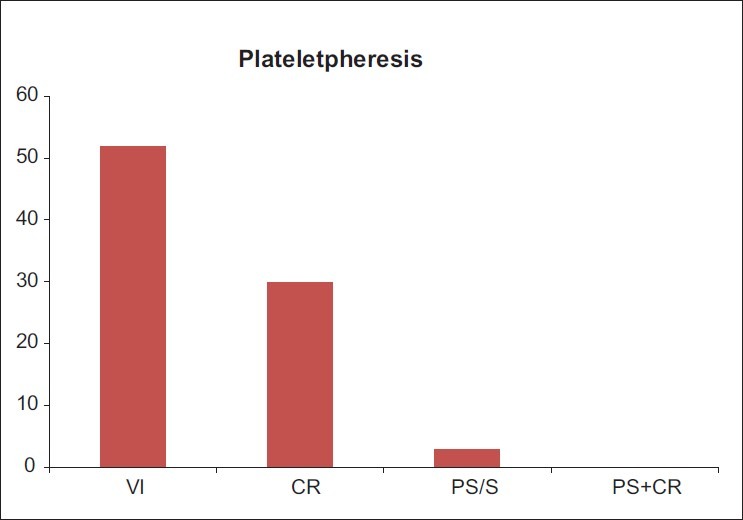
AEs occurring during and after the apheresis procedures (n = 3,120) and (AEs = 85)
Figure 4.
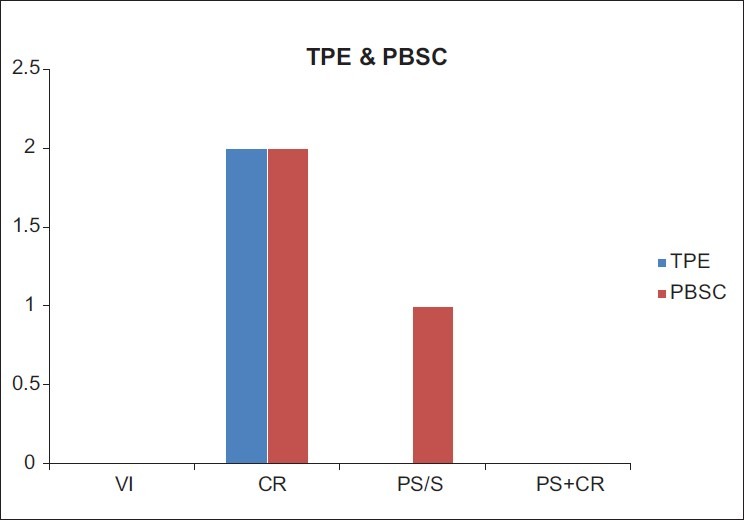
AEs occurring during and after the TPE and PBSC procedures (n = 247) and (AEs = 05)
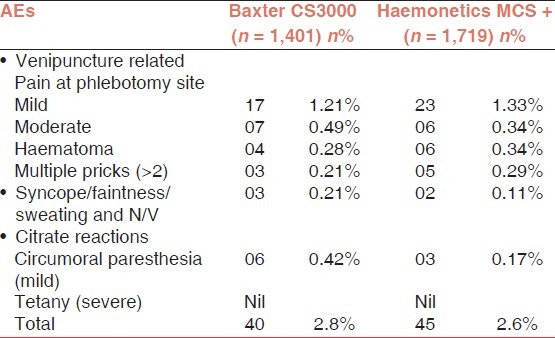
Both cell separators were equally safe during plateletpheresis procedures, when AEs were compared with each other. Out of 85 AEs in plateletpheresis, 40 AEs (2.85%) were on CS 3000 and 45 AEs (2.61%) were on MCS+ [Table 5].
Table 5.
Adverse events associated with cell separators during Plateletpheresis
Discussion
A decreasing blood donor pool in the presence of increasing blood transfusion demands has resulted in the need to maximally utilize each blood donor. This has led to a trend in the increasing use of automated blood collections. These collection methods share many of the same reactions and injuries seen with whole blood donation but also have unique complications due to the collection method and the frequency at which donation can occur.
While apheresis donation shares many reactions and injuries with whole blood donation, because of the differences, unique complications also exist. Overall, evidence in the literature suggests that the frequency of reactions to apheresis donation is less than that seen in whole blood donation.[1,2,6] The most common apheresis-specific reaction is hypocalcaemia due to citrate anticoagulation, which, while usually mild, has the potential for severely injuring the donor. Other reactions to apheresis donation are uncommon (e.g., hypotension) or rare (e.g., air embolism).
The frequency of acute reactions among donors undergoing apheresis procedures was found by McLeod et al. to be 2.18% in a multi-institutional study.[1] Donors collected with the Fenwal CS3000 had fewer reactions than those collected with the Haemonetics instruments. It was felt that the larger extracorporeal volumes seen with the single-needle procedures might have contributed to hypotensive reactions.[1,2] However our experience has shown otherwise as discussed below.
The adverse events during the process of plateletpheresis using CS3000 and MCS+ has been broadly divided into,
-
(i)
Venipuncture related,
-
(ii)
Syncope/faintness/sweating and N/V and,
-
(iii)
Citrate reactions [Table 5].
Pain at the site of venipuncture was noted to be slightly more while using CS3000. This may be related to the increased sensation of vibrations induced by the subjectively cruder centrifugation forces of CS3000. The increased percentage of haematoma while using MCS+ Haemonetics; could be as a result of the fact that the same vein in one arm is used for inflow and return, resulting in trauma and resulting haematoma to the vein.
Citrate is used as the primary anticoagulant in donor apheresis procedures. The anticoagulant effect of citrate results from its ability to chelate calcium ions resulting in the calcium ions being unavailable to participate in biological reactions such as the coagulation cascade. Within the apheresis instrument, plasma citrate concentrations reach 15 to 24 mmol/L, lowering the calcium ion concentration below 0.2 to 0.3 mmol/L, the level necessary for clotting to occur.[4,5,7] This level of anticoagulation requires the infusion of approximately 500 ml of ACD-A solution and it would be expected that the infusion of this volume of solution into a donor would result in a calcium ion concentration of 0.2 mmol/l; a level incompatible with life. This does not occur, however, for a number of reasons. First, when blood returns from the apheresis instrument to the donor, the citrate present in the blood is diluted throughout total extracellular fluid, not just the intravascular space. In addition, the liver, kidneys, and muscles rapidly metabolize citrate, releasing the bound calcium.[8] Despite compensatory mechanisms, citrate infusion can result in the decrease in ionized calcium levels to a point where symptoms develop in the donor. In our study we did not determine the ionised calcium level pre-procedure but in a study of the effects of citrate on apheresis platelet donors, Bolan et al. found an average fall in ionized calcium of 33% from baseline.[8] The result of such a decrease in ionized calcium is that the excitability of nerve membranes increases to the point where spontaneous depolarization can occur.[8] This produces the signs and symptoms of citrate toxicity including perioral paresthesias, shivering, light-headedness, twitching, and tremors. In addition, some patients also experience nausea and vomiting. As the ionized calcium levels fall further, these symptoms may progress to carpopedal spasm, tetany, and seizure.[8,9] It is, therefore, important to elicit the presence of the early symptoms from the donor so that interventions can occur prior to the more severe symptoms. In our study calcium supplementation was given to donors when they complained about paraesthesia/tingling or numbness sensations. All these citrate reactions in donors were mild. There was no severe form of reaction noted in our study. Hypotension may also be seen with citrate reactions and may be due to the depressed myocardial function as well as to vascular smooth muscle relaxation.[9]
Factors that have been found to influence the rate of citrate reactions in donor and therapeutic apheresis include alkalosis due to hyperventilation, the type of anticoagulant solution used with ACD-A having more reactions than ACD-B, the rate of infusion of the anticoagulant solution, the amount of citrate infused, and the donor's serum albumin level prior to the start of the collection procedure.[8,9] It has also been reported that intermittent flow hemapheresis procedures tend to have a greater frequency of citrate reactions, as there is a higher rate of citrate infusion when the separation chamber is emptied, as compared to continuous apheresis procedures. In our study, as far as citrate reactions in donors were considered, there were no severe reactions suggesting hypocalcaemic tetany, in donors taken on both the machines. However, circumoral paraesthesia, labelled as a mild citrate reaction was of a higher percentage in CS3000 as compared to MCS+. In 1,401 procedures conducted for plateletpheresis using MCS+, the average volume of ACD transfused was 312 ml. In 1,719 procedures conducted for plateletpheresis using CS3000, the average volume of ACD transfused was 448 ml. This shows the ACD consumption while using the two different machines. It can be clearly seen that the ACD consumption was significantly more in procedures conducted by CS3000 in nearly all procedures conducted on it. The above would have contributed greatly in presenting a slightly increased percentage of overall AEs in CS3000 vis-a-vis MCS+.
The treatment of citrate reactions is relatively simple when the reactions are identified early. The treatment includes slowing the re-infusion rate to allow for dilution and metabolism of the citrate, increasing donor blood to citrate ratio to decrease the amount of citrate infused, giving oral calcium supplement, and if required giving intravenous calcium.[3–5,7] The administration of oral calcium carbonate and its effects on citrate toxicity have recently been examined by Bolan et al.[8] These authors found that the administration of 2 g of calcium carbonate was associated with a statistically significant reduction in the severity of paresthesia.[8,9] In our study, we gave calcium supplementation in the form of 1 gm capsules of calcium carbonate orally. While improving paresthesias, in multivariate analysis, the oral administration of calcium was not associated with a reduction in overall symptom development and did not affect the occurrence of more severe symptoms.[2,6,8] The administration of intravenous calcium, in the form of calcium gluconate or calcium chloride is usually not necessary in donor procedures and, therefore, has not been studied in this setting. In hematopoietic progenitor cell (HPC) collections, the continuous infusion of either calcium gluconate or calcium chloride has been found to prevent hypocalcemic symptom development with calcium chloride maintaining higher ionized calcium levels.[8,9] In a comparison with a continuous infusion of calcium gluconate, prophylactically at the start of HPC collection, or at the time of symptom development, continuous infusion maintained higher calcium levels with insignificant changes seen in the other two modes of administration. In our study we also started the calcium gluconate infusion prophylactically at the beginning of PBSC collection and TPE procedure. Continuous infusion should not be necessary in normal apheresis donation. When IV supplementation is necessary, such as in severe reactions, the usual dose of intravenous calcium is 10 ml of 10% calcium gluconate IV infused over 10 to 15 minutes. Too rapid of an infusion can result in hypotension and is to be avoided.[5,7] In our study, we have not encountered any kind of severe form of citrate AEs.
Another mechanism causing hypotension during apheresis procedures is the vasovagal reaction. In this reaction, hypovolemia results in a decrease in blood pressure. The compensatory response for this volume depletion is to increase sympathetic nervous system output with physiologic compensation as previously described. During a vasovagal reaction, however, parasympathetic output that normally counteracts sympathetic output increases, resulting in a slowing of heart rate and decreased vascular tone. This results in hypotension. Tomita et al. examined the incidence of vasovagal reactions among apheresis donors and whole blood donors at the same collection centre. They found the incidence of vasovagal reactions among female apheresis donors and female whole blood donors to be 1.25 and 4.17%, respectively. The rate among male donors was 0.83 and 0.99%, respectively.[10] Tomita et al. noted that the incidence of vasovagal reactions increased with age among apheresis donors, unlike what has been reported with whole blood donors.
In our study, vasovagal reactions (syncope/faintness/sweating) occur mainly in the form of sweating. There were relatively larger percentage of donors with features of sweating 03 in CS3000 and 02 in MCS+. This can possibly be attributed to apprehension due to mechanical and psychological factors, and was more in CS3000, possibly attributable to the noise generated by the machine, the increased sensations of conducted vibrations induced by the cruder centrifuge of the machine, and due to the fact that over a period of time, the donor got more stressed, since both his arms were being utilized for conducting the procedure.
Tomita et al. hypothesized that the higher incidence in women and the increasing frequency with age were related to a lower circulating blood volume in these donor groups with a resulting greater percentage of the donor's blood being within the extracorporeal circuit during collection. This resulted in a greater drop in blood pressure during collection leading to more vasovagal reactions. Tomita et al. also noted that the incidence of these reactions increased with increasing cycles during a collection. Based upon this, they theorized that hypocalcaemia may also be involved in the onset of vasovagal reactions in apheresis donors.[10]
In our study, no patient developed frank hypotension. However three donors on CS3000 and 02 donors on MCS+ had features of sweating and uneasiness. This would be multifactorial. It will be noted that severe hypotension during the process was not observed even while using MCS+, even though the fact that this was an intermittent flow process, which would in turn have resulted in increased extracorporeal blood volume. (The extracorporeal blood volume using MCS+ is approximately 360 ml and the extracorporeal blood volume using CS3000 is 200 to 250 ml).
Hypovolemic and vasovagal reactions are treated similarly. The procedure should be temporarily paused and a fluid infusion should be started. If the reaction is due to hypovolemia, the blood pressure should increase and the pulse rate should decrease in response to this intervention. If the reaction is due to a vasovagal reaction, this may not occur. Additional treatments for vasovagal reactions include placing the donor in trendelenburg position (head down below the level of the heart), applying cold compresses to the forehead and neck, and reassuring the donor.
The overall rate of acute adverse reactions, among healthy donors undergoing plateletpheresis procedures in our study was 2.72% (85/3,120) and among patients undergoing therapeutic apheresis procedures (PBSC and TPE) was 2.02% (05/247). This is more or less same (2.18%) as found by McLeod et al. in a multi-institutional study.[1] Frequency of VI, CR and PS/S in plateletpheresis was 1.6%, 1% and 0.09% respectively, in our study, which is almost equal in comparison to study done by McLeod et al. 1.15%, <1% and 0.39% respectively.[1,7] Frequency of PS/S was 0.83% in a study done by Tomita et al.,[10] which is more in comparison to our study (0.11%). Frequencies of these AEs studied by other authors are almost equal to the results seen in our study.[7]
Overall, apheresis donations performed on cell separators are safe, and have acute reaction rates less than those seen with whole blood donation, though the frequency of reactions requiring hospitalization appears to be greater. The acute effects of donation are relatively mild and easily treated. Recent evidence suggests, however, that repeated apheresis donation may produce adverse long-term effects in donors such as bone demineralization and cataract formation.[2] Additional research is needed to ascertain the risks of long-term apheresis donation.
Footnotes
Source of Support: No
Conflict of Interest: None declared.
References
- 1.Mcleod BC, Price TH, Owen H, Ciavarella D, Sniecinski I, Randels MJ, et al. Frequency of immediate adverse effects associated with apheresis donation. Transfusion. 1998;38:938–43. doi: 10.1046/j.1537-2995.1998.381098440858.x. [DOI] [PubMed] [Google Scholar]
- 2.Winters JL. Complications of donor apheresis. J Clin Apher. 2006;21:132–41. doi: 10.1002/jca.20039. [DOI] [PubMed] [Google Scholar]
- 3.Brecher ME, Leger RM. AABB technical manual. 15th ed. Bethesda: American Association of Blood Banks; 2005. [Google Scholar]
- 4.Simon TL, Dzik WH. Rossi's principles of transfusion medicine. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 5.Crookes RL, Hillyer CD. Blood banking & transfusion medicine. 2nd ed. Philadelphia: Churchill Livingstone; 2009. [Google Scholar]
- 6.Despotis GJ, Goodnough LT, Dynis M, Baorto D, Spitznagel E. Adverse events in platelet apheresis donors: A multivariate analysis in a hospital-based program. Vox Sang. 1999;77:24–32. doi: 10.1159/000031070. [DOI] [PubMed] [Google Scholar]
- 7.Klein HG, Anstee DJ. Mollison's blood transfusion in clinical medicine. 11th ed. Bristol (UK): Blackwell Publishing Ltd; 2005. [Google Scholar]
- 8.Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 9.Bell AM, Nolen JF, Knudson CM. Severe citrate toxicity complicating volunteer apheresis platelet donation. J Clin Apher. 2007;22:15–6. doi: 10.1002/jca.20107. [DOI] [PubMed] [Google Scholar]
- 10.Tomita T, Takayanagi M, Kiwada K, Mieda A, Takahashi C, Hata T. Vasovagal reactions in apheresis donors. Transfusion. 2002;42:1561–6. doi: 10.1046/j.1537-2995.2002.00241.x. [DOI] [PubMed] [Google Scholar]


