Abstract
Background:
Hepatitis G virus (HGV) is newly identified virus, transmitted by infected blood and blood products. Effect of HGV infection on liver diseases is not well known.
Aims:
Co-infection of HGV with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection has been reported however; very limited data is available from India. Therefore, we have performed a pilot study for the presence of co-infection of HGV in chronic liver disease patients.
Setting and Design:
The study was performed in research laboratory at P.D. Hinduja National hospital and Medical research center, Mahim, Mumbai. Prospective study was designed.
Methods and Materials:
Forty HBV, HCV related chronic liver disease patients were studied. Forty randomly selected voluntary healthy blood donors visiting our blood bank were included as controls. Serum bilirubin, alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were estimated. HGV infection was detected by using reverse transcriptase molony murine leukemia virus (M-MLV) with the help of HGV 340/625IC kit (Sacace, Italy).
Results and Conclusion:
One HCV positive patient had infection with HGV among 40 HBV/HCV chronic liver disease patients.
Keywords: Hepatitis B virus, hepatitis C virus, hepatitis G virus, reverse transcription PCR
Introduction
Hepatitis G virus (HGV) is a newly identified RNA virus belonging to flaviviridae family.[1] It can be transmitted efficiently by blood transfusion, sexual route and other parenteral mechanism.[2] Available reports suggest that HGV is more prevalent in high risk groups such as hemodialysis patients, hemophiliacs and intravenous drug abusers.[2] Infection of HGV has also been detected in chronically infected patients with other hepatotrophic viruses such as HBV or HCV. Its prevalence ranges from 3.2-21% in HBV/ HCV positive chronic liver disease patients all over the world.[3–5] The virus has a global distribution and is reported to be present within 0%-4.95% in healthy blood donors.[6–8] The pathogenic implication of HGV infection in liver disease is controversial.[9] In India, very little data is available on prevalence of HGV in hepatitis patients.[10,11] We undertook a pilot study to detect the presence of HGV viremia among patients with HBV or HCV positive chronic liver disease and in healthy blood donors. As routine blood donors screening procedure does not include testing for HGV, it will be worthwhile to detect its presence in healthy blood donors as it might help in reducing the possible risk of transmission of HGV in blood units.
Materials and Methods
Sample population: During the year 2008-2010, forty consecutive HBV/HCV patients with chronic liver disease and forty randomly selected healthy blood donors (controls) were studied. Patients with HBV/HCV chronic liver disease were selected on the basis of positive HBsAg/anti HCV serological test. Blood donors (age range 17-59 years) referring to Blood Transfusion Centre of P.D. Hinduja National Hospital and MRC were selected as per routine blood donor screening criteria. None of the donors had a past history of transfusion, jaundice and viral hepatitis. Donor's positive for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis and malaria were excluded from the control group. The study has been approved by institutional review board (IRB) and ethics committee (EC).
Biochemical testing: Serum levels of total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) were estimated in patients and controls using SYNCHRON CX® System(s).
Molecular Methods: HGV RNA was assayed by reverse transcription PCR (RT PCR) (using HGV340/625 IC kit, Sacace, Italy). RNA was extracted from plasma samples using “Ribo-Sorb Extraction kit (‘Sacace, Italy)” as described in the manufacturer's instructions. The extracted RNA was subjected to reverse transcription (by “Reveta R kit”, Sacace, Italy), in which cDNA synthesis was carried out at 37°C for 30 minutes using deoxyoligonucleotide primer and M-MLV reverse transcriptase enzyme (provided by the kit) using a thermal cycler (MJ thermal cycler). This was followed by amplification conditions (as per ‘HGV 340/625IC kit’, Sacace, Italy): initial denaturation at 95°C for 5 minutes; 42 cycles each of 95°C for 10 seconds, 67°C for 10 seconds, 72°C for 10 seconds and final extension at 72°C for 1 min (MJ thermal cycler). Negative control of amplification and positive control of amplification were maintained as provided by the kit. Internal control which serves as amplification control was used to amplify 625 bp regions in order to identify possible reaction inhibition. DNA amplicons were analyzed by 2% agarose gel electrophoresis and visualized by ultraviolet fluorescence. Presence of 340 base pair band was considered positive for HGV RNA. The sensitivity of this assay was 100 viral copies/ml and specificity was 100%. To prevent cross contamination, RNA extraction, preparation of reaction mixture, PCR amplification and handling of PCR product were done in separate rooms. Furthermore, all protocols were performed in laminar hood irradiated with UV light before use. HGV 340/625IC kit (Sacace, Italy) has been validated using known positive serum sample of HGV infected person having viral load of 107 copies/ml. This sample was gifted by National Institute of Health; Bethesda (U.S.) The sample was run along with the positive and negative controls of the kit.
Result
Among forty HBV/HCV positive patients group, one HCV related cirrhosis patient was found to be co-infected with HGV [Figure 1]. This was confirmed by repeat testing. Serum levels of liver function tests of this patient were towards higher side of normal as follows: serum bilirubin (2.30 mg/dl, NR: 0.20-1.0), AST (103 mg/ dl, NR: 15.0-48.0), ALT (43 mg/dl, 10.0-40.0) and ALP (124 mg/dl, 40.0- 120.0). None of the healthy blood donors were positive for the presence of HGV RNA.
Figure 1.
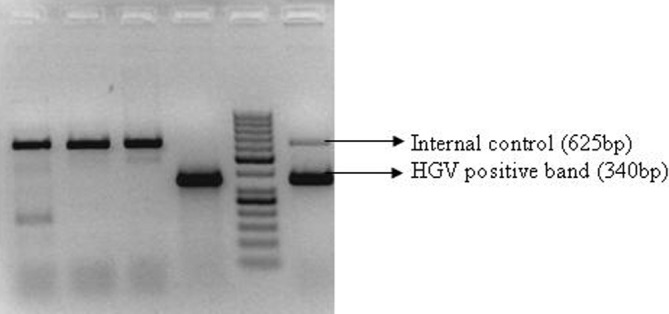
Gel Picture: HGV-HCV co-infection Lane 1 to lane 3: samples no 27 to 29 Lane 4: positive control of amplification Lane 5: 50 bp DNA ladder Lane 6: sample no. 30 (HCV related chronic liver disease)
Discussion
In the present study, HGV co-infection was detected in only one HCV chronic liver disease patient. However, prevalence of HGV in HBV/HCV positive patients was reported to be higher in other parts of the world as tabulated below [Tables 1 and 2].
Table 1.
Prevalence of HGV in HCV chronic liver disease patients
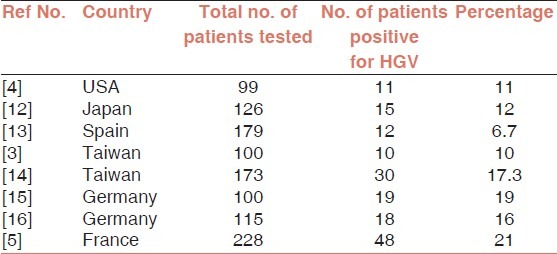
Table 2.
Prevalence of HGV in HBV chronic liver disease patients
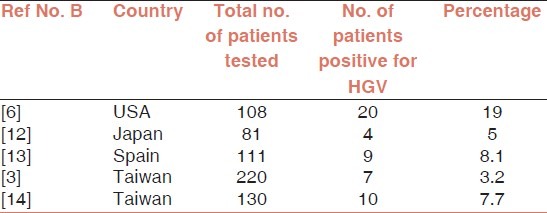
Based on the literature reviewed, HGV/HCV co-infection was found to be more prevalent than HGV/HBV co-infection.
Indian study by Jain, et al. reported HGV prevalence of 12% (6/50) in virus related cirrhosis patients. In another study from India, HGV infection was tested in high risk groups.[10] It was reported that, HGV RNA was detected in 17.7% (16/90) multi-transfused patients and 23% (15/65) intravenous drug abusers. Of these, 93.5% HGV positive patients were found to be co-infected with either HBV (38.7%) or HCV (74.1%). Thus the dual infection was reported in 15.5% of multi-transfused patients and 10.7% of intravenous drug abusers. HGV was therefore found to be co-infected more frequently with HCV than with HBV even in India.[11] However, very little data is available on this issue.
Similarly, in the present study, HGV positive infection was found to be co-infected with a HCV positive chronic liver disease patient.
In healthy blood donors, the prevalence of HGV ranges from 0.9% to 4.95% all over the world.[6–8]
In an Indian study by Jain, et al., 4% (2/50) prevalence of HGV was reported among healthy blood donors.[10] Another Indian study by Thakur, et al., have reported the HGV prevalence of 0.9% (2/221) among healthy blood donors. Of these two donors, one had dual infection of HGV and HBV.[17] Praharaj, et al. reported 2.6% (13/500) HGV prevalence in voluntary blood donors in armed forces. Among these 13 HGV positive blood donors, 5 were co-infected with HBV and 2 with HCV.[18]
According to the reported data, the prevalence of HGV in healthy blood donors’ population was lower than HBV/HCV positive liver disease patients. In our study, all the controls (healthy blood donors) were negative for HGV infection. Currently, there is no serological assay available for the routine detection of HGV in blood donors. Screening of donors for HGV by PCR method does not seem to be beneficial in terms of time and cost. Therefore, it can be said that screening of blood donors will be justified only if, in future, more data on prevalence of HGV in healthy blood donors will become available along with the significance of the virus with specific disease.
In the present pilot study, we have detected one HCV-HGV positive co-infection in 40 HBV/HCV chronic liver disease patients. However, clinical correlation of HGV with liver disease has been reported to be insignificant. Our sample size was too small to determine the prevalence of HGV; hence, large scale studies and follow-up are needed to understand the occurrence of HGV in HBV/HCV patients and its clinical implications.
Acknowledgment
We are extremely grateful to Dr. Robert Purcell, Dr. Kyle Dora and Dr. Alicia Brockington Laboratory of Infectious Diseases 50 South Drive; Bldg. 50, Rm. 6523 National Institute of Allergy and Infectious Diseases National Institutes of Health Bethesda, USA for providing us serum sample of hepatitis G virus positive patient.
Footnotes
Source of Support: National Health and Education Society, P.D. Hinduja National Hospital and MRC.
Conflict of Interest: No.
References
- 1.Linnen J, Wages J, Jr, Zhang-Keck ZY, Fry KE, Krawczynski KZ, Alter H, et al. Molecular cloning and disease association of hepatitis G virus: A transfusion-transmissible agent. Science. 1996;271:505–8. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 2.Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725–34. doi: 10.3748/wjg.14.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao JH, Chen PJ, Lai MY, Chen W, Liu DP, Wang JT, et al. GB virus-C/hepatitis G virus infection in an area endemic for viral hepatitis, chronic liver disease, and liver cancer. Gastroenterology. 1997;112:1265–70. doi: 10.1016/s0016-5085(97)70139-2. [DOI] [PubMed] [Google Scholar]
- 4.Marrone A, Shih JW, Nakatsuji Y, Alter HJ, Lau D, Vergalla J, et al. Serum hepatitis G virus RNA in patients with chronic viral hepatitis. Am J Gastroenterol. 1997;92:1992–6. [PubMed] [Google Scholar]
- 5.Martinot M, Marcellin P, Boyer N, Detmer J, Pouteau M, Castelnau C, et al. Influence of hepatitis G virus infection on the severity of liver disease and response to interferon-alpha in patients with chronic hepatitis C. Ann Intern Med. 1997;126:874–81. doi: 10.7326/0003-4819-126-11-199706010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Masuko K, Mitsui T, Iwano K, Yamazaki C, Okuda K, Meguro T, et al. Infection with hepatitis GB virus C in patients on maintenance hemodialysis. N Engl J Med. 1996;334:1485–90. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- 7.Schlueter V, Schmolke S, Stark K, Hess G, Ofenloch-Haehnle B, Engel AM. Reverse transcription-PCR detection of hepatitis G virus. J Clin Microbiol. 1996;34:2660–4. doi: 10.1128/jcm.34.11.2660-2664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson GJ, Schlauder GG, Pilot-Matias TJ, Thiele D, Leary TP, Murphy P, et al. Prevalence studies of GB virus-C infection using reverse transcriptase-polymerase chain reaction. J Med Virol. 1996;50:97–103. doi: 10.1002/(SICI)1096-9071(199609)50:1<97::AID-JMV16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Alter HJ, Nakatsuji Y, Melpolder J, Wages J, Wesley R, Shih JW, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–54. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Kar P, Gopalkrishna V, Gangwal P, Katiyar S, Das BC. Hepatitis G virus (HGV) infection and its pathogenic significance in patients of cirrhosis. Indian J Med Res. 1999;110:37–42. [PubMed] [Google Scholar]
- 11.Asim M, Potukuchi SK, Arora A, Singh B, Kar P. Hepatitis-G virus infection in multi-transfused patients and intravenous drug abusers: New Delhi experience. Dig Dis Sci. 2008;53:1383–9. doi: 10.1007/s10620-007-0004-1. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuji Y, Shih JW, Tanaka E, Kiyosawa K, Wages J, Jr, Kim JP, et al. Prevalence and disease association of hepatitis G virus infection in Japan. J Viral Hepat. 1996;3:307–16. doi: 10.1111/j.1365-2893.1996.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 13.Guilera M, Sáiz JC, López-Labrador FX, Olmedo E, Ampurdanés S, Forns X, et al. Hepatitis G virus infection in chronic liver disease. Gut. 1998;42:107–111. doi: 10.1136/gut.42.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JF, Dai CY, Chuang WL, Lin WY, Lin ZY, Chen SC, et al. Prevalence and clinical significance of HGV/GBV-C infection in patients with chronic hepatitis B or C. Jpn J Infect Dis. 2006;59:25–30. [PubMed] [Google Scholar]
- 15.Schleicher S, Chaves RL, Dehmer T, Gregor M, Hess G, Flehmig B. Identification of GBV-C hepatitis G RNA in chronic hepatitis C patients. J Med Virol. 1996;50:71–4. doi: 10.1002/(SICI)1096-9071(199609)50:1<71::AID-JMV12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Berg T, Dirla U, Naumann U, Heuft HG, Kuther S, Lobeck H, et al. Responsiveness to interferon alpha treatment in patients with chronic hepatitis C co-infected with hepatitis G virus. J Hepatol. 1996;25:763–8. doi: 10.1016/s0168-8278(96)80250-9. [DOI] [PubMed] [Google Scholar]
- 17.Thakur V, Guptan RC, Sarin SK. Prevalence of hepatitis GB virus C/hepatitis G virus infection in blood donors in India. J Assoc Physicians India. 2000;48:818–9. [PubMed] [Google Scholar]
- 18.Praharaj AK, Tripathy S, Kalghatgi AK, Nagendra AB. Hepatitis G virus: Prevalence in blood donors in armed forces. Med J Armed Forces India. 2005;61:333–5. doi: 10.1016/S0377-1237(05)80057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


