Abstract
Background:
Although, in the past the risk of transfusion transmitted viral infections were high in hemophilia patients, but introduction of viral inactivation methods in1985,decreased the risk of human immunodeficiency and hepatitis C and B viruses transmission significantly. The aim of study was seroprevalence of hepatitis B surface antigen (HBs Ag), hepatitis C virus antibody (HCV Ab) and human immunodeficiency virus antibody (HIVAb) in hemophiliacs in west Azarbaijan of Iran, born in 1985-2010.
Materials and Methods:
In a cross-sectional study, fifty patients with hereditary bleeding disorders born in 1985-2010, from total 250 patients who had been registered in Urmia Hemophilia Society were enrolled through the year 2010 to assess their seroprevalence for HCV Ab, HIV Ab and HBs Ag. Thirty five of 50 patients had hemophilia. Also; we performed a subset analysis for hemophilia patients.
Results:
All 50 patients with hereditary bleeding disorders including 35 patients with hemophilia were seronegative for HIV Ab and HBs Ag. HCV-Ab was detected in serum of 3 of 50 (6%) patients with bleeding disorders. After subset analysis for hemophilia (A and B) patients, we found HCV infection in 8.57% (3 of 35) of hemophiliacs.
Conclusion:
In this study prevalence of HCV infection was very smaller than similar studies in Iran and other countries. This study shows the safety of using viral inactivated factor concentrates and recombinant factors after year 1985. None of Hemophiliacs were seropositive for HIV Ab and HBs Ag.
Keywords: Hepatitis B surface antigen, hepatitis C virus antibody, hemophilia, human immunodeficiency virus antibody, west Azarbaijan
Introduction
In the past hemophilia replacement therapies were included fresh frozen plasma (FFP), cryoprecipitate and blood derived products without any viral inactivation. In 1950, plasma became available for treating hemophilia. In1965, cryoprecipitate was used as a treatment for hemophilia. FIX and FVIII concentrates used for hemophilia patients in 1968. FIX and FVIII genes were cloned in 1982. Viral inactivated factor concentrates became available in 1985 and recombinant product became available in 1992.[1]
Before year 1985, using human's plasma derived factor concentrates which did not undergo viral inactivation increased the risk of transfusion transmitted viral infections in hemophiliacs.[2–6]
Introduction of viral inactivation methods in 1985 decreased the risk of human immunodeficiency and hepatitis C and B viruses’ transmission significantly.
In this study we aimed to assess seroprevalence of HBs Ag, HCV Ab and HIV Ab in patients with hereditary bleeding tendency born 1985-2010 in west Azarbaijan of Iran.
Materials and Methods
In a cross-sectional study fifty patients with hereditary bleeding disorders born in 1985-2010, from the total, 250 patients had been registered in Urmia Hemophilia Society were enrolled through the year 2010 to assess their seroprevalence for HCV Ab, HIVAb and HBs Ag.
Questionnaires including age, sex, presence of possible exclusion criteria (Hetero or homosexuality, IV drug abuse, high risk jobs e.g. health center staff, jailor and history of seropositivity for HCVAb, HBs Ag and HIV Ab in their first degree relative), type of bleeding disorder and severity of it, type of blood product administration (FFP, cryoprecipitate and blood derived and/or recombinant factors) are arranged. These questionnaires were filled out by patients’ physician or an educated nurse during communication with patients and/or their parents.
They were tested for HBs Ag, human immuno-deficiency virus (HIV) Ab and hepatitis C virus antibody with enzyme linked immunosorbant assay (ELISA) and positive cases for HCV Ab would be confirmed with recombinant immunoblot assay (RIBA) and HCV PCR. All analysis was performed by SPSS (version 17) soft ware. Thirty five of 50 patients had hemophilia. We performed a subset analysis for hemophilia patients.
Ethical review
This study is approved in ethical committee of Urmia University of Medical Sciences. Informed consent was completed and signed by all patients, implying their determent right in any part of the study. All information received from patients and their laboratory results are stored confidentially and with a code given to each participant.
Results
Fifty patients with hereditary bleeding disorders, who were born since 1985 from total 250 patients who were registered at Urmia Hemophilia Society were enrolled in the study including 43 (86%) male, and 7 (14%) female. The mean age of patients was 10.3 years (ranges 3 to 25 years). None of them had high risk behaviors (Hetero or homosexuality, IV drug abuse, high risk jobs e.g. health center staff, jailor, etc) and history of seropositivity for HCV, HBs Ag and HIV Ab in their spouse and/or first degree relative.
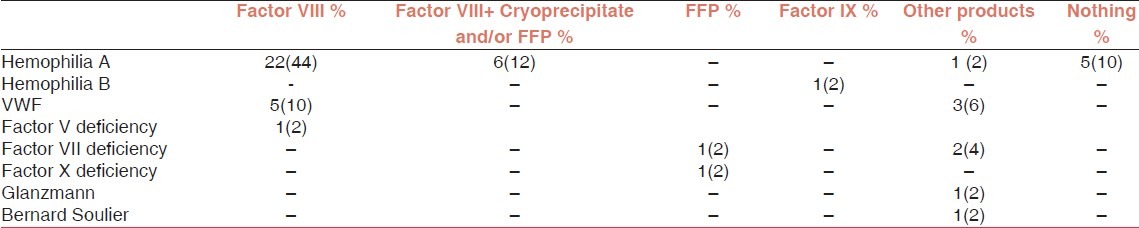
Among our studied population, 34(68%) had hemophilia A, 1 (2%) hemophilia B, 8 (16%) VWF, 3(6%) factor VII deficiency, 1 (2%) factor V deficiency, 1 (2%) factor X deficiency, 1 (2%) Glanzmann thrombasthenia and 1 (2%) had Bernard Soulier syndrome. Duration of receiving coagulation factors until 2010 was less than 10 years in 41 patients (82%) and more than 10 years in 9(18%).
28 patients (56%) had been received only factor VIII, 6 (12%) both factor VIII and cryoprecipitate or FFP, 2(4%) only fresh frozen plasma (FFP), 1(2%) only factor IX and 8(16%) other blood concentrates [including platelet 2(4%), Factor VIIa 4(8%) and Humate P 2(4%)] [Table 1].
Table 1.
We analyzed data in 50 patients with hereditary bleeding disorder who born 1985-2010
HCV-Ab was detected in serums of 3 of 50(6%) patients with bleeding disorders [Table 2]. These 3 patients with HCV infection had severe hemophilia A (Factor VIII level <1%) and had been received not only factor VIII but also cryoprecipitate and/or FFP. All of 3 patients with positive HCV Ab had documented hepatitis C and 2 of them had been received interferon therapy for hepatitis C.
Table 2.
Data analysis of 50 patients, with bleeding disorders
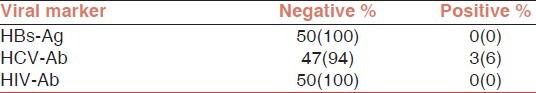
All 50 with bleeding disorders patients were seronegative for HBs Ag and HIV Ab. In a subset analysis we assessed seroprevalence of HBs Ag, HCV Ab and HIV Ab only for patients with only hemophilia A and B.
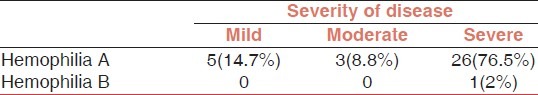
Hemophilia patients are divided in three groups according to factor VIII or IX level [Table 3]: Mild hemophilia (factor level 5-25%), moderate hemophilia (factor level: 1-5%) and severe hemophilia (factor level<1%).
Table 3.
Severity of hemophilia
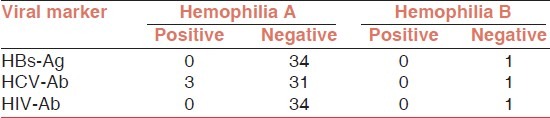
After subset analysis, we found HCV infection in 8.57% (3 of 35) of hemophilia A and B patients [Table 4], all of them had severe hemophilia A and had history of cryoprecipitate and/ or FFP transfusion in addition of factor VIII concentrates injection.
Table 4.
Data analysis in hemophilia A and B patients
Among 35 patients with hemophilia A and B only 30 patients had been received factor derivates and/or other blood products [Table 5].
Table 5.
Hemophilia patients according to blood products and factor concentrate administration

3/30 (10%) of 30 hemophiliacs who had received factor concentrates and/or other blood products had HCV infection, but none of them were seropositive for HIV Ab and/or HBS Ag.
Discussion
In the past hemophilia replacement therapies included blood products without any viral inactivation. Viral inactivated factor concentrates become available in 1985 and recombinant product becomes available in 1992.[1] Administration of human's plasma derived factor concentrates without viral inactivation before 1985 increased the risk of transfusion transmitted viral infections in hemophiliacs.[2–6]
Nassiri Toosi, et al., conducted a study in Iranian hemophilia patients through the year 2003 in Tehran University and concluded that 4.7% (10/211) patients tested were HIV seropositive and 83.3% were HCV seropositive.[3]
Vinelli, et al., conducted a multi center cross-sectional study through 2002-2003 on multi-transfused patients. Five hundred and two patients were enrolled; 11% were HBsAg positive; 27% for anti-HBc; 7% for HCV Ab and 1% for HIV Ab. In this study 1.6% of hemophilia patients were positive for HBsAg; 38% were positive for anti-HBc and 26.9% were seropositive for HCV Ab.[4]
Alavian, et al. from Iran in a cross sectional study assessed 176 hemophiliacs through 2010-2011. Out of them 106 patients (60.2%) were anti-HCV Ab positive and four patients were anti- HIV Ab positive.[5]
In another study conducted by Borhany, et al., on 173 multi-transfused male hemophiliacs 51.4% were seropositive for HCV, 1.73% for HBV and none of them for HIV Ab.[6]
Windyga, et al. studied transfusion transmitted viral infections in hemophilia patients in 2 age groups (1935-90, 1991-2002) according to the type of replacement therapy. 95% of 172 patients born in 1935-90 were seropositive for HCV Ab and in 77.3% patients, HCV RNA was detected. HBs Ag was positive in 8.7% of them. Only one of them was HIV positive. Viral markers (HCV Ab and HCV RNA) were detected in only one (2.4%) of 41 patients who was born 1991-2002.[7]
Chow, et al., in 1991 studied on 11 severe hemophiliacs who had received a long period factor VIII product. The rate of seropositivity for HIV and HCV were 82% (9/11) and 100% (11/11). The seropositivity rate of hepatitis B surface antigen was only 9% (1/11).[8]
Prevalence of HCV seropositivity in hemophilia patients treated before 1985 was high (ranging 92-100%).[8–10] In another series prevalence of HCV was from 43.9% to 84%.[7,3,11–15]
Because of risk of transfusion transmitted viral infections in hemophiliacs, another choices such as recombinant clotting factor took place. Safety and efficacy of recombinant factors was showed in a study conducted by Lusher, et al. on 93 hemophiliacs treated with recombinant factors.[16]
Zhubi, et al., assessed 75 patients with hemophilia A or B. They showed that HCV infection was positive in 38.7% (29 cases), while infection with HBV and HIV were 2.7% and 1.4%. HCV infections in cases of age under 18 years was only 9.5%.[17] Morfini, et al. from Italy studied on 708 hemophiliacs patients, infused for the first time before 1985 (group A) and 80 patients, infused for the first time between 1985 and 1991 (group B). The prevalence of anti-HCV was 83% (591/708) in group A and 6% (5/80) in group B.[18]
We studied 50 patients with hereditary bleeding disorders born in 1985-2010 for HCV Ab, HBs Ag and HIV Ab seropositivity. HCV infection was found in 6% (3/50) of them. None of them had positive serology for HIV Ab and HBs Ag. After subset analysis for only hemophilia A and B, we found HCV infection in 8.57% (3 of 35) of hemophiliacs. Although, 30 of 35 patients with hemophilia A and B had history of taking factor and/or other blood products but only 3/30 (10%) had been infected with HCV and none of them were seropositive for HIV and HBs Ag. In our study prevalence of HCV infection was very smaller than similar studies in Iran and other countries, which shows the safety of using viral inactivated factors and recombinant factors after year 1985.
Conclusion
Three of 50 cases (6%) with hereditary bleeding disorders, had HCV infection. All of 3 patients had severe hemophilia A and had history of not only factor VIII concentrates but also cryoprecipitate and/or FFP injection. All 50 patients were seronegative for HIV Ab and HBs Ag. We analyzed data in 35 patients with Hemophilia (34 hemophilia A and 1 hemophilia B) and we found HCV infection in 8.57% (3/35) of hemophiliacs. None of 35 hemophiliacs were seropositive for HIV Ab and HBs Ag. 30 of 35 hemophiliacs had received factor concentrates and/or blood products and only 3/30(10%) had been infected with HCV but none of them with HBV and/or HIV. In this study prevalence of HCV infection was very smaller than similar studies in Iran and other countries, which show the safety of using viral inactivated factor concentrates and recombinant factors after year 1985. None of Hemophiliacs were seropositive for HIV Ab and HBs Ag.
Acknowledgment
We want to thank Urmia Hemophilia society and Urmia University of Medical Sciences who helped us in this study.
Footnotes
Source of Support: Urmia University of Medical Sciences
Conflict of Interest: None declared.
References
- 1.Hough C, Lillicrap D. Gene therapy for hemophilia: An imperative to succeed. J Thromb Haemost. 2005;3:1195–205. doi: 10.1111/j.1538-7836.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- 2.Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort haemophilic patients between 1961 and 1985. Gut. 2000;47:845–51. doi: 10.1136/gut.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NassiriToosi M, Lak M, Karimi K, Managhchi M, Samimi-Rad K, Abdollahi A, et al. Seroprevalence of human immunodeficiency virus (HIV) and hepatitis C infection in hemophilic patients in Iran. Iran J Pathol. 2008;3:119–24. [Google Scholar]
- 4.Vinelli E, Lorenzana I. Transfusion-transmitted infections in multi-transfused patients in Honduras. J Clin Virol. 2005;34(Suppl 2):S53–60. doi: 10.1016/s1386-6532(05)80035-6. [DOI] [PubMed] [Google Scholar]
- 5.Alavian SM, Ardeshiri A, Hajarizadeh B. Prevalence of HCV, HBV, HIV infections among hemophiliacs. Hakim. 2003;6:45–51. [Google Scholar]
- 6.Borhany M, Shamsi T, Boota S, Ali H, Tahir N, Naz A, et al. Transfusion transmitted infections in patients with hemophilia of Karachi, Pakistan. Clin Appl Thromb Hemost. 2011;17:651–5. doi: 10.1177/1076029611398122. [DOI] [PubMed] [Google Scholar]
- 7.Windyga J, Grabarczyk P, Stefańska E, Buczma A, Szczepanik AB, Klukowska A, et al. Prevalence of HCV, HBV and HIV infections among severe Polish haemophiliacs. Przegl Epidemiol. 2008;62:415–23. [PubMed] [Google Scholar]
- 8.Chow MP, Lin CK, Lin JS, Chau WK, Ho CH, Chen SY, et al. HIV, HBV and HCV seropositivity in hemophiliacs. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1991;24:339–44. [PubMed] [Google Scholar]
- 9.Laursen AL, Scheibel E, Ingerslev J, Clausen NC, Wantzin P, Ostergaard L, et al. Alpha interferon therapy in Danish haemophiliac patients with chronic hepatitis C: Results of a randomized controlled open label study comparing two different maintenance regimens following standard interferon-alpha-2b treatment. Haemophilia. 1998;4:25–32. doi: 10.1046/j.1365-2516.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 10.Foster P, Mcintosh RV, Macleod AJ. Hepatitis C and haemophilia. BMJ. 1995;311:754–5. doi: 10.1136/bmj.311.7007.754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brettler DB, Alter HJ, Dienstag JL, Forsberg AD, Levine PH. Prevalence of hepatitis C virus antibody in a cohort of hemophilia patients. Blood. 1990;76:254–6. [PubMed] [Google Scholar]
- 12.Yamada-Osaki M, Sumazaki R, Kajaiwara Y, Miyakawa T, Shirahata A, Matsui A. Naturalcourse of HGV infection in haemophiliacs. Br J Haematol. 1988;102:616–21. doi: 10.1046/j.1365-2141.1998.00793.x. [DOI] [PubMed] [Google Scholar]
- 13.Bauduer F, Degioanni A, Ducout L, Scribans C, Dutour O. Distribution of haemophilia in the French Basque Country. Haemophilia. 2002;8:735–9. doi: 10.1046/j.1365-2516.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- 14.Makris M, Preston FE, Triger DR, Underwood JC, Choo QL, Kuo G, et al. Hepatitis C antibody and chronic liver disease in haemophilia. Lancet. 1990;335:1117–9. doi: 10.1016/0140-6736(90)91124-s. [DOI] [PubMed] [Google Scholar]
- 15.Maisonneuve P, Laurian Y, Guerois C, Verroust F, Ferrer Le Coeur F, Couroucé AM, et al. Antibody to hepatitis C (anti C 100-3) in French hemophiliacs. Nouv Rev Fr Hematol. 1991;33:263–6. [PubMed] [Google Scholar]
- 16.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with Hemophilia A.Safety, efficacy and development of inhibitors. N Engl J Med. 1993;328:453–9. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 17.Zhubi B, Mekaj Y, Baruti Z, Bunjaku I, Belegu M. Transfusion transmitted Infections in Haemophilia patients. Bosn J Basic Med Sci. 2009;9:271–7. doi: 10.17305/bjbms.2009.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morfini M, Mannucci PM, Ciavarella N, Schiavoni M, Gringeri A, Rafanelli D, et al. Prevalence of infection with the hepatitis C virus among Italian hemophiliacs before and after the introduction of virally inactivated clotting factor concentrates: A retrospective evaluation. Vox Sang. 1994;67:178–82. doi: 10.1111/j.1423-0410.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]


