Abstract
A centralized hemovigilance program to assure patient safety and to promote public health has been launched for the first time in India on Dec 10, 2012 in 60 medical colleges in the first phase along with a well-structured program for monitoring adverse reactions associated with blood transfusion and blood product administration. National Institute of Biologicals (NIB) will be the National Coordinating Centre for Hemovigilance. This program will be implemented under overall ambit of Pharmacovigilance Program of India (PvPI), which is being coordinated by Indian Pharmacopoeia Commission (IPC). All medical colleges of the country will be enrolled in this program by the year 2016 in order to have a National Centre of Excellence for Hemovigilance at NIB, which will act as a global knowledge platform.
Keywords: Hemovigilance, India, Medical colleges, Transfusion Reaction Reporting Form
Introduction
Hemovigilance was first introduced in France in 1993 with mandatory reporting and in United Kingdom (UK) with first voluntary reporting system in 1996. Most of the developed countries like Canada, Ireland, Netherlands, and Denmark have a voluntary reporting requirement. Hemovigilance program in these countries is linked to International Hemovigilance Network, which presently has 28 members.
Hemovigilance systems, depending upon the country, are governed either by regulators (e.g., France, Germany, Switzerland), blood manufacturers (e.g., Japan, Singapore, South Africa), medical societies (e.g., Netherlands, UK), or public health authorities including regulators (e.g., Canada).[1]
Member states of the European Union have to implement hemovigilance program with reporting to a Central Office as per the commission directive.[2–4] Among the Asian countries, a well-established hemovigilance system is lacking and there is paucity of data on hemovigilance data except for Japan, which has published a report on adverse reactions.[5] Hemovigilance system is required in the country to have a comprehensive approach to address the issues of adverse reaction following blood transfusion and blood product administration.
Hemovigilance program of India
A Hemovigilance program as an integral part of pharmacovigilance program of India at a national level has been launched on December 10, 2012 with a road map of 5 years, i.e., year 2012–17, with four phases, i.e., initiation phase, expansion and consolidation phase, expansion and maintenance phase, and optimization phase. A core group to coordinate the activities of hemovigilance between the medical colleges and National Coordinating Centre at IPC has been constituted. Furthermore, an advisory committee has also been constituted to a) finalize hemovigilance—Transfusion Reaction Reporting Form (TRRF) to be introduced in the country, b) give expert opinion for collection, collation, and analysis of hemovigilance data and development of the software for the same, c) monitor the functioning and quality of the data collected by the Adverse Transfusion Reaction Reporting Centres, i.e., ADR Monitoring Centres of PvPI, d) develop training modules and guidelines for implementation of hemovigilance program under PvPI, and e) develop a roadmap for linking hemovigilance program under PvPI with International Haemovigilance Network.
Initially, 60 medical colleges that are already enrolled under pharmacovigilance program of India have been brought under the ambit of this program. This number will be increased to a total of 90 medical colleges by March 2013. Hemovigilance program has been launched with the following objectives:
Monitor transfusion reactions
Create awareness among health care professionals
Generate evidence-based recommendations
Advise Central drugs standard control organization (CDSCO) for safety related regulatory decisions
Communicate findings to all key stakeholders
Createnationaland international linkages
The Medical Colleges enrolled under hemovigilance program will collect data in respect of adverse reactions associated with blood transfusion and blood product administration in TRRF from their respective Department of Transfusion Medicine or the blood bank. The information collected in TRRF will be forwarded to the coordinating Centre NIB through a software developed in-house by NIB Information technology division. This data will be collated and analyzed to identify trends and recommend best practices and interventions required to improve patient care and safety. These recommendations will be forwarded to national coordinating Centre IPC, PvPI for onward transmission to Drugs Controller General (India), Central Drugs Standard Control Organization. These recommendations will be used to formulate safety related regulatory decisions on blood and blood products transfusion that will be communicated to various stake holders. The flow of information at various stages from hemovigilance centre located in medical colleges to stake holders is depicted in Figure 1.
Figure 1.
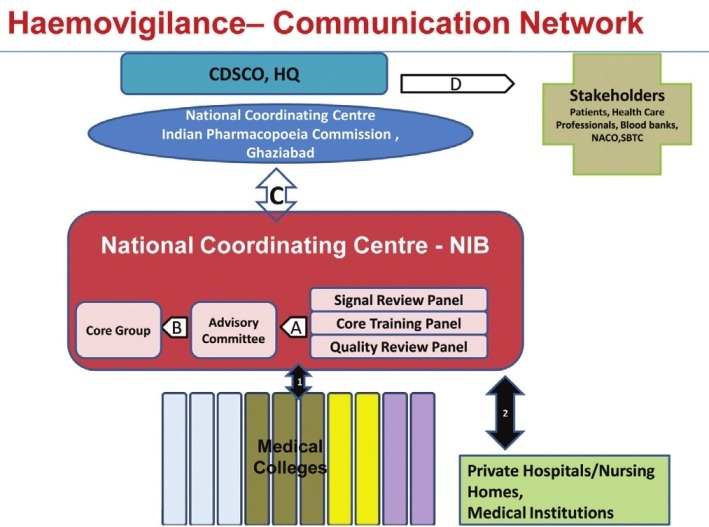
Communication Network–Hemovigilance program in India
Conclusion
Hemovigilance program is an integral part of pharmacovigilance program of India and is a comprehensive, centralized, and a well-structured approach to collect, collate, and analyze data to address the issues of adverse reactions associated with blood transfusion and blood product administration by generating evidence-based recommendations for transfusion safety and preventive measures.
Footnotes
Source of Support: Nil
Conflicting Interest: None.
References
- 1.Public Health Service (PHS) Biovigilance Working Group. Department of Health and Human Services Washington, DC20201. Biovigilance in the United States: Efforts to bridge a critical gap in patient safety and donor health. 2009:16. [Google Scholar]
- 2.Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards traceability requirements and notification of serious adverse reactions and events. Official Journal of the European Union [Google Scholar]
- 3.Faber JC. Haemovigilance procedure in transfusion medicine. Haematol J. 2004;5:S74–82. doi: 10.1038/sj.thj.6200427. [DOI] [PubMed] [Google Scholar]
- 4.Faber JC. The European blood directive: A new era of blood regulation has begun. Transfus Med. 2004;14:257–73. doi: 10.1111/j.0958-7578.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 5.Jain A, Kaur R. Hemovigilance and blood safety. Asian J Transfus Sci. 2012;6:137–8. doi: 10.4103/0973-6247.98911. [DOI] [PMC free article] [PubMed] [Google Scholar]


