Vol. 198 No. 1, July 9, 2012. Pages 103–114.
The actin panel in the original version of Fig. 5 B was flipped horizontally, and the Fig. 5 B and Fig. 6 C legends did not indicate the intentional duplication of the 3 and 4 d actin data. In addition, the HIF-2α panel in the original version of Fig. 7 C was a duplicate of the HIF-2α panel in Fig. 6 F. The authors have indicated that these issues were due to clerical errors during figure and manuscript preparation. Corrected versions of Fig. 5 B and Fig. 7 C and of the Fig. 5 B and Fig. 6 C legends are shown below.
Figure 5.
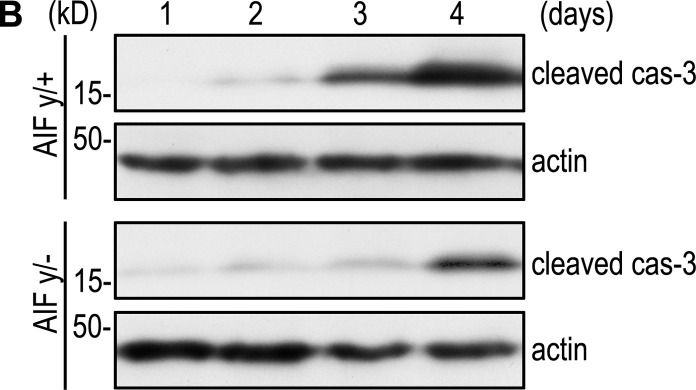
(B) EBs were cultured for 1–4 d and analyzed by immunoblotting for cleaved caspase-3 (cas-3) and actin. Ablation of AIF inhibited caspase-3 activation. The 3 and 4 d actin data are the same as those in Fig. 6 C, where the same blots were analyzed for Bnip3 levels.
Figure 6.
(C) AIFy/+ and AIFy/− EBs were cultured for 3–5 d and analyzed for Bnip3 by immunoblotting. Bnip3 expression was significantly reduced in the absence of AIF. The 3 and 4 d actin data are the same as those in Fig. 5 B, where the same blots were analyzed for cleaved cas-3 levels.
Figure 7.
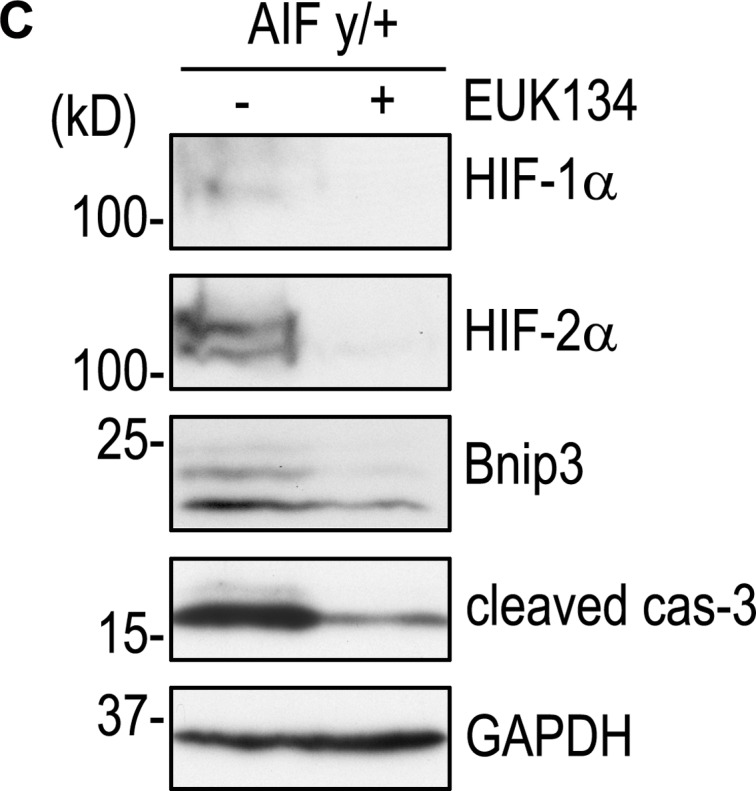
(C) 2-d AIFy/+ EBs were treated with or without 2 µM EUK134 for 24 h. Immunoblots show that EUK134 treatment reduced the expression of HIF-1α, HIF-2α, Bnip3, and cleaved caspase-3.
The html and pdf versions of this article have been corrected. The errors remain only in the print version.


