A human Aurora A kinase engineered to be specifically inhibited by the ATP analog 1-Na-PP1 allows dissection of a novel role for this protein in central spindle assembly.
Abstract
Knowledge of Aurora A kinase functions is limited to premetaphase events, particularly centrosome maturation, G2/M transition, and mitotic spindle assembly. The involvement of Aurora A in events after metaphase has only been suggested because appropriate experiments are technically difficult. We report here the design of the first human Aurora A kinase (as-AurA) engineered by chemical genetics techniques. This kinase is fully functional biochemically and in cells, and is rapidly and specifically inhibited by the ATP analogue 1-Naphthyl-PP1 (1-Na-PP1). By treating cells exclusively expressing the as-AurA with 1-Na-PP1, we discovered that Aurora A is required for central spindle assembly in anaphase through phosphorylation of Ser 19 of P150Glued. This paper thus describes a new Aurora A function that takes place after the metaphase-to-anaphase transition and a new powerful tool to search for and study new Aurora A functions.
Introduction
The mitotic spindle is a highly specialized macromolecular machinery that ensures equal segregation of genetic information between daughter cells during mitosis. The assembly and operation of this complex and very dynamic structure have to be appropriately regulated to avoid generation of aneuploid cells. Numerous signaling molecules participate in this regulation, and they include the Aurora A kinase. Some of the functions of the Aurora A kinase during mitotic spindle assembly are now well established; they operate in G2 when the kinase is recruited to centrosomes. Aurora A participates in centrosome maturation by recruiting NDLE1 (Mori et al., 2007) and TACC3 (Kinoshita et al., 2005). In prometaphase, Aurora A participates in regulation of microtubule dynamics by contributing to the recruitment of factors involved in the dynamic instability of microtubules, including DDA3 (Jang and Fang, 2011), MCAK (Zhang et al., 2008), ch-TOG (De Luca et al., 2008; Asteriti et al., 2011), and KIF2A (Jang et al., 2009). Aurora A is also involved in recruitment of proteins that move along microtubules, for example EG5 (Castro et al., 2003) and P150Glued (Romé et al., 2010). In addition to these well-established functions in the initial phase of mitosis, there are some clues implicating Aurora A in late mitosis. First, before the metaphase-to-anaphase transition, the functions of Aurora A are closely related to its localization. During late mitotic phases, Aurora A localizes to the central spindle in anaphase and telophase and then to the midbody during cytokinesis, thus suggesting that the kinase may have regulatory functions during mitotic exit. Second, Marumoto et al. (2003) showed that injection of antibodies against Aurora A into G2 cells caused cytokinesis failure and suggested that this consequence was directly linked to Aurora A inhibition. Third, Hégarat et al. (2011) recently showed, by using a conditional knockout of Aurora A, that Aurora A and B cooperate to trigger chromosome segregation in anaphase through spindle microtubules depolymerization.
Although it has long been suggested that Aurora A might play an important role during mitotic exit, only limited information is available concerning the involvement of Aurora A in late mitotic phases. Studies investigating the functions of Aurora A have involved modifying Aurora A activity by depleting the protein through RNAi, by overexpression (Pascreau et al., 2008; Roghi et al., 1998), and/or by the use of mutants (active, inactive, hyperactive, or nondegradable; Hannak et al., 2001; Berdnik and Knoblich, 2002; Giet et al., 2002; Marumoto et al., 2002; Anand et al., 2003; Marumoto et al., 2003). The major finding of such experiments is the failure of centrosome maturation (Hannak et al., 2001). During G2, the cell prepares to enter mitosis, and numerous proteins required for microtubule nucleation are recruited to centrosomes to participate in mitotic spindle assembly. Defects in centrosome maturation frequently result in a longer G2/M transition, perturb mitotic spindle assembly, and activate the spindle assembly checkpoint (SAC). Activation of the SAC arrests the cells before the metaphase-to-anaphase transition and thereby prevents investigation of Aurora A functions beyond this step. One way to avoid this problem would be to use small molecules that rapidly inhibit Aurora A in anaphase, once the SAC has been satisfied. Compounds that inhibit Aurora A activity in vitro have been described (Manfredi et al., 2007; Maris et al., 2010), but their specificities in vivo remain poorly documented. One other solution is a chemical genetics approach to modify the catalytic domain of the kinase to make it sensitive to small molecules that have no effect on the wild-type (WT) kinase (Bishop et al., 1998; Liu et al., 1998). The major advantage of this approach is that the WT kinase could be used as a negative control, such that any off-target effects of the inhibitors could be easily identified. Only 20 kinases have been studied using chemical genetics approaches of this type. One of the impediments is that mutations in the catalytic domain often result in a less active or completely inactive kinase.
Here, we present strong evidence for the involvement of Aurora A in central spindle assembly during the early steps of anaphase. We used chemical genetics techniques to develop an Aurora A kinase variant (as-AurA) that retained full activity in vitro and was fully functional in U2OS cells. as-AurA is specifically inhibited by the ATP analogue 1-Naphthyl-PP1 (1-Na-PP1), which had no effect on the WT kinase. Experiments in cells and in vitro using as-AurA showed that Aurora A is involved in central spindle assembly at anaphase onset through phosphorylation of Ser 19 of P150Glued. This is the first documentation of a function of Aurora A after the metaphase-to-anaphase transition and the mechanism underlying this function. This work also clearly illustrates that as-AurA is a powerful new tool for studying Aurora A functions in late mitosis.
Results
Generation of an analogue-sensitive Aurora A kinase
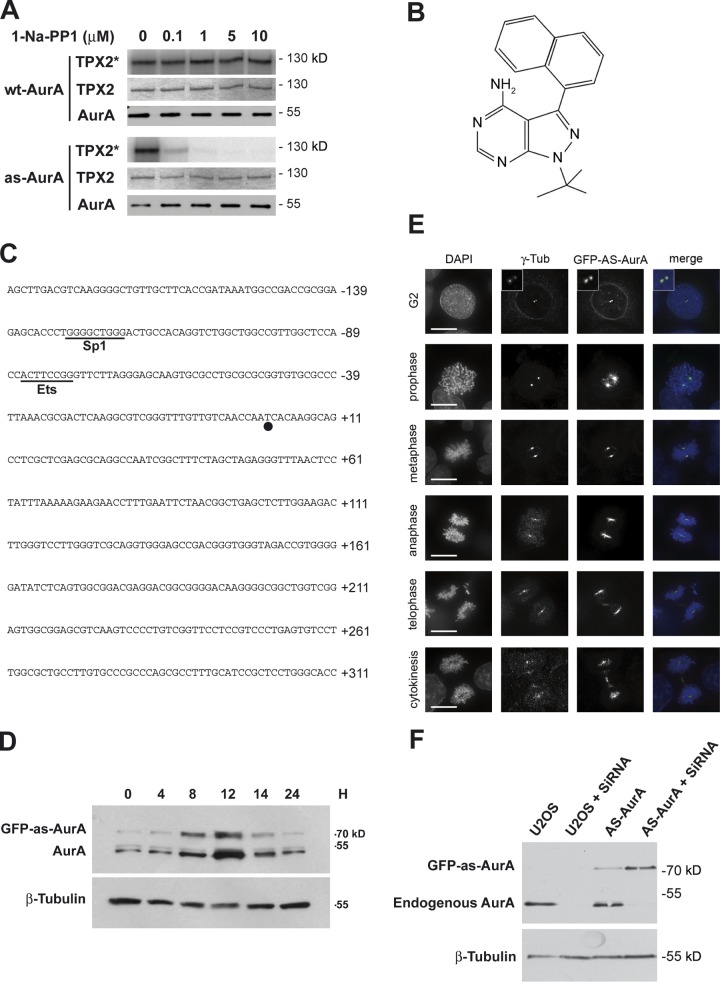
To generate an Aurora A variant with an enhanced sensitivity to ATP analogues, we changed the specificity of the ATP-binding pocket by converting an amino acid residue with a bulky side chain, the so-called gatekeeper residue, to alanine. The gatekeeper residue of the Aurora A kinase was identified from the crystal structure as the amino acid Leu 210; a genetic construct was produced encoding an L210A mutant. This mutant protein is referred to hereafter as analogue-sensitive Aurora A (as-AurA). In vitro, recombinant as-AurA was as active as wt-AurA and was still able to phosphorylate TPX2, a well-characterized Aurora A substrate (Fig. 1 A, first well; and Fig. S1). We then screened ATP analogues for an appropriate as-AurA inhibitor. 1-Na-PP1 (Fig. 1 B) was one of the most potent inhibitors of as-AurA and had no effect on wt-AurA; 10 µM 1-Na-PP1 completely inhibited as-AurA activity but had no effect on wt-AurA activity (Fig. 1 A).
Figure 1.
as-AurA retains full activity in vitro and behaves as wt-AurA when expressed in U2OS cells. (A) 0.5 pmol of each wt- and as-AurA were incubated with 20 pmol of TPX2 and the indicated amount of 1-Na-PP1. TPX2 phosphorylation (TPX2*) was assessed by autoradiography (top), and the presence of TPX2 and Aurora A was assessed by Coomassie blue staining (middle) and Western blotting (bottom), respectively. (B) The chemical structure of the as-AurA–specific inhibitor 1-NA-PP1. (C) Nucleotide sequence of the −139 to +311 transcriptional regulatory region of Aurora A. The putative binding sites for transcriptional factors are underlined. The major transcriptional initiation site (circle) is designated +1. (D) Western blot showing the expression of GFP-as-AurA and endogenous Aurora A in U2OS cells. Each lane corresponds to a time point (in hours) after release from double thymidine block. β-Tubulin serves as a loading control. (E) IF microscopy of AS-AurA cells at each phase of mitosis. Insets show enlargements of the centrosome area. Bars, 10 µm. (F) Protein lysates prepared from U2OS control and AS-AurA cells, depleted or not of endogenous Aurora A, were immunoblotted with anti–Aurora A (top) and anti–β-tubulin antibodies (bottom).
Cloning of the Aurora A promoter and characterization of wt- or as-AurA expression in human U2OS cells
To study the effect of specific inhibition of as-AurA by 1-Na-PP1 during mitosis, wt-AurA and as-AurA alleles needed to be expressed at a physiological level. Aurora A overexpression from the cytomegalovirus (CMV) promoter leads to nonphysiological features during cell division and even cell mortality (Goepfert et al., 2002; Anand et al., 2003; Dutertre et al., 2004). Therefore, we used the Aurora A minimal transcriptional regulatory region, previously described by Tanaka et al. (2002; Fig. 1 C). This DNA segment was amplified by PCR and ligated upstream from the genes encoding wt-AurA or as-AurA (fused to the GFP coding sequence) in a peGFP-C1 plasmid, replacing the CMV promoter. U2OS cells were then transfected with these constructs, and endogenous Aurora A, GFP-wt-AurA, and GFP-as-AurA expression levels were determined during the cell cycle in synchronized cells. Although the GFP-as-AurA expression level was slightly weaker than that of endogenous Aurora A, the kinetics of accumulation in the cells were similar: levels were low between 0 and 4 h after release from double thymidine block, corresponding to S phase; they increased between 8 and 12 h after release, during the G2/M phases, and then returned to the basal level (Fig. 1 D). We determined the cellular localization of as-AurA (Fig. 1 E): GFP-as-AurA was associated with centrosomes during G2 phase; it was then associated with centrosomes and the mitotic spindle from prophase to telophase and with the midbody during telophase and cytokinesis. This pattern was entirely consistent with that described for Aurora A (Marumoto et al., 2005). Silent mutations were then introduced into the wt-AurA and as-AurA constructs to make them resistant to the RNAi used to deplete endogenous Aurora A: the sequence 5′-CCTGTCTTACTGT-3′, between positions 729 and 741, was replaced with 5′-ACTATCATATTGC-3′. We generated U2OS cells stably expressing the resulting GFP-wt-AurA construct and the GFP-as-AurA construct (named WT-AurA and AS-AurA, respectively).
Cellular validation of the chemical genetics approach
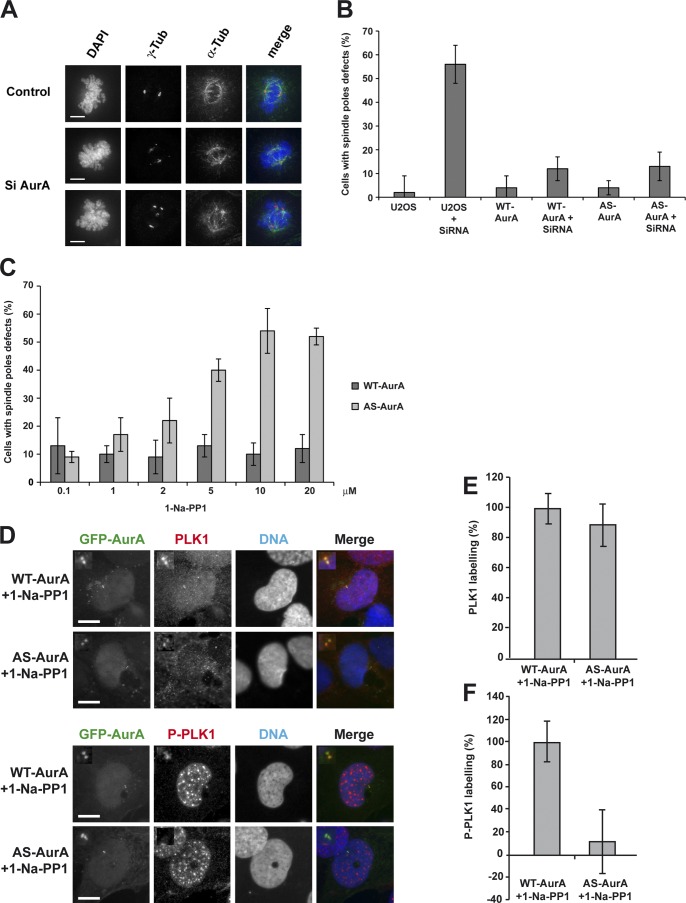
First, we validated our chemical genetics approach. Depletion of Aurora A by RNAi or injection of cultured cells with anti–Aurora A antibodies results in centrosome amplification leading to multiple spindle poles, or spindle pole fragmentation (Marumoto et al., 2003; De Luca et al., 2008). We therefore compared the effect of RNAi depletion of endogenous Aurora A in U2OS cells and WT-AurA or AS-AurA cells with and without 1-Na-PP1 treatment. Endogenous Aurora A was effectively depleted by RNAi in all of the U2OS, WT-AurA, and AS-AurA cells, and interestingly, the GFP-wt-AurA and GFP-as-AurA protein levels slightly increased to reach the endogenous Aurora A expression level (Fig. 1 F and 6 B). As previously observed (Asteriti et al., 2011), depletion of Aurora A for 24 h in U2OS cells led to the apparition of metaphase cells with multiple or fragmented spindle poles (56 ± 7%; Fig. 2, A and B). Depletion of endogenous Aurora A in WT-AurA or AS-AurA cells did not trigger any significant increase in mitotic spindle pole defects (Fig. 2 B), showing that GFP-wt-AurA and GFP-as-AurA each efficiently rescued endogenous Aurora A depletion. We then tested the effect of 1-Na-PP1 on WT-AurA and AS-AurA cells depleted of endogenous Aurora A (Fig. 2 C). 1-Na-PP1 (from 0.1 µM to 20 µM) had no significant effect on mitotic spindle poles in WT-AurA cells, but substantially increased the percentage of multipolar or fragmented spindle poles in AS-AurA cells. The maximum effect was obtained at 10 µM 1-Na-PP1, and this concentration was systematically used for all the following experiments. At last, to extensively validate the inhibition of as-AurA by 1-Na-PP1, we verified the phosphorylation state of a well-described Aurora A substrate, the Thr 210 (T210) of PLK1. This residue was shown to be phosphorylated by Aurora A at centrosomes in G2 cells (Macůrek et al., 2008). Fig. 2 D (top) and Fig. 2 E show that WT- or AS-AurA cells depleted of endogenous Aurora A and treated with 1-Na-PP1 for 24 h did not modify the level of PLK1 at centrosomes in G2 cells. On the opposite side, T210 phosphorylation was strongly decreased in AS-AurA cells when compared with WT-AurA cells (Fig. 2 D, bottom; and Fig. 2 F), thus indicating a strong inhibition of the activity of the kinase.
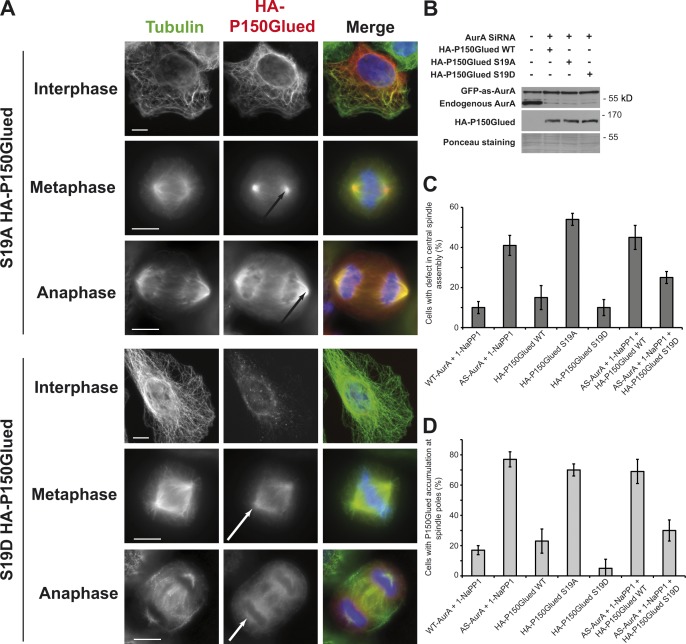
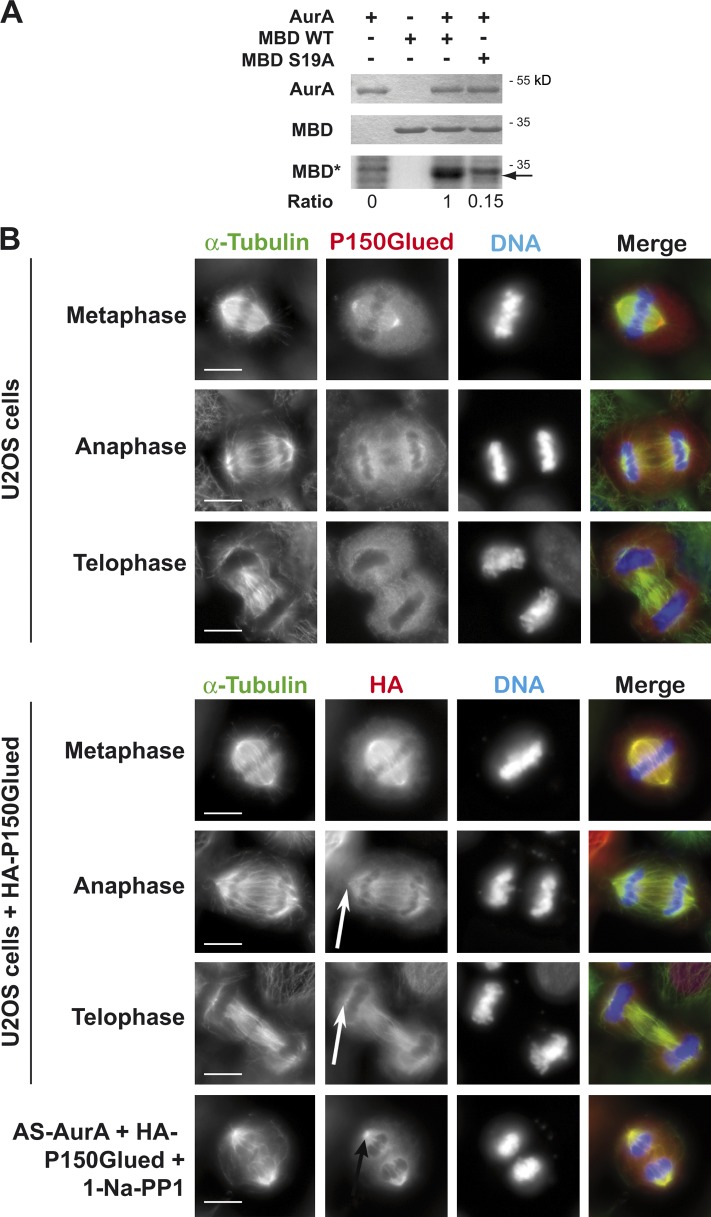
Figure 6.
Phosphorylation by Aurora A of P150Glued Ser 19 is required for central spindle assembly. (A) IF microscopy of cells showing the localization of S19A (top) or S19D (bottom) HA-P150Glued during interphase, metaphase, or anaphase. White arrows show mitotic spindle poles in anaphase or telophase cells without accumulation of HA-P150Glued, whereas the black arrows indicate accumulation of HA-P150Glued. Bars, 5 µm. (B) Western blot showing depletion of endogenous Aurora A from cells expressing S19 mutant versions of P150Glued. Ponceau staining serves as a loading control. (C) Histogram presenting the percentage of cells with defects in central spindle assembly. Values are means of three independent experiments; 50 cells were counted for each experiment. (D) Histogram presenting the percentage of cells with accumulation of P150Glued at spindle poles. Values are means of three independent experiments; 50 cells were counted for each experiment. Error bars represent standard deviations.
Figure 2.
as-AurA is a fully functional kinase in cultured cells and is efficiently inhibited by 1-Na-PP1. (A) IF microscopy of control or Aurora A–depleted (Si AurA) U2OS cells in metaphase. Bars, 5 µm. (B) Histogram presenting the percentage of cells with fragmented or multiple spindle poles. Values are means of three independent experiments; 100 cells were counted for each experiment. (C) Histogram presenting the percentage of cells with fragmented or multiple spindle poles, according to the 1-Na-PP1 concentration, in WT-AurA or AS-AurA cells depleted of endogenous Aurora A. Values are means of three independent experiments. 100 cells were counted for each experiment. (D) IF microscopy of WT- or AS-AurA cells depleted of endogenous Aurora A and treated for 24 h with 10 µM 1-Na-PP1. Cells were labeled with anti-PLK1 (top) or anti-T210 P-PLK1 antibody. Insets show enlargements of the centrosome area. Bars, 7 µm. (E) Histogram presenting the intensity of PLK1 labeling at centrosomes, in percentages, relative to the control (WT-AurA + 1-Na-PP1). (F) Histogram presenting the intensity of T210 P-PLK1 labeling at centrosomes, in percentages, relative to the control (WT-AurA + 1-Na-PP1). Values are means of three independent experiments. Labeling intensity was measured in 20 cells for each experiment. Error bars represent standard deviations.
Specific inhibition of Aurora A activity in early anaphase triggers a central spindle assembly defect
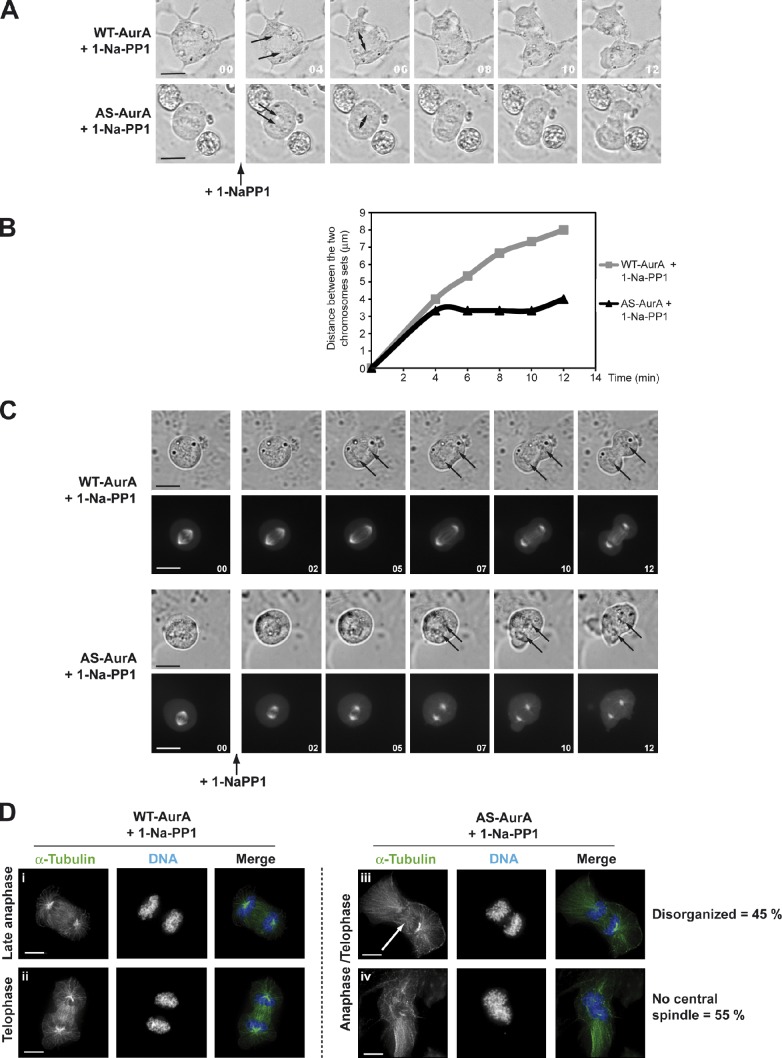
We used time-lapse video microscopy to film proliferating WT-AurA and AS-AurA cells, depleted of endogenous Aurora A and treated with 10 µM 1-Na-PP1 (Fig. 3 A). Addition of 1-Na-PP1 to control WT-AurA cells at the time of anaphase initiation had no effect: the two sets of chromosomes separated and cells underwent normal telophase and cytokinesis (n = 13). In AS-AurA cells treated with 1-Na-PP1 at anaphase, the chromosomes separated but rapidly stopped and cells did not undergo telophase or cytokinesis, leading to generation of binucleated cells (60 ± 6%, n = 9). The distance between the two sets of chromosomes increased until the end of telophase in WT-AurA control cells, but rapidly stopped increasing in AS-AurA cells, which indicates a probable defect in anaphase B (Fig. 3 B). During anaphase A, chromosome movement in HeLa cells is generated by pulling forces due to depolymerization of kinetochore microtubules. In anaphase B, the spindle poles move further apart, through the action of the midzone microtubules that grow by polymerization at their plus ends, whereas the cross-linked motor proteins travel toward the plus ends, thereby pushing the overlapping polar microtubules past each other and the spindle poles further apart. This event requires a functional central spindle, so we studied central spindle assembly in the context of Aurora A inhibition. We generated WT-AurA or and AS-AurA cells stably expressing GFP-tubulin and filmed them with a confocal spinning-disc time-lapse video microscope. As previously described, cells were first depleted of endogenous Aurora A and then treated with 1-Na-PP1 at the onset of anaphase (Fig. 3 C). Central spindles formed normally in WT-AurA cells but not in AS-AurA cells, implicating Aurora A activity in central spindle assembly. Interestingly, addition of 1-Na-PP1 to AS-AurA cells already engaged in anaphase B, with the first central spindle microtubules growing, did not perturb spindle assembly (unpublished data). To better characterize this phenotype, morphologically and statistically, we synchronized the cells in anaphase and observed them by immunofluorescence (IF). To this end, WT-AurA or AS-AurA cells depleted of endogenous Aurora A were blocked in late G2 phase by addition of the CDK1 inhibitor RO 3306 (Vassilev, 2006), and then released into mitosis by washing with fresh medium. Once the majority of the cells had entered early steps of anaphase (75 min), they were treated with 1-Na-PP1. 12 min after metaphase-to-anaphase transition, WT-AurA cells entered telophase, whereas AS-AurA cells presented substantial defects of central spindle assembly (Fig. 3, A and C). We thus fixed cells at this precise time point and analyzed the anaphase/telophase state by IF. Fig. 3 D shows that inhibition of Aurora A in early anaphase resulted in substantial central spindle defects (42 ± 6% in AS-AurA cells compared with 10 ± 3% for WT-AurA control cells, similar to nontreated U2OS cells). Among cells presenting this phenotype, 45% showed a strong central spindle disorganization and 55% showed a total absence of central spindle. In those with disorganized spindles, few microtubules were present and midzone microtubules were not cross-linked to form a functional central spindle (Fig. 3 D, compare i and ii with iii). In cells with no central spindle, the two chromosomes sets appeared very close to each other and were misoriented (Fig. 3 D, iv). We did not notice the presence of cells with lagging chromosomes.
Figure 3.
Inhibition of Aurora A at anaphase onset causes central spindle assembly defects. (A) Time-lapse microscopy images showing late mitotic phases in WT-AurA and AS-AurA cells depleted of endogenous Aurora A and treated with 10 mM 1-Na-PP1. Time is given in minutes. The two arrows indicate separating chromosome sets. The double arrow indicates the distance measured and reported in B. (B) Graph showing the evolution of the distance (in micrometers) between the two sets of chromosomes of the WT-AurA and AS-AurA cells presented in A. Each curve is the recording of 1 experiment representative of 13 and 9 experiments, respectively. (C) Fluorescence and DIC time-lapse microscopy images showing late mitotic phases of WT-AurA and AS-AurA cells expressing GFP-tubulin, depleted of endogenous Aurora A and treated with 10 µM 1-Na-PP1. The two arrows indicate separating chromosome sets. Time is given in minutes. (D) IF microscopy of WT-AurA and AS-AurA cells depleted of endogenous Aurora A and treated with 10 µM 1-Na-PP1 at anaphase onset. The arrow shows the disorganized microtubules in the central spindle. Bars: (A and C) 10 µm; (D) 7 µm.
Inhibition of Aurora A in early anaphase strongly impairs MKLP1 and P150Glued localization
Central spindle assembly is a complex process involving diverse molecules with very specific functions. The evolutionarily conserved Centralspindlin complex is central to this process. It is composed of a plus end–directed kinesin 6 protein, called MKLP1 in mammals and Pavarotti kinesin-like protein (Pav-KLP) in Drosophila melanogaster, and a Rho family GTPase-activating protein (GAP), known as RacGAP1 in mammals and RacGAP50C in Drosophila (Adams et al., 1998; Hirose et al., 2001; Mishima et al., 2002). Appropriate localization of Centralspindlin in Drosophila depends on the dynactin complex, and depletion of the dynactin subunit P150Glued by RNAi in Drosophila S2 cells perturbs Pav-KLP localization and central spindle organization (Delcros et al., 2006). P150Glued is a microtubule-associated protein that binds to dynein (Vaughan and Vallee, 1995) and is implicated in bidirectional transport of cargo along microtubules (Deacon et al., 2003). Recently, the microtubule-binding domain (MBD) of P150Glued in Drosophila was shown to be phosphorylated by Aurora A during prometaphase. This event is necessary for P150Glued to be unloaded from mitotic microtubules and for correct metaphasic mitotic spindle assembly. Depletion of Aurora A by RNAi in S2 or Drosophila embryonic cells triggers P150Glued accumulation at spindle poles (Romé et al., 2010). We therefore investigated the cellular localizations of P150Glued and MKLP1 during anaphase/telophase under conditions of Aurora A inactivation. We observed by IF that inhibition of Aurora A activity in early anaphase triggered mislocalization of MKLP1 and accumulation of P150Glued at mitotic spindle poles (55 ± 8% and 77 ± 5%, respectively; Fig. 4, A and B). Interestingly, depletion of P150Glued by siRNA triggered apparition of cells presenting a strong central spindle assembly defect (Fig. 4, C and D), which resembles the effect of an Aurora A inhibition. We also tested the effect of Aurora B inhibition because this kinase was shown to be involved in central spindle organization through recruitment of bundling factors such as Pav-KLP (Giet and Glover, 2001; Glotzer, 2009) and in chromosomes segregation in collaboration with Aurora A (Hégarat et al., 2011). As described before, we treated cells with RO3306 and released them into late mitosis after washes. When a majority of cells entered early anaphase, they were treated with 3 µM ZM447439. Although inhibition of Aurora B at this precise time point triggered apparition of lagging chromosomes and a disorganization of the central spindle, we could not detect any P150Glued accumulation at spindle poles nor any cells without microtubule into the midzone (Fig. S2).
Figure 4.
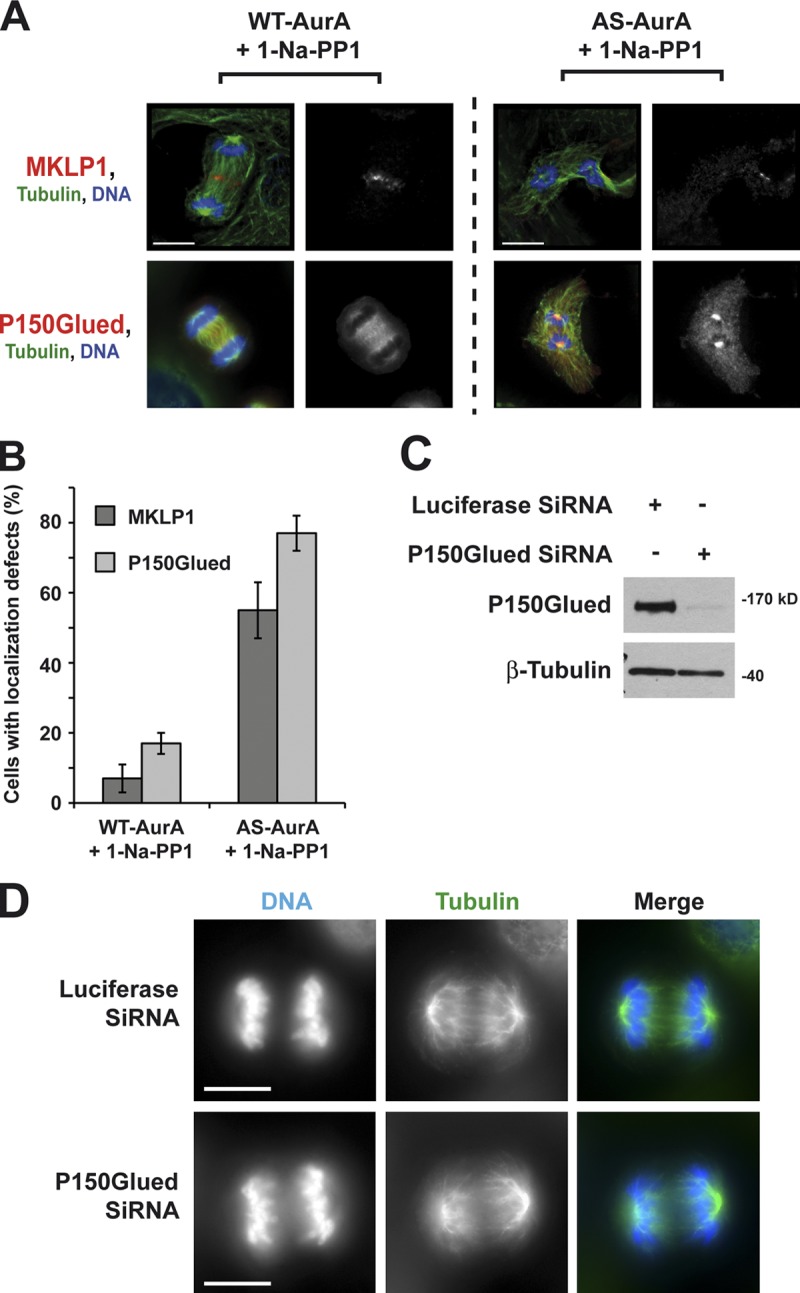
Inhibition of Aurora A at anaphase onset triggers mislocalization of MKLP1 and the accumulation of P150Glued at spindle poles. (A) IF microscopy of WT- and AS-AurA cells depleted of endogenous Aurora A and treated with 10 µM 1-Na-PP1 at anaphase onset. Bars, 10 µm. (B) Histogram presenting the percentage of cells with mislocalized MKLP1 or P150Glued in the WT- and AS-AurA cells shown in A. Values are means of three independent experiments; 30 cells were counted for each experiment. Error bars represent standard deviations. (C) Western blot showing depletion of P150Glued in U2OS cells. Luciferase siRNA serves as a negative control. β-Tubulin serves as a loading control. (D) IF microscopy of cells depleted of P150Glued by siRNA for 24 h. Bars, 10 µm.
Phosphorylation of P150Glued Ser 19 by Aurora A is required for central spindle assembly
In Drosophila, eight serines in the MBD of P150Glued are phosphorylated by Aurora A (Romé et al., 2010), but these serines are not conserved in humans. To identify the residues that are phosphorylated by Aurora A in human P150Glued, we produced recombinant Aurora A and human P150Glued MBD (amino acids 1–213). We used phosphoproteomic analysis to determine which MBD residues were phosphorylated by Aurora A in vitro. We obtained >85% of sequence coverage in tandem mass spectrometry (MS/MS) analyses and thereby identified Ser 19 as being the only residue phosphorylated by Aurora A in the MBD. To confirm this result, we produced an S19A mutant (alanine is nonphosphorylatable) MBD construct and conducted in vitro phosphorylation assays (Fig. 5 A). The control P150Glued MBD was phosphorylated by Aurora A, and the S19A mutation strongly abolished this MBD phosphorylation: the 32P signal was more than six times weaker in the S19A mutant compared with control. This confirms that residue S19 of the P150Glued MBD is directly phosphorylated by Aurora A in vitro. To verify the effect of P150Glued S19 phosphorylation on central spindle assembly, we expressed the WT and S19 mutated versions (S19A as a nonphosphorylatable form of P150Glued and S19D as a mimicking a constitutively phosphorylated serine) of full-length HA-tagged P150Glued (HA-P150Glued) in U2OS cells. HA-P150Glued localized on microtubules similarly to the endogenous protein (Fig. 5 B, compare bottom and top panels). Inhibition of Aurora A led to HA-P150Glued accumulation at spindle poles in anaphase/telophase and defects in central spindle assembly (Fig. 5 B bottom; and Fig. 6, C and D). Localizations of the S19A and S19D mutants were significantly different. S19A P150Glued strongly accumulates on interphase microtubules and on mitotic spindle poles in metaphase and anaphase (Fig. 6 A, top; and Fig. 6 C). Contrary to S19A, binding to microtubules of the S19D mutant was strongly reduced in interphase and we could not detect any accumulation of S19D P150Glued to spindle poles in metaphase or anaphase (Fig. 6 A, bottom; and Fig. 6 D). Interestingly, if the expression of HA-P150Glued S19D mutant did not trigger any significant effect on central spindle organization (Fig. 6, A and C), that of HA-P150Glued S19A was associated with a large increase in the percentage of cells presenting central spindle defects (54 ± 3%, Fig. 6, A and C). To confirm that phosphorylation of S19 by Aurora A is required for normal central spindle assembly, we tested whether the expression of the S19D mutant was able to rescue Aurora A inhibition. AS-AurA cells were depleted of endogenous Aurora A for 24 h, and then transfected with WT or S19D HA-P150Glued plasmids. After 24 h, the cells were synchronized in early anaphase with RO 3306, treated with 1-Na-PP1 for 12 min, and then fixed. We first verified that endogenous Aurora A was fully depleted and that WT and S19D HA-P150Glued were appropriately produced when 1-Na-PP1 was added (Fig. 6 B). We then quantified anaphase/telophase cells with defects in central spindle assembly or HA-P150Glued accumulation at spindle poles (Fig. 6, C and D). The percentages of anaphase cells presenting central spindle assembly defects or accumulation of HA-P150Glued at the spindle pole were significantly reduced by the expression of HA-P150Glued S19D but not HA-P150Glued WT. This confirmed that the phospho-mimicking version of HA-P150Glued (the S19D mutant) was able to rescue Aurora A inhibition, at least partially.
Figure 5.
P150Glued is phosphorylated by Aurora A on Ser 19 in vitro. (A) 50 pmol of MBD of P150Glued (MBD) was incubated with 20 pmol wt-AurA or as-AurA. MBD phosphorylation (MBD*) was assessed by autoradiography (bottom), and the presence of MBD and AurA was assessed by Coomassie blue staining (top). The arrow indicates the place of MBD on the autoradiography. The number below the figure represents the ratio of phosphorylation in the first, third, and fourth well compared with the third well. The areas and the formula used for calculation are, respectively, shown in Fig. S3 (A and B). Fig. S3 A presents the same data, though describing explanations concerning the calculation of phosphorylation rates. (B) IF microscopy of cells showing localization of endogenous P150Glued (top) and HA-P150Glued (bottom, expression of HA-P150Glued in cells still expressing endogenous P150Glued) in late mitotic phases. White arrows show mitotic spindle poles in anaphase or telophase cells without accumulation of HA-P150Glued, whereas the black arrow indicates accumulation of HA-P150Glued in AS-AurA cells depleted of endogenous Aurora A and treated with 10 µM 1-Na-PP1 at anaphase onset. Bars, 10 µm.
Discussion
An L210A mutation results in a functional Aurora A kinase that is specifically inhibited by 1-Na-PP1
Aurora A kinase has been studied for more than 15 years (Kimura et al., 1997), and many of its functions are now well established. All these documented functions concern events before the metaphase-to-anaphase transition. Here, we describe an approach that allowed the analysis of Aurora A function after the metaphase-to-anaphase transition. We thereby identified a new Aurora A function in late mitosis and described the underlying mechanism.
During early phases of mitosis, the functions of Aurora A are strictly related to its localization. Aurora A localizes to the centrosomes to ensure centrosome maturation, then to the spindle poles to ensure bipolar spindle assembly. Loss of Aurora A function immediately causes spindle abnormalities and subsequent cell cycle arrest in prometaphase. After the metaphase-to-anaphase transition, Aurora A is found on the central spindle in anaphase and on the midbody in telophase and cytokinesis, without any identified function. This absence of information is essentially due to the lack of a specific Aurora A inhibitor allowing inhibition and consequently analysis of Aurora A functions at times after its contributions to early mitotic events. Chemical genetics have been developed (Bishop et al., 1998; Liu et al., 1998) to circumvent the absence of specific kinase inhibitors. Although this approach is promising, only about 20 kinases have been studied by this approach, and most are in unicellular eukaryotes. The main reason for this is that it has been difficult to maintain full kinase activity after the introduction of mutations into the active site of the enzyme.
We report the first human Aurora A kinase variant (as-AurA) obtained by a chemical genetics approach: the leucine 210 of the ATP-binding pocket was replaced with an alanine. In vitro as-AurA retained full kinase activity and was specifically inhibited by 10 µM 1-Na-PP1 (which had no inhibitory effects on the WT kinase, or wt-AurA). Overexpression of Aurora A triggers mitotic defects, so we produced as-AurA under the control of the minimal endogenous Aurora A transcriptional regulatory region (Tanaka et al., 2002). This construct allowed production of physiological amounts of the as-kinase once the endogenous Aurora-A has been depleted by RNAi. The localization of as-AurA was similar to that of wt-AurA, and rescue experiments indicated that as-AurA was fully functional in cultured cells. The concentration of 1-Na-PP1 used (10 µM), inhibiting as-AurA in cells without any effects on wt-AurA, was similar to concentrations used in previous studies involving as-kinases (Liu et al., 1998; Chen et al., 2005; Kenski et al., 2005; Endo et al., 2006; Burkard et al., 2007).
Aurora A is required at anaphase onset for central spindle assembly
Inhibition of Aurora A immediately after anaphase onset led to an anaphase B defect due to the failure of central spindle assembly. Few studies have reported or suggested late mitotic functions for Aurora A. Marumoto et al. (2003) injected G2 cells with antibodies against Aurora A, and observed that cells underwent normal mitosis until cytokinesis, at which point the cleavage furrow regressed to give a binucleated cell. This result is surprising because we observed an earlier defect in central spindle assembly at the onset of anaphase in our model system. Possibly, antibody injection only partially inhibits kinase activity, such that the residual activity is sufficient for central spindle assembly, but not for completion of cytokinesis. Motegi et al. (2006) observed cleavage furrow defects in Caenorhabditis elegans air-1 mutants (AIR-1 in C. elegans is the orthologue of Aurora A in human), and suggested that they were related to microtubule behavior. Also, a proteomic approach identified midbody proteins and suggested that Aurora A participates in the regulation of cytokinesis through phosphorylation of CRMP-1 (Collapsin response mediator protein 1; Chen et al., 2009). At last, a recent study, using conditional knockout of Aurora A in DT40 cells, showed that Aurora A collaborates with Aurora B to control spindle microtubules depolymerization in anaphase (Hégarat et al., 2011). Indeed, Hégarat et al. (2011) showed that simple depletion of Aurora A was not sufficient to trigger central spindle disorganization and that Aurora B should be inhibited concomitantly to obtain an effect. Although conditional knockout is an interesting strategy, it does not allow precise time point inhibition of Aurora A, which could explain the discrepancies observed between that work and our present study. We effectively observed that simple inhibition of Aurora A activity at the onset of anaphase was sufficient to strongly impair central spindle assembly (a majority of cells showed an absence of microtubule in the midzone) and trigger P150Glued accumulation at spindle poles. Contrary to Aurora A inhibition, our study shows that Aurora B inhibition at the anaphase onset did not impair microtubule growth in the midzone and did not provoke any accumulation of P150Glued at spindle poles. It nevertheless triggered apparition of lagging chromosomes and a defect in central spindle organization corresponding to previously published data (Giet and Glover, 2001; Glotzer, 2009). These results collectively indicate that if Aurora A and B could cooperate in late anaphase it is likely not the case in early anaphase. The central spindle is composed of interpolar microtubules emanating from centrosomes and interzone microtubules nucleated near chromosomes (Uehara and Goshima, 2010). The fact that inhibition of Aurora A after apparition of the first microtubules composing the central spindle did not disturb further central spindle assembly could suggest that Aurora A is implicated in a pathway regulating initial microtubule nucleation. Several molecules are required for central spindle microtubule nucleation and bundling. They include the Centralspindlin complex, composed of the MKLP1 kinesin and the RacGAP1 Rho family GAP, necessary for the initial steps of central spindle assembly (Mishima et al., 2002). Centralspindlin must be appropriately located for microtubule nucleation and bundling in the central spindle (Mishima et al., 2002), and this depends on the dynactin subunit P150Glued (Delcros et al., 2006). In Drosophila, phosphorylation of P150Glued by Aurora A is necessary to regulate binding of the dynactin complex to the mitotic spindle in metaphase (Romé et al., 2010). In particular, Romé et al. (2010) showed that P150Glued accumulates at spindle poles in cells depleted of Aurora A. As described for metaphase spindles, we found that inhibition of Aurora A triggered the accumulation of P150Glued at spindle poles of anaphase/telophase cells and mislocalization of MKLP1. Interestingly, our results show that depletion of P150Glued triggers a strong defect in central spindle assembly and thus mimics the specific inhibition of Aurora A.
Phosphorylation by Aurora A of P150Glued Ser 19 is required for central spindle assembly
Aurora A phosphorylates eight serines in the Drosophila P150Glued, all on the N-terminal part of the protein in a region called the MBD (Romé et al., 2010). Expression of a nonphosphorylatable form of MBD or P150Glued (all the eight serines mutated into alanines) resulted in disconnection of centrosomes from the metaphase spindle and accumulation of the protein at spindle poles. MBD is poorly conserved between flies and humans, therefore we mapped Aurora A phosphorylation sites in human MBD, and identified Ser 19 as an Aurora A target. This Ser is included into a sequence (underlined, SRMSAE) that fits the Aurora A consensus sequence (RXp(S/T)) described by Kettenbach et al. (2011). Mutation of Ser 19 into alanine (S19A mutant) resulted in P150Glued accumulation at spindle poles, but unlike in Drosophila cells, not centrosome disconnection. This mutation was also associated with defects in central spindle assembly, similar to those resulting from Aurora A inhibition. Phosphorylation of S19 by protein kinase A (PKA) has been shown to be involved in regulating the affinity of P150Glued for microtubules, and the S19A mutation caused an accumulation of P150Glued on interphase microtubules (Vaughan et al., 2002). Here, we show that Aurora A can also phosphorylate S19 during mitosis. We also show that a P150Glued S19D mutant that mimics constitutive phosphorylation of the Ser 19 significantly rescued the Aurora A inhibition. This strongly indicates that Aurora A participates in central spindle assembly by phosphorylating P150Glued. However, the fact that the rescue was only partial suggests that Aurora A may phosphorylate other substrates involved in central spindle assembly; indeed, Hice1, a subunit of the Augmin complex, is involved in central spindle assembly (Uehara et al., 2009) and is phosphorylated by Aurora A. This phosphorylation is involved in regulating Hice1 affinity for microtubules and is necessary for mitotic spindle assembly before metaphase (Tsai et al., 2011). Similarly, HURP is involved in mitotic and central spindle assembly (Koffa et al., 2006; Uehara and Goshima, 2010) and is phosphorylated by Aurora A. This phosphorylation is necessary for cells to proliferate in low-serum environments (Yu et al., 2005). Although sites of microtubule nucleation responsible for central spindle growth have been identified, the regulatory mechanisms necessary for correct assembly remain largely unknown. P150Glued interacts with EB1, a microtubule-associated protein involved in microtubule nucleation (Berrueta et al., 1999; Askham et al., 2002); interaction between P150Glued and EB1 is necessary for microtubule binding to centrosomes (Askham et al., 2002) and for microtubule nucleation (Hayashi et al., 2005; Strickland et al., 2005; Manna et al., 2008). The C terminus of EB1 binds to the N terminus of P150Glued, and this decreases microtubule shortening and increases rescue frequency and the growth rate of microtubules, thereby favoring microtubule elongation (Askham et al., 2002; Manna et al., 2008). Aurora A depletion results in disconnection of centrosomes from mitotic spindle poles in Drosophila (Romé et al., 2010), and inhibition of Aurora A seems to be involved in central spindle microtubule nucleation (this paper). Both these effects resemble those of EB1 inactivation (Askham et al., 2002; Hayashi et al., 2005; Strickland et al., 2005; Manna et al., 2008). Consequently, phosphorylation of S19 in P150Glued by Aurora A could be involved in central spindle assembly through an EB1 function.
as-AurA kinase is a potent new tool for studying Aurora A functions and validating Aurora A inhibitors
Various Aurora A inhibitors with good efficiency in vitro are now available. However, it is important to be cautious when interpreting findings with such tools, because the molecular environment of the kinase may considerably modify the kinase activity and sensitivity to inhibitory molecules. For instance, binding of TPX2 to Aurora A strongly decreases the inhibitory effect on Aurora A of chemical inhibitors (Anderson et al., 2007). Millennium Laboratories identified MLN8054 as an interesting inhibitory molecule. Although this drug is >40-fold more selective for Aurora A than Aurora B in vitro, strong Aurora A inhibition requires 1 µM MLN8054, a concentration that also affects Aurora B (Manfredi et al., 2007). Consequently, interpreting results involving Aurora A inhibition by such drugs may be complicated by low levels of Aurora B inhibition (Taylor and Peters, 2008). One problem with this type of chemical biology approach is the absence of negative controls to identify off-target effects. A recent paper has tried to solve this problem by mutating Aurora A to prevent inhibition by MLN8054 or its close analogue MLN8237. Although this study strongly suggests that the main target of these molecules is Aurora A, these mutants are only partially resistant to the drugs such that they are unsatisfactory for cell biology (Sloane et al., 2010). The chemical genetics approach we present is a good alternative to chemical biology and allowed strong, specific, and rapid inhibition of Aurora A. In particular, the existence of a true negative control allowed the observed effects to be attributed to specific inhibition of Aurora A and not to off-target effects.
Aurora kinases have central roles in mitosis and are frequently overexpressed in many types of tumors. Consequently, their specific inhibition is a major strategy for combating cancer (Sloane et al., 2010). New antiproliferative drugs are required to treat varieties of cancers that are resistant to classical chemotherapy. It would therefore be valuable to identify specific Aurora A or B inhibitors for use as chemotherapy. as-AurA could thus be used to study the specificity of candidate drugs and contribute to the validation of new pharmacological tools.
Materials and methods
DNA constructs, siRNA sequences, and protein purification
For purification of recombinant proteins, Aurora A and P150Glued MBD cDNAs were inserted into the vector pET29. The P150Glued and MBD coding sequences were isolated by PCR amplification of the corresponding sequences in the pTB701HA plasmid were provided by M. Takahashi (Teikyo Heisei University, Ichihara, Japan). Aurora A L210A and P150Glued mutants (S19A, S19D) were generated with the QuikChange mutagenesis kit (Agilent Technologies). Aurora A was depleted from U2OS cells as described in Reboutier et al. (2012). In brief, cells were transfected for 24 h using Jetprime (Polyplus Transfection), according to the manufacturer’s instructions, with an siRNA oligonucleotide coding the following sequence: 5′-AUGCCCUGUCUUACUGUCA-3′. Depletion of endogenous Aurora A was systematically verified by Western blotting for all experiments. wt-AurA and as-AurA cDNA were inserted into a pEGFP-C1 vector under the Aurora A promoter sequence to drive physiological levels of expression. WT P150Glued and mutant versions were inserted into a pCDNA3.1 vector. For production of recombinant proteins, BL21 Escherichia coli were transformed with plasmids, induced by 1 mM IPTG for 4 h, pelleted, and frozen at −80°C. Histidine-tagged proteins (human Aurora A, TPX2) were purified as described previously (Roghi et al., 1998). In brief, bacterial pellets were sonicated in lysis buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4,1.8 mM KH2PO4, pH 7.3, 0.1% Triton X-100, and 1 mg/ml lysozyme) and loaded onto Talon beads (Takara Bio Inc.); the samples were washed and proteins were eluted with buffer containing 50 mM imidazole. Purified proteins were stored at −80°C or in 50% glycerol at −20°C. For P150Glued depletion, cells were transfected for 24 h by using Jetprime according to the manufacturer’s instructions with a siRNA oligonucleotide coding the following sequence: 5′-GGCCCAUGCCAAGGCAAUU-3′ (for Luciferase, the sequence is 5′-CGUACGCGGAAUACUUCGA-3′).
Cell culture, synchronization, and transfection
Human U2OS cells were grown in McCoy’s medium with penicillin and streptomycin (Invitrogen) and 10% fetal calf serum (PAA). Cells were transfected in culture medium using Jetprime according to the manufacturer’s instructions. The medium was changed after 24 h. For anaphase/telophase synchronization, cells were arrested in G2 by 16 h of treatment of 10 µM RO3306 (EMD Millipore) and then washed three times for 30 s with fresh prewarmed medium. The first cells enter anaphase ∼1 h after the first wash.
IF
Cells were first plated onto glass coverslips, fixed with methanol at −20°C for 10 min (or with 4% paraformaldehyde for 10 min for native GFP observation), and then washed three times in PBS and saturated with PBS-BSA 1% for 1 h at room temperature. Antibodies in 1% PBS-BSA were added onto the cells: rat anti–α-tubulin 1:1,000 (clone YL1/2; EMD Millipore); mouse anti–γ-tubulin 1:1,000 (clone GTU88; Sigma-Aldrich); mouse anti–Aurora A (clone 35C11:20; Cremet et al., 2003); rabbit anti-MKLP1 1:1,000 (Santa Cruz Biotechnology, Inc.); mouse anti-P150Glued (BD); mouse anti-HA 1/1,000 (Covance); mouse anti-PLK1 (Abcam); and mouse anti-T210 P-PLK1 (BD). The cells were incubated with antibodies overnight at 4°C, and then washed three times and incubated in the dark with secondary antibodies (anti–mouse or anti–rabbit Alexa Fluor 488 or 555 1:1,000; Invitrogen) for 1 h at room temperature. After three final washes with PBS, the coverslips were mounted with ProLong Gold (Invitrogen) with 1 µg/ml DAPI (Sigma-Aldrich). The cells were examined using a fluorescent microscope (DMRXA2; Leica) with a 63× oil immersion objective lens, and images were processed using MetaMorph software (Molecular Devices).
Live cell imaging
Cells were grown in Lab-Tek I chambered coverglass (Thermo Fisher Scientific). Before microscopy, the medium was changed to CO2-independent medium supplemented with 10% FBS and 200 mM l-glutamine (Invitrogen). For 1-Na-PP1 treatment, we selected metaphasic cells, waited until they entered anaphase, and then immediately added the drug. Time-lapse images were acquired using a Plan Apo 60×/1.4 NA objective lens on an Eclipse Ti-E microscope (Nikon) equipped with a spinning disk (CSU-X1; Yokogawa Corporation of America), a thermostatic chamber (Life Imaging Service), a Z Piezo stage (Märzhäuser Wetzlar), and a charge-coupled device camera (CoolSNAP HQ2; Roper Scientific). MetaMorph software was used to collect the data. Frames were recorded every minute. Images are maximum projections of 20 z planes acquired 0.6 mm apart. Time-lapse data were processed using MetaMorph.
Western blot analysis
Cells were lysed in RIPA buffer containing anti-protease (Complete; Roche), and lysates were clarified by centrifugation (13,000 rpm, 30 min, 4°C). Proteins were assayed by Bradford assay (Bio-Rad Laboratories). Equal amounts of protein lysates in Laemmli buffer were resolved by 12.5% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked for 1 h at room temperature with TBST 4% milk, and then incubated overnight with the following antibodies: mouse anti–β-tubulin 1:2,000 (Sigma-Aldrich), mouse anti–Aurora A (clone 35C1 1:100; Cremet et al., 2003), and mouse anti-HA (Covance). Membranes were incubated with secondary antibodies coupled to HRP (Jackson ImmunoResearch Laboratories, Inc.) for 1 h, and antibody binding was detected by enhanced chemiluminescence (Pico or Dura; Thermo Fisher Scientific).
In vitro kinase assays
Amounts of recombinant Aurora A, MBD, and TPX2, as indicated in the figures, were mixed in 50 mM Tris HCl, pH 7.5, 25 mM NaCl, 1 mM DTT, 0.01% Triton X-100, and 10 mM MgCl2. Then, 100 µM ATP and 2 µCi γ-[32P]ATP were added and the reaction mixtures were incubated at 37°C for 10 min. Reactions were stopped by the addition of Laemmli buffer, and the samples were boiled for 5 min and resolved by 12.5% SDS-PAGE. Gels were stained with Coomassie blue, fixed, dried and autoradiographed with Storm 840 (Molecular Dynamics).
Mass spectrometry analysis
Purified proteins were separated by SDS-PAGE on a 12% precast gel (NuPAGE; Invitrogen). The gel was stained using the EZBlue gel staining reagent (Sigma-Aldrich), and gel slices corresponding to p150Glued were destained and washed with a series of acetonitrile (ACN) 100 mM NH4HCO3 solutions. Proteins were then reduced with 65 mM DTT for 15 min at 37°C and alkylated with 135 mM iodoacetamide in the dark at room temperature. The samples were washed with a series of ACN/100 mM NH4HCO3 solutions and dehydrated with 100% ACN. Proteins were digested overnight at 37°C with 4 ng/µl of modified trypsin (Promega) in 50 mM NH4HCO3. The proteolytic products were extracted from the gel by sequential incubations in 70:30:0.1 ACN/H2O/HCOOH (vol/vol/vol) and 100% ACN and concentrated in a Speed Vac (Thermo Fisher Scientific) to a final volume of 40 µl. The peptides were analyzed using a nanoflow high-performance liquid chromatography system (Ultimate 3000; Dionex) connected to a hybrid LTQ-OrbiTrap XL (Thermo Fisher Scientific) equipped with a nanoelectrospray ion source. Peptides were loaded onto a C18 (PepMap; Dionex) trapping precolumn (5 mm, 300-µm inner diameter, 300-Å pore size, 5 µm) and separated on a C18 (PepMap) analytical column (15 cm, 300-µm inner diameter, 300-Å pore size, 5 µm). Mobile phases A (0.1% HCOOH in water) and B (0.1% HCOOH in ACN) were delivered by an Ultimate 3000 nanoflow LC system (Dionex). Peptides were eluted with a gradient from 2–35% B from 0–60 min, 35–60% B from 60–85 min, and 60–90% B from 85–105 min. The MS instrument was operated in its data-dependent decision tree mode by automatically switching between full survey scan MS and consecutive MS/MS acquisition. Survey full scan MS spectra (mass range 400–2,000) were acquired in the OrbiTrap section of the instrument with a resolution of R = 60,000 at m/z 400. The seven most intense peptide ions with a charge state ≥2 were sequentially isolated by collision-induced dissociation (CID) with normalized collision energy at 35% and an activation time of 30 ms. Electron transfer dissociations (ETD) were automatically triggered if the peptide precursor ion had a charge state of +3 and <650 m/z, a charge state of +4 and <900 m/z, or a charge state of +5 and <950 m/z with a maximum ETD reaction time of 100 ms.
The Xcalibur software (Thermo Fisher Scientific) was used to create the peak list from raw data. MS/MS spectra were searched against the Swiss-Prot database filtered with the E. coli taxonomy (release February 2, 2012; 4,948 sequences; 1,569,896 residues) and completed with the sequences of P150Glued and common protein contaminants using the Proteome Discoverer software (version 1.2), supported by Mascot (Matrix Science) and SEQUEST database search engines for peptide and protein identification. Mass tolerances for the precursor and fragment ions were set to 10 ppm and 0.5 D, respectively. The enzyme selectivity was set to full trypsin with one missed cleavage allowed. Protein modifications were fixed carbamidomethylation of cysteines, variable oxidation of methionine, variable acetylation of lysine, and variable phosphorylation of serine, threonine, and tyrosine. Identified peptides were filtered based on Xcorr values and the Mascot score to obtain a false discovery rate of 1% and a false positive rate of 5%.
Online supplemental material
Fig. S1 shows that kinase activity of wt- and as-Aurora A is similar in vitro. Fig. S2 shows that inhibition of Aurora B in early anaphase triggers different effects on central spindle when compared with Aurora A. Fig. S3 present the method of calculation of the relative phosphorylation intensities of mutated versions of MBD. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201210060/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Chao Zhang and Dr. Kevan M. Shokat for design of the L210A mutation and for providing ATP analogues. We thank Dr. Mélanie Lagarrigue for proteomics analysis (Plateforme protéomique Biogenouest). The microscopy work was performed on the IBiSA platform MRic (Microscopy Rennes Imaging Center). We thank Dr. Mikiko Takahashi for providing pTB701HA, the plasmid encoding full-length P150Glued. We thank Dr. Regis Giet and Dr. Christelle Benaud for fruitful discussions.
This work was supported by the Agence Nationale de la Recherche. D. Reboutier, M.-B. Troadec, P. Salaun, and C. Prigent are coinventors of a patent application involving as-Aurora A and its specific inhibitor 1-Na-PP1, and the construct allowing physiological expression of as-Aurora A in culture cells. The authors have no other conflicting financial interests.
Author contributions: D. Reboutier designed and performed experiments of molecular biology (cloning and mutagenesis of p150Glued), cell biology (validation of the AS- and WT-AurA cell lines, discovery and characterization of the mitotic spindle assembly defect, generation of the GFP-tubulin–expressing cell lines, cell synchronization, and rescue experiments), biochemistry (purification of recombinant proteins, in vitro kinase assays), and proteomics. D. Reboutier also analyzed data and wrote the paper. M.-B. Troadec set up the AS-AurA cell line (cloning of the Aurora regulatory region, screening of ATP analogs, generation of the AS- and WT-AurA cell lines, and validation of AS-AurA localization). J.-Y. Cremet performed cloning and mutagenesis of Aurora A and p150Glued and purified recombinant proteins. L. Chauvin cloned the Aurora regulatory region and performed cell synchronization and validation of as-AurA localization. V. Guen performed cell synchronization and characterization of the mitotic spindle assembly defect. P. Salaun generated Aurora A–expressing constructs. C. Prigent supervised the project, designed experiments, and wrote the paper.
Footnotes
Abbreviations used in this paper:
- 1-Na-PP1
- 1-Naphthyl-PP1
- ACN
- acetonitrile
- GAP
- GTPase-activating protein
- IF
- immunofluorescence
- MBD
- microtubule-binding domain
- Pav-KLP
- Pavarotti kinesin-like protein
- SAC
- spindle assembly checkpoint
- WT
- wild type
References
- Adams R.R., Tavares A.A., Salzberg A., Bellen H.J., Glover D.M. 1998. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12:1483–1494 10.1101/gad.12.10.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S., Penrhyn-Lowe S., Venkitaraman A.R. 2003. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 3:51–62 10.1016/S1535-6108(02)00235-0 [DOI] [PubMed] [Google Scholar]
- Anderson K., Yang J., Koretke K., Nurse K., Calamari A., Kirkpatrick R.B., Patrick D., Silva D., Tummino P.J., Copeland R.A., Lai Z. 2007. Binding of TPX2 to Aurora A alters substrate and inhibitor interactions. Biochemistry. 46:10287–10295 10.1021/bi7011355 [DOI] [PubMed] [Google Scholar]
- Askham J.M., Vaughan K.T., Goodson H.V., Morrison E.E. 2002. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol. Biol. Cell. 13:3627–3645 10.1091/mbc.E02-01-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriti I.A., Giubettini M., Lavia P., Guarguaglini G. 2011. Aurora-A inactivation causes mitotic spindle pole fragmentation by unbalancing microtubule-generated forces. Mol. Cancer. 10:131 10.1186/1476-4598-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik D., Knoblich J.A. 2002. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 12:640–647 10.1016/S0960-9822(02)00766-2 [DOI] [PubMed] [Google Scholar]
- Berrueta L., Tirnauer J.S., Schuyler S.C., Pellman D., Bierer B.E. 1999. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 9:425–428 10.1016/S0960-9822(99)80190-0 [DOI] [PubMed] [Google Scholar]
- Bishop A.C., Shah K., Liu Y., Witucki L., Kung C., Shokat K.M. 1998. Design of allele-specific inhibitors to probe protein kinase signaling. Curr. Biol. 8:257–266 10.1016/S0960-9822(98)70198-8 [DOI] [PubMed] [Google Scholar]
- Burkard M.E., Randall C.L., Larochelle S., Zhang C., Shokat K.M., Fisher R.P., Jallepalli P.V. 2007. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 104:4383–4388 10.1073/pnas.0701140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A., Mandart E., Lorca T., Galas S. 2003. Involvement of Aurora A kinase during meiosis I-II transition in Xenopus oocytes. J. Biol. Chem. 278:2236–2241 10.1074/jbc.M207894200 [DOI] [PubMed] [Google Scholar]
- Chen X., Ye H., Kuruvilla R., Ramanan N., Scangos K.W., Zhang C., Johnson N.M., England P.M., Shokat K.M., Ginty D.D. 2005. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 46:13–21 10.1016/j.neuron.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Chen T.C., Lee S.A., Hong T.M., Shih J.Y., Lai J.M., Chiou H.Y., Yang S.C., Chan C.H., Kao C.Y., Yang P.C., Huang C.Y. 2009. From midbody protein-protein interaction network construction to novel regulators in cytokinesis. J. Proteome Res. 8:4943–4953 10.1021/pr900325f [DOI] [PubMed] [Google Scholar]
- Cremet J.Y., Descamps S., Vérite F., Martin A., Prigent C. 2003. Preparation and characterization of a human aurora-A kinase monoclonal antibody. Mol. Cell. Biochem. 243:123–131 10.1023/A:1021608012253 [DOI] [PubMed] [Google Scholar]
- Deacon S.W., Serpinskaya A.S., Vaughan P.S., Lopez Fanarraga M., Vernos I., Vaughan K.T., Gelfand V.I. 2003. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 160:297–301 10.1083/jcb.200210066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcros J.G., Prigent C., Giet R. 2006. Dynactin targets Pavarotti-KLP to the central spindle during anaphase and facilitates cytokinesis in Drosophila S2 cells. J. Cell Sci. 119:4431–4441 10.1242/jcs.03204 [DOI] [PubMed] [Google Scholar]
- De Luca M., Brunetto L., Asteriti I.A., Giubettini M., Lavia P., Guarguaglini G. 2008. Aurora-A and ch-TOG act in a common pathway in control of spindle pole integrity. Oncogene. 27:6539–6549 10.1038/onc.2008.252 [DOI] [PubMed] [Google Scholar]
- Dutertre S., Cazales M., Quaranta M., Froment C., Trabut V., Dozier C., Mirey G., Bouché J.P., Theis-Febvre N., Schmitt E., et al. 2004. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J. Cell Sci. 117:2523–2531 10.1242/jcs.01108 [DOI] [PubMed] [Google Scholar]
- Endo S., Satoh Y., Shah K., Takishima K. 2006. A single amino-acid change in ERK1/2 makes the enzyme susceptible to PP1 derivatives. Biochem. Biophys. Res. Commun. 341:261–265 10.1016/j.bbrc.2005.12.179 [DOI] [PubMed] [Google Scholar]
- Giet R., Glover D.M. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682 10.1083/jcb.152.4.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R., McLean D., Descamps S., Lee M.J., Raff J.W., Prigent C., Glover D.M. 2002. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156:437–451 10.1083/jcb.200108135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. 2009. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat. Rev. Mol. Cell Biol. 10:9–20 10.1038/nrm2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert T.M., Adigun Y.E., Zhong L., Gay J., Medina D., Brinkley W.R. 2002. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 62:4115–4122 [PubMed] [Google Scholar]
- Hannak E., Kirkham M., Hyman A.A., Oegema K. 2001. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155:1109–1116 10.1083/jcb.200108051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I., Wilde A., Mal T.K., Ikura M. 2005. Structural basis for the activation of microtubule assembly by the EB1 and p150Glued complex. Mol. Cell. 19:449–460 10.1016/j.molcel.2005.06.034 [DOI] [PubMed] [Google Scholar]
- Hégarat N., Smith E., Nayak G., Takeda S., Eyers P.A., Hochegger H. 2011. Aurora A and Aurora B jointly coordinate chromosome segregation and anaphase microtubule dynamics. J. Cell Biol. 195:1103–1113 10.1083/jcb.201105058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Kawashima T., Iwamoto I., Nosaka T., Kitamura T. 2001. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J. Biol. Chem. 276:5821–5828 10.1074/jbc.M007252200 [DOI] [PubMed] [Google Scholar]
- Jang C.Y., Fang G. 2011. DDA3 associates with MCAK and controls chromosome congression. Biochem. Biophys. Res. Commun. 407:610–614 10.1016/j.bbrc.2011.03.081 [DOI] [PubMed] [Google Scholar]
- Jang C.Y., Coppinger J.A., Seki A., Yates J.R., III, Fang G. 2009. Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J. Cell Sci. 122:1334–1341 10.1242/jcs.044321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenski D.M., Zhang C., von Zastrow M., Shokat K.M. 2005. Chemical genetic engineering of G protein-coupled receptor kinase 2. J. Biol. Chem. 280:35051–35061 10.1074/jbc.M507594200 [DOI] [PubMed] [Google Scholar]
- Kettenbach A.N., Schweppe D.K., Faherty B.K., Pechenick D., Pletnev A.A., Gerber S.A. 2011. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 4:rs5 10.1126/scisignal.2001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Kotani S., Hattori T., Sumi N., Yoshioka T., Todokoro K., Okano Y. 1997. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J. Biol. Chem. 272:13766–13771 10.1074/jbc.272.21.13766 [DOI] [PubMed] [Google Scholar]
- Kinoshita K., Noetzel T.L., Pelletier L., Mechtler K., Drechsel D.N., Schwager A., Lee M., Raff J.W., Hyman A.A. 2005. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 170:1047–1055 10.1083/jcb.200503023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa M.D., Casanova C.M., Santarella R., Köcher T., Wilm M., Mattaj I.W. 2006. HURP is part of a Ran-dependent complex involved in spindle formation. Curr. Biol. 16:743–754 10.1016/j.cub.2006.03.056 [DOI] [PubMed] [Google Scholar]
- Liu Y., Shah K., Yang F., Witucki L., Shokat K.M. 1998. Engineering Src family protein kinases with unnatural nucleotide specificity. Chem. Biol. 5:91–101 10.1016/S1074-5521(98)90143-0 [DOI] [PubMed] [Google Scholar]
- Macůrek L., Lindqvist A., Lim D., Lampson M.A., Klompmaker R., Freire R., Clouin C., Taylor S.S., Yaffe M.B., Medema R.H. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 455:119–123 10.1038/nature07185 [DOI] [PubMed] [Google Scholar]
- Manfredi M.G., Ecsedy J.A., Meetze K.A., Balani S.K., Burenkova O., Chen W., Galvin K.M., Hoar K.M., Huck J.J., LeRoy P.J., et al. 2007. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc. Natl. Acad. Sci. USA. 104:4106–4111 10.1073/pnas.0608798104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna T., Honnappa S., Steinmetz M.O., Wilson L. 2008. Suppression of microtubule dynamic instability by the +TIP protein EB1 and its modulation by the CAP-Gly domain of p150glued. Biochemistry. 47:779–786 10.1021/bi701912g [DOI] [PubMed] [Google Scholar]
- Maris J.M., Morton C.L., Gorlick R., Kolb E.A., Lock R., Carol H., Keir S.T., Reynolds C.P., Kang M.H., Wu J., et al. 2010. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr. Blood Cancer. 55:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T., Hirota T., Morisaki T., Kunitoku N., Zhang D., Ichikawa Y., Sasayama T., Kuninaka S., Mimori T., Tamaki N., et al. 2002. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 7:1173–1182 10.1046/j.1365-2443.2002.00592.x [DOI] [PubMed] [Google Scholar]
- Marumoto T., Honda S., Hara T., Nitta M., Hirota T., Kohmura E., Saya H. 2003. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J. Biol. Chem. 278:51786–51795 10.1074/jbc.M306275200 [DOI] [PubMed] [Google Scholar]
- Marumoto T., Zhang D., Saya H. 2005. Aurora-A - a guardian of poles. Nat. Rev. Cancer. 5:42–50 10.1038/nrc1526 [DOI] [PubMed] [Google Scholar]
- Mishima M., Kaitna S., Glotzer M. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell. 2:41–54 10.1016/S1534-5807(01)00110-1 [DOI] [PubMed] [Google Scholar]
- Mori D., Yano Y., Toyo-oka K., Yoshida N., Yamada M., Muramatsu M., Zhang D., Saya H., Toyoshima Y.Y., Kinoshita K., et al. 2007. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol. Cell. Biol. 27:352–367 10.1128/MCB.00878-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Velarde N.V., Piano F., Sugimoto A. 2006. Two phases of astral microtubule activity during cytokinesis in C. elegans embryos. Dev. Cell. 10:509–520 10.1016/j.devcel.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Pascreau G., Delcros J.G., Morin N., Prigent C., Arlot-Bonnemains Y. 2008. Aurora-A kinase Ser349 phosphorylation is required during Xenopus laevis oocyte maturation. Dev. Biol. 317:523–530 10.1016/j.ydbio.2008.02.053 [DOI] [PubMed] [Google Scholar]
- Reboutier D., Troadec M.B., Cremet J.Y., Fukasawa K., Prigent C. 2012. Nucleophosmin/B23 activates Aurora A at the centrosome through phosphorylation of serine 89. J. Cell Biol. 197:19–26 10.1083/jcb.201107134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghi C., Giet R., Uzbekov R., Morin N., Chartrain I., Le Guellec R., Couturier A., Dorée M., Philippe M., Prigent C. 1998. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111:557–572 [DOI] [PubMed] [Google Scholar]
- Romé P., Montembault E., Franck N., Pascal A., Glover D.M., Giet R. 2010. Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J. Cell Biol. 189:651–659 10.1083/jcb.201001144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane D.A., Trikic M.Z., Chu M.L., Lamers M.B., Mason C.S., Mueller I., Savory W.J., Williams D.H., Eyers P.A. 2010. Drug-resistant aurora A mutants for cellular target validation of the small molecule kinase inhibitors MLN8054 and MLN8237. ACS Chem. Biol. 5:563–576 10.1021/cb100053q [DOI] [PubMed] [Google Scholar]
- Strickland L.I., Wen Y., Gundersen G.G., Burgess D.R. 2005. Interaction between EB1 and p150glued is required for anaphase astral microtubule elongation and stimulation of cytokinesis. Curr. Biol. 15:2249–2255 10.1016/j.cub.2005.10.073 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ueda A., Kanamori H., Ideguchi H., Yang J., Kitajima S., Ishigatsubo Y. 2002. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J. Biol. Chem. 277:10719–10726 10.1074/jbc.M108252200 [DOI] [PubMed] [Google Scholar]
- Taylor S., Peters J.M. 2008. Polo and Aurora kinases: lessons derived from chemical biology. Curr. Opin. Cell Biol. 20:77–84 10.1016/j.ceb.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Tsai C.Y., Ngo B., Tapadia A., Hsu P.H., Wu G., Lee W.H. 2011. Aurora-A phosphorylates Augmin complex component Hice1 protein at an N-terminal serine/threonine cluster to modulate its microtubule binding activity during spindle assembly. J. Biol. Chem. 286:30097–30106 10.1074/jbc.M111.266767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R., Goshima G. 2010. Functional central spindle assembly requires de novo microtubule generation in the interchromosomal region during anaphase. J. Cell Biol. 191:259–267 10.1083/jcb.201004150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R., Nozawa R.S., Tomioka A., Petry S., Vale R.D., Obuse C., Goshima G. 2009. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 106:6998–7003 10.1073/pnas.0901587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L.T. 2006. Cell cycle synchronization at the G2/M phase border by reversible inhibition of CDK1. Cell Cycle. 5:2555–2556 10.4161/cc.5.22.3463 [DOI] [PubMed] [Google Scholar]
- Vaughan K.T., Vallee R.B. 1995. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131:1507–1516 10.1083/jcb.131.6.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P.S., Miura P., Henderson M., Byrne B., Vaughan K.T. 2002. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J. Cell Biol. 158:305–319 10.1083/jcb.200201029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.T., Hsu J.M., Lee Y.C., Tsou A.P., Chou C.K., Huang C.Y. 2005. Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A. Mol. Cell. Biol. 25:5789–5800 10.1128/MCB.25.14.5789-5800.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ems-McClung S.C., Walczak C.E. 2008. Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Mol. Biol. Cell. 19:2752–2765 10.1091/mbc.E08-02-0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.