Abstract
Our understanding of cancer risk from ionizing radiation is largely based on studies of populations exposed at high dose and high dose rates. Less certain is the magnitude of cancer risk from protracted, low-dose and low-dose-rate radiation exposure. We estimated the dose-response relationship for solid cancer mortality in a cohort of 29,730 individuals who lived along the Techa River between 1950 and 1960. This population was exposed to both external γ radiation and internal 90Sr, 137Cs and other radionuclides after the release of radioactive waste into the river by the Mayak Radiochemical Plant. The analysis utilized the latest individualized doses from the Techa River Dosimetry System (TRDS) 2009. We estimated excess relative risks (ERRs) per Gy for solid cancer mortality using Poisson regression methods with 95% confidence intervals (CIs) and P values based on likelihood ratio tests. Between 1950 and 2007, there were 2,303 solid cancer deaths. The linear ERR/Gy = 0.61 (95%; CI 0.04–1.27), P = 0.03. It is estimated that approximately 2% (49.7) of solid cancers deaths were associated with the radiation exposure. Our results, based on 2,303 solid cancer deaths and more than 50 years of follow-up, support an increased risk of solid cancer mortality following protracted radiation exposure from the Techa River contamination. The wide confidence interval of our estimate reflects the challenges of quantifying and describing the shape of the dose-response relationship in the low dose range. Nevertheless, the risk estimates provide important information concerning health risks from whole-body radiation exposure that can occur from accidents that result in wide-scale environmental contamination.
INTRODUCTION
Our understanding of the dose response for cancer risk from ionizing radiation is largely based on studies of populations exposed at high dose and high dose rates, including patients treated with radiotherapy for benign and malignant disease and atomic bomb survivors (1–4). Less certain is the magnitude of risk from protracted, low-dose and low-dose-rate radiation exposure, particularly among general populations, as occurs from environmental radiation exposure (4).
Between 1949 and 1956, the Mayak Radiochemical Plant released approximately 1017 Bq of uranium fission products into the Techa River in the Southern Urals, with 95% of the releases between September 1950 and November 1951 (5, 6). Those residing along the river received external γ-radiation exposure from contaminated river sediments and flood plain soil and internal exposure from 90Sr, 137Cs and other radionuclides from the consumption of contaminated water, milk and food products. The first investigation of radioactive contamination of water and bottom sediments in the Techa River was performed in June 1950, and systematic monitoring of the water and sediment contamination started in July 1951 (5).
In 1967, the Urals Research Center for Radiation Medicine (URCRM) initiated a study of the long-term health effects of protracted low-dose-rate environmental radiation exposure in this population (7). The Techa River Cohort (TRC) [previously referred to as the Extended Techa River Cohort (8)] study provides a unique opportunity to quantify the long-term effects of chronic, low-dose-rate exposure in a large, unselected population.
Analyses of all solid cancer mortality from 1950–1999 (8) demonstrated a clear dose-response relationship based on dose estimates computed using the Techa River Dosimetry System-2000 (TRDS-2000) (9, 10). Statistically significant dose-response functions have also been reported for solid cancer and leukemia incidence (11, 12). Here, we estimate the dose-response relationship for solid cancer mortality using dose estimates from a revised dosimetry system, TRDS-2009 (13), and include an additional 8 years of follow-up. We also compare these results with those based on TRDS-2000 and evaluate the effect of the additional follow-up. The excess risk of all solid cancers from radiation is an important measure in this context of whole-body exposure (4).
MATERIALS AND METHODS
The methods used to establish the cohort and to carry out follow-up for mortality and cancer incidence among TRC members have been described in detail (7, 8). This study was approved by the Institutional Review Board of the Urals Research Center for Radiation Medicine
Cohort Definition
Individuals who were born before 1950 and lived in any of the 41 villages situated along the Techa River between 1950–1960 were eligible for inclusion in the TRC. URCRM conducted extensive review of official documents (including taxation books, vital statistics and medical records) between the late 1960s and the 1980s to identify cohort members. As of October 2010, the TRC numbered 29,730 persons, with women accounting for a somewhat greater proportion (58%) of cohort members than men. Approximately 40% of the cohort was first exposed before age 20, 28% at ages 20–40 and 32% after age 40 years. The cohort is 80% Slav and 20% Tatar and Bashkir.
Cohort Follow-Up and Ascertainment of Cancer Mortality
URCRM staff conducted regular, systematic follow-up of the cohort to ascertain vital status and cause of death for deceased members. The catchment area for deaths included Chelyabinsk Oblast (region) and Kurgan Oblast. At the end of follow-up, approximately 16% of the cohort had migrated out of the catchment area (i.e., distal migrants). The primary sources of vital status information are the regional address bureaus and the office of the civil registrar but additional sources of information included the “Unified Computer Registry ‘Mayak’” (which provided vital status and cause of death information for exposed residents of Techa riverside villages who continue to reside in areas near the Techa) and reports from next-of-kin who are seen by URCRM staff during clinical and field examinations. Cause of death as ascertained from review of death certificates was available for 91% of the deceased non-migrants and was coded using ICD-9 by trained URCRM nosologists. Solid cancers (ICD9 codes 140–199 and 239.6) listed as an underlying cause of death were considered cases for the solid cancer mortality analyses. Table 1 provides an overview of the vital status and availability of death certificates for the present analyses.
TABLE 1.
Follow-Up Status for the Techa River Cohort as of December 31, 2007
| Status at end of follow-up | People |
|---|---|
| Alive in catchment area | 5,684 |
| Dead | 17,307 |
| % Known cause | 91% |
| Lost to follow-upa | 6,739 |
| Total | 29,730 |
Lost to follow-up includes 4,696 distal migrants and 2,043 nonmigrants who were last known to be living in the catchment area prior to the end of 2006. All people whose date of last know vital status was 2007 were treated as being alive at the end of 2007.
Dosimetry
Radiation exposures to the TRC members resulted from a combination of external γ-radiation exposure from contaminated river sediments and flood plain soil and internal exposure from the consumption of contaminated water, milk and food products. Internal exposures were primarily from 90Sr and 137Cs but also 89Sr and to a lesser extent, from other uranium fission products. Dose was unevenly distributed throughout the body due to the intake of 89,90Sr, which resulted in elevated levels of absorbed dose to the bone marrow, bone surfaces and the large intestine. Compared to other soft tissues, the intestines also received a larger dose from radionuclides with poor intestinal absorption (such as 144Ce, 95Zr, 95Nb). Exposures to other tissues, mainly from external exposures and 137Cs intake, were lower and relatively homogenous.
Within the entire TRC, 16% of members were also exposed in the East Urals Radioactive Trace (EURT), an area that was contaminated by an accidental discharge of 7.4 × 1016 Bq of uranium fission products into the atmosphere from the Mayak in September 1957 (14). Recent dose assessments, however, indicate that dose received from presence on the EURT was a small fraction of the total dose received by TRC members (14).
The Techa River Dosimetry System (TRDS) was developed to support epidemiologic studies of the TRC (9, 10, 15). The TRDS makes use of large numbers of measurements of long-lived radionuclides in the human body and in the environment, as well as measurements of external exposure rates in places where the TRC members lived. The traditional approach of analyzing all steps of the pathway of exposure is only used as a backup when other approaches are exhausted, in particular for reconstruction of doses in the first two years of exposure. Earlier analyses of solid cancers risks in the cohort (8) made use of the TRDS version created in 2000 (TRDS-2000) (9, 10). An improved dosimetry system (TRDS-2009) was used for the current analyses, and recent improvements in the dosimetry have been described in refs. (5, 6, 15–18). Here, we briefly summarize the essence of dose reconstruction efforts.
External exposure was the major pathway for residents of villages located in the upper Techa region close to the site of the releases. The external dose rate peaked in 1951 and subsequently declined with time. Doses from external exposure decreased markedly with the distance along the Techa River. Additional factors that influenced the external dose in a particular village were the distance of the household from the contaminated river shore and the steepness of the riverbank. Doses also depended on age-dependent lifestyle factors. The conversion factors used to convert absorbed dose in air to absorbed dose in organs were also age-dependent. The TRDS makes use of village-average external dose rates near the shoreline and within residential areas together with individual information about age and residence history to provide annual age-dependent site-specific estimates of individual external dose. Small differences in external dose estimates between the previous and current dosimetry systems occurred due to improved parameters of the source term that have been used in TRDS-2009 and described recently (5).
Major pathways of internal exposure of the TRC members were intakes of radionuclides into the body through the consumption of the Techa River water and cow milk. Radionuclide intake decreased with the distance from the release site and also depended on the availability of drinking water sources other than the contaminated river (such as wells) in the riverside villages (17, 18). The intake estimates were derived from numerous data on tooth-beta counts and whole-body counts (WBC) for the TRC members. From the data, it is possible to derive three kinds of estimates: (1) individual estimates for those who have WBC measurements; (2) household-mean estimates based on WBC data for inhabitants of the same household; and (3) village-mean estimates based on WBC data for residents of the same village (16). Thus, it is possible to assign household-mean or village-mean intake estimates for unmeasured TRC members. A detailed description of the methodology used for intake reconstruction is given in refs. (17, 18).
While TRDS-2000 made use of individual age and residence history data to provide annual age-dependent site-specific estimates of individual internal dose for all members of the TRC, the system did not directly use the results of individual WBC measurements of internal exposure. In the TRDS-2009, individual data on the results of WBC measurements of a person and his/her cohabitants were used to provide individual or household-average internal exposure estimates.
Statistical Methods
Cohort members accrued person-time from after January 1, 1950, or the date the individual first lived in a riverside village until the date of death, date of migration out of the Chelyabinsk or Kurgan oblasts, date of last known vital status or December 31, 2007, whichever occurred first. Individuals who moved in and out of the catchment area only contributed person-years to the follow-up during periods of residence in the catchment area (Chelyabinsk or Kurgan oblasts). We used stomach dose as a representative dose in these analyses of total solid cancer mortality. In light of the non-uniformities of internal dose as noted above, one might expect colon and bone cancer risks to differ from those for other solid cancers. Therefore, we also conducted an analysis based on solid cancers excluding these two sites.
Data organization
The data were organized as a highly stratified person-year table in which person-years and cases were stratified on both TRDS-2009 and TRDS-2000 cumulative stomach dose estimates with a five-year lag. There was a zero dose category (which included the first five years of follow-up for each person) and 13 additional dose categories with lower bounds 0, 0.002, 0.004, 0.008, 0.01, 0.05, 0.075, 0.1, 0.15, 0.2, 0.25, 0.3 and 0.5 Gy. Additional stratifying factors were gender, ethnicity, entry period (1950–1952, 1953–1960), calendar time (13 categories with cut points at January 1st of 1950, 1953, 1956, 1960, 1965, 1970, 1975, 1980, 1985, 1990, 1995, 2000 and 2005), attained age (16 five-year categories for ages 0–79 and a ≥80 years category), age at entry (8 categories with cut points at 10, 15, 20, 30, 40, 50 and 60), and time since first exposure (11 categories with cut points at 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 years).
Excess relative risk models
Analyses were conducted using Poisson regression methods [Epicure, AMFIT module (19)]. All analyses were based on parametric baseline rate models with radiation effects modeled in terms of excess relative risk (ERR) models. The basic linear dose-response model had the form λ0(a,s,e,b,o)(1 βD). The baseline rate, λ0(a,s,e,b,o), is described as a function of attained age (a), sex (s), ethnicity (e), birth cohort (b), and oblast of initial exposure (o). βD estimated the ERR as a function of cumulative 5-year lagged dose from contamination of the Techa River. We also considered models in which the ERR was linear-quadratic or quadratic in dose, or was allowed to vary across dose categories and present some of these findings in the Results section. We evaluated potential modification of the ERR by factors including attained age, age at entry, gender and time since entry using models of the form λ0(a,s,e,b,o)(1 + βcDeγf(z)) where βc indicates that the dose effect might differ for different groups (e.g., men and women) and f(z) is a function of some factors of interest (e.g., attained age, age at exposure or time since exposure). For comparison, we fit models using TRDS-2009 and TRDS-2000 doses. We also fit models in which the follow-up period was restricted to December 31, 1999 to examine the effect of additional follow-up time in the current analysis. Hypothesis tests and confidence intervals were based on likelihood ratio tests and direct evaluation of the profile likelihood. In some cases, we also present the asymptotic standard error after the ERR as indicated by the “±” sign. Two-sided P < 0.05 were considered statistically significant.
RESULTS
During the follow-up period, 1950–2007, there were 2,303 solid cancer deaths registered in the mortality catchment area with 927,743 person-years. Among men, lung, stomach and esophagus cancers were the most frequent causes of solid cancer death. For women, stomach, uterine (corpus and cervix) and breast cancers accounted for the largest number of solid cancer deaths. Cohort distribution and crude solid cancer mortality rates by select characteristics are presented in Table 2.
TABLE 2.
Cohort Distribution and Crude Solid Cancer Mortality Rates by Select Characteristics Among Techa River Cohort Members
| Characteristic | Person-years | Solid cancer cases | Rate per 10,000 person years |
|---|---|---|---|
| Total | 927,743 | 2,303 | 24.8 |
| Sex | |||
| Men | 373,599 | 1,188 | 31.8 |
| Women | 554,144 | 1,115 | 20.1 |
| Age at entry (year) | |||
| 0–19 | 438,753 | 558 | 12.7 |
| 20–39 | 327,647 | 951 | 29.0 |
| 40+ | 161,343 | 794 | 49.2 |
| Entry oblast | |||
| Chelyabinsk | 620,809 | 1,654 | 26.6 |
| Kurgan | 306,933 | 649 | 21.1 |
| Entry period | |||
| 1950–1952 | 795,920 | 1,941 | 24.4 |
| 1953–1960 | 131,823 | 362 | 27.5 |
| Ethnicity | |||
| Slav | 706,698 | 1,803 | 25.5 |
| Tatar/Bashkir | 221,045 | 500 | 22.6 |
| Attained age (year) | |||
| 0–19 | 100,137 | 3 | 0.3 |
| 20–39 | 254,725 | 67 | 2.6 |
| 40–59 | 331,223 | 687 | 20.7 |
| 60–69 | 136,055 | 760 | 55.9 |
| 70–79 | 77,622 | 613 | 79.0 |
| 80+ | 27,981 | 173 | 61.8 |
Baseline risk
The baseline rate was described by a model with gender-specific log-quadratic splines in log-attained age, a log-linear birth cohort effect (independent of gender), a multiplicative effect for ethnicity (Slav vs. Tartar-Bashkir), a binary time period effect (1970–1989 vs. other time periods) and an age-dependent oblast effect. The highly significant (P < 0.001) difference in age-specific cancer rates in Chelyabinsk and Kurgan Oblast (independent of exposure) has been noted previously (8). Further examination of the baseline rates suggested that this difference was more pronounced at older ages. In particular, solid cancer death rates for Kurgan residents under age 70 were estimated to be approximately 16% less than those for Chelyabinsk residents (P = 0.004) but were approximately 45% less than those for Chelyabinsk (P < 0.001) after age 70. These differences were independent of dose, but failure to allow for this effect led to an overestimation of the ERR per Gy (using either TRDS-2009 or TRDS-2000), because doses for TRC members exposed in Kurgan were much lower than for those exposed in Chelyabinsk. The quadratic splines in log attained age captured the marked decline in rates after age 80.
Excess relative risk from radiation
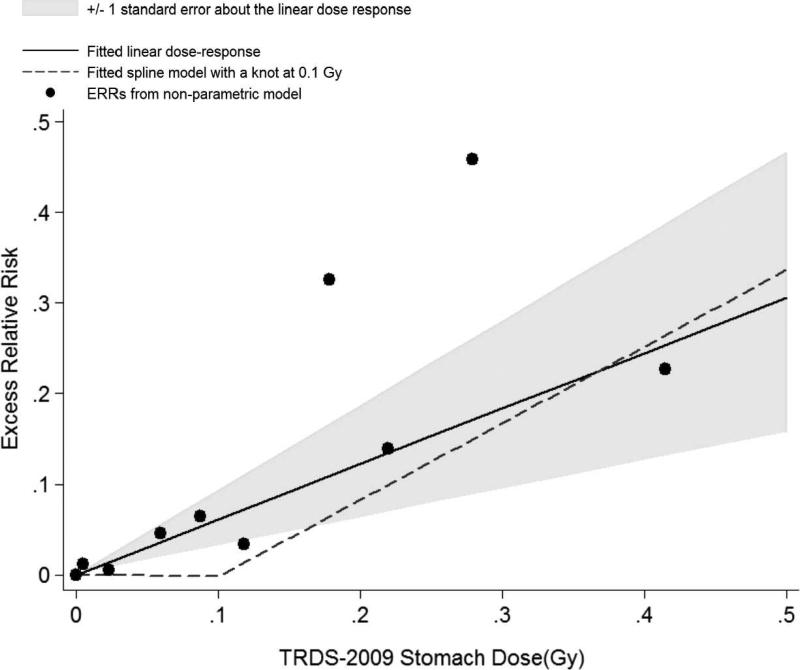
The mean stomach cumulative doses five years prior to the end of follow-up was 0.035 (range 0–0.96 Gy) in TRDS-2009 and 0.028 (range 0–0.44 Gy) in TRDS-2000. The ERR as a function of TRDS-2009 doses is shown in Fig. 1. The ERR/Gy for all solid cancers was 0.61 (± 0.31; 95% CI 0.04–1.27; P = 0.03). While allowing for a linear-quadratic dose response provided no evidence against linearity (P > 0.5), a pure quadratic dose response described the data as well as a simple linear model. The maximum likelihood estimate for a threshold was 0.05 Gy but there was no evidence to support a non-zero threshold; the P value for the test that the threshold is 0 was 0.46. As less than 10% of the person-years were at doses ≥0.1 Gy, we also fit a spline model with a knot at 0.1 Gy to examine the influence of that 10% of the data on the linear fit. The dose response below the knot was –0.02 with large uncertainty (95% CI <0–2.2) and that above the knot above 0.1 was 0.86 (95% CI 0.14–1.87). This spline model did not fit the data significantly better than a simple linear dose response model (P > 0.5). Figure 1 includes the fitted linear dose-response function and the spline model together with nonparametric estimates of the dose response based on a categorical dose-response model. Based on the linear ERR model, it is estimated that about 2% (49.7) of solid cancers deaths were associated with the radiation exposure (Table 3). The ERR estimates in linear dose-response models did not change greatly when the analyses excluded the 22 bone and 73 colon cancers (ERR/Gy 0.54, P = 0.07).
FIG. 1.
Solid cancer mortality dose response based on TRDS-2009 stomach doses. Circles represent ERR by dose categories from a nonparametric model. Fitted linear dose response (solid line) and fitted spline model with a knot at 0.1 Gy (dashed line) is also shown. The shaded area indicates the uncertainty (±1 standard error) about the linear dose response.
TABLE 3.
Comparison of Fitted Background and Excess Solid Cancer Cases in the Techa River Cohort by Dose Category Using Techa River Dosimetry System-2009 and Techa River Dosimetry-2000
| TRDS-2009 |
TRDS-2000 |
|||||
|---|---|---|---|---|---|---|
| Dose (Gy) Category | Person-years | Excess cases | Background cases | Person-years | Excess cases | Background cases |
| 0 | 132,593 | 0 | 149.7 | 132,669 | 0 | 149.9 |
| <0.01 | 386,880 | 2.9 | 951.9 | 535,211 | 3.9 | 1430.4 |
| 0.01 – <0.05 | 273,436 | 10.2 | 777.9 | 173,454 | 7.6 | 453.2 |
| 0.05 – <0.1 | 63,297 | 6.8 | 174.4 | 21,484 | 2.3 | 50.8 |
| 0.1 – <0.15 | 27,651 | 5.8 | 80.3 | 26,451 | 6.1 | 73.5 |
| 0.15 – <0.3 | 21,707 | 6.5 | 51.5 | 16,626 | 5.0 | 34.1 |
| 0.3 – <0.5 | 21,074 | 16.1 | 63.7 | 21,848 | 18.0 | 68.2 |
| ≥0.5 | 1105 | 1.4 | 4.0 | 0 | 0 | 0 |
| Total | 927,743 | 49.7 | 2,253.4 | 927,743 | 42.9 | 2260.1 |
Note. TRDS, Techa River dosimetry system.
The risk estimate based on the TRDS-2000 doses [ERR/Gy = 0.66 (±0.33; 95% CI 0.07–1.34; P = 0.03)] was similar to that obtained using TRDS-2009. There was virtually no difference in the estimated number of radiation-associated cases (Table 3). Irrespective of dosimetry system, the ERR/Gy was increased when the period of follow-up was restricted to 1999, as in ref. (8). Using the current data with follow-up through 1999, the ERR/Gy estimate was 0.90 (±0.37; 95% CI 0.23–1.68) when the TRDS-2009 doses were used and 0.96 (±0.38; 95% CI 0.27– 1.77) using the TRDS-2000 doses.
We also examined whether the ERR/Gy, based on TRDS-2009, varied by age at entry, attained age, time since entry or gender. There were suggestions that the ERR/Gy increased with either older age at first exposure (P = 0.05) or older attained age (P = 0.10). When age at first exposure was used as an effect modifier, the ERR/Gy was estimated to increase by a factor of 2.2 (95% CI 1.0–8.0) for each decade increase in age at first entry. With log attained age as an effect modifier, the increase in the ERR/Gy with increasing age was estimated to be proportional to age to the power 5.1 (95% CI –0.7–16.9). Estimates of the attained age effect on the ERR/Gy depended rather markedly on how the effects of attained age and oblast differences were addressed in the baseline rate model; this complicates the quantification and interpretation of this effect. Using a model that stratified on age, gender and oblast, rather than modeling those parameters, the linear ERR with no effect modification was unchanged as was the age-at-entry effect. The attained age effect on the ERR decreased to approximately half that observed in the main model and the P value increased, but the estimate remained positive. There were no indications of statistically significant variability in the ERR/Gy with time since first exposure (P = 0.2), gender (P > 0.5) or ethnicity (P = 0.3).
DISCUSSION
The Techa River Cohort is one of the few studies in which the long-term effects of chronic, low-dose-rate radiation exposure can be evaluated for a large, unselected population. Our results, based on 2,303 solid cancer deaths and more than 50 years of follow-up, support an increased risk of solid cancer mortality following protracted whole-body radiation exposure from the Techa River contamination. The data were well described by a linear dose-response model. While neither pure quadratic nor spline dose-response models led to significant improvement, they suggest that the risk could be lower than the linear model risk at doses below 0.1 Gy. Uncertainty in the shape of the dose-response curve reflects the challenge of quantifying and describing the relationship in the low dose range; over 90% of the person-years were at doses below 0.1 Gy.
As evident from the similar estimates of the ERR/Gy obtained from TRDS-2009 (0.61; 95% CI 0.04–1.27) and TRDS-2000 (0.66; 95% CI 0.07–1.34), improvements to the dosimetry system had little impact on the risk estimates. This is not surprising given the similarity of typical doses to most soft tissues (such as stomach and lung) in TRDS-2000 and TRDS-2009 (13). Improvements in the TRDS-2009 system primarily had an impact on the internal dose estimates and thus had a greater impact on bone (surface and marrow) dose (13). Updated leukemia risk estimates as well as solid cancer incidence risks will be presented in subsequent manuscripts. Efforts are also underway to better characterize the uncertainty in individual dose estimates. The effect of such uncertainties on risk estimates will be addressed in future analyses using the Monte Carlo dosimetry-based system that is being developed at this time.
The additional follow-up had a greater effect on the risk estimates. Using both TRDS-2009 and TRDS-2000, the ERR/Gy was increased when the period of follow-up was restricted to 1999 [the follow-up in ref. (8)]. This may be explained in part by the shifting age distribution between the old and new follow-up periods. Cohort members who were younger at entry now contributed more cases to the analysis. Although age at initial exposure did not significantly modify the risk estimate, there was a suggestion that the ERR was lower for those who were younger at the time of their initial exposure than for those who were initially exposed later in life. The observed differences could also be due to chance, as the estimates based on restricted and full follow-up periods differ by only one standard error.
Our analysis of effect modification by attained age and age at entry suggests that the ERR/Gy may be increasing with increasing attained age or age at exposure. Although these effects were not statistically significant, these patterns were in the opposite direction of those observed in the atomic bomb survivors data (2, 3) and the Mayak Worker Cohort (20). However, an increase of the ERR with increasing attained age and age at first exposure has been reported in some studies of nuclear workers (21). Concerned that under-ascertainment of baseline cancer rates at older ages could affect the inference about age effects on the ERR, we conducted analyses restricted to attained age <80 but this did not change the age pattern of the ERR. Similar patterns were also observed using a baseline model that stratified on age, gender and oblast, rather than modeling those parameters. Our results concerning modification of the dose response should be interpreted cautiously. It should also be noted that it is not possible to separate the effects of age at entry or attained age in this population due to their strong correlation.
As discussed at length in ref. (8), there has been concern about possible confounding of the environmental exposure ERR by diagnostic radiation exposure. TRDS-2009 includes doses from diagnostic medical exposures conducted at the URCRM clinic for approximately 22% of the cohort. Routine annual chest fluorography, as was conducted in the former Soviet Union, was not included in the medical dose estimates (22). Preliminary analyses of medical exposure produced extremely large ERR estimates with respect to solid cancer mortality. This suggests that medical diagnostic dose could be more of an indication of ill health than a cause of subsequent cancer risk and therefore it may not be appropriate to adjust for it in analyses of environmental exposure. Methodological issues related to medical dose data will be considered in a separate manuscript.
In the context of whole-body exposure, as occurred in the Techa River Cohort, total solid cancer incidence or mortality is a commonly studied outcome and such estimates form the basis of radiation protection standards (23). Analyses of all solid cancer combined, however, do not take into account heterogeneity of the magnitude and/or shape of the dose-response curve across tumor sites. Unfortunately, power to predict site-specific risk estimates is extremely limited in the Techa River Cohort and estimated site-specific risks have very large uncertainty, as demonstrated in a recent publication that included data from the TRC based on follow-up through 2003 (24).
Loss to follow-up, largely due to migration, is a limitation of this study. Due in large part to data access restrictions, information on vital status or cause of death for individuals who migrated out of the catchment area was not widely available. Therefore, it was necessary to censor these individuals at the time of migration. Although loss to follow-up could potentially bias the ERR, it would have to depend on both radiation dose and cancer mortality risk for such a bias to occur. In other words, within a given level of exposure, the migration or loss to follow-up patterns would have to differ between those who did and did not subsequently die of solid cancer. Further, these differences would have to vary between dose groups. There was little variation in the mean dose between those who were lost to follow-up, those who were known to have migrated and those who were neither lost nor had migrated. Nonetheless, the potential for bias cannot be completely dismissed. Missing cause of death for approximately 9% of the deaths is another limitation, but there were no consistent patterns by dose. The proportion of deaths with missing cause has declined over calendar time, likely reflecting general societal improvements rather than some function of dose. Death certificate coding regulations and practices have also changed over calendar time, likely accounting for some of the fluctuations in cancer mortality rates in Russia in general, particularly among the elderly (25). The loss of solid cancer cases due to migration, missing cause of death, and underreporting of cancer deaths led to some reduction in the precision of our estimates.
There are also several strengths of this study including the range of doses, inclusion of males and females at all ages at exposure, and long-term follow-up (1950–2007). Another strength of this study is the latest dosimetry system, which is based on more individual-level data than the previous system and addresses previous concerns about the source term (26).
In summary, data from the TRC study demonstrate that all solid cancer mortality risk increases with radiation dose. The data were consistent with a linear dose-response model, but could also be described by models in which the risk at low doses (<0.1 Gy) is less than that predicted by the linear model. While challenges remain to quantify and describe the dose-response relationship at low doses, risk estimates from this study provide important information concerning health risks from whole-body radiation exposure as can occur from accidents resulting in wide-scale environmental contamination.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The Federal Medical Biological Agency of the Russian Federation has also provided support for this study. The authors thank the U.S. Department of Energy (DOE) under the auspices of the Joint Coordinating Committee for Radiation Effects Research, which supports the dosimetry program. We acknowledge the many contributions of Elaine Ron, Catherine Zhidkova, Nikolai Startsev and Mira Kossenko.
REFERENCES
- 1.Ron E. Cancer risks from medical radiation. Health Phys. 2003;85(1):47–59. doi: 10.1097/00004032-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 3.Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162(4):377–89. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]
- 4.National Research Council (U.S.) Health risks from exposure to low levels of ionizing radiation: Beir VII Phase 2. National Academies Press; Washington, D.C.: 2006. Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. [PubMed] [Google Scholar]
- 5.Degteva MO, Shagina NB, Vorobiova MI, Anspaugh LR, Napier BA. Reevaluation of waterborne releases of radioactive materials from the Mayak production association into the Techa River in 1949–1951. Health Phys. 2012;102(1):25–38. doi: 10.1097/HP.0b013e318228159a. [DOI] [PubMed] [Google Scholar]
- 6.Shagina NB, Vorobiova MI, Degteva MO, Peremyslova LM, Shishkina EA, Anspaugh LR, et al. Reconstruction of the contamination of the Techa River in 1949–1951 as a result of releases from the “MAYAK” Production Association. Radiat Environ Biophys. 2012 doi: 10.1007/s00411-012-0414-0. [DOI] [PubMed] [Google Scholar]
- 7.Kossenko MM, Thomas TL, Akleyev AV, Krestinina LY, Startsev NV, Vyushkova OV, et al. The Techa River Cohort: Study design and follow-up methods. Radiat Res. 2005;164(5):591–601. doi: 10.1667/rr3451.1. [DOI] [PubMed] [Google Scholar]
- 8.Krestinina LY, Preston DL, Ostroumova EV, Degteva MO, Ron E, Vyushkova OV, et al. Protracted radiation exposure and cancer mortality in the Techa River Cohort. Radiat Res. 2005;164(5):602–11. doi: 10.1667/rr3452.1. [DOI] [PubMed] [Google Scholar]
- 9.Degteva MO, Vorobiova MI, Kozheurov VP, Tolstykh EI, Anspaugh LR, Napier BA. Dose reconstruction system for the exposed population living along the Techa River. Health Phys. 2000;78(5):542–54. doi: 10.1097/00004032-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Degteva MO, Vorobiova MI, Tolstykh EI, Shagina NB, Shishkina EA, Anspaugh LR, et al. Development of an improved dose reconstruction system for the Techa River population affected by the operation of the Mayak Production Association. Radiat Res. 2006;166:255–70. doi: 10.1667/RR3438.1. [DOI] [PubMed] [Google Scholar]
- 11.Krestinina L, Preston DL, Davis FG, Epifanova S, Ostroumova E, Ron E, et al. Leukemia incidence among people exposed to chronic radiation from the contaminated Techa River, 1953–2005. Radiat Environ Biophys. 2010;49(2):195–201. doi: 10.1007/s00411-009-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krestinina LY, Davis F, Ostroumova E, Epifanova S, Degteva M, Preston D, et al. Solid cancer incidence and low-dose-rate radiation exposures in the Techa River Cohort: 1956–2002. Int J Epidemiol. 2007;36(5):1038–46. doi: 10.1093/ije/dym121. [DOI] [PubMed] [Google Scholar]
- 13.Degteva MO, Shagina NB, Tolstykh EI, Vorobiova MI, Anspaugh LR, Napier BA. Individual dose calculations with use of the revised Techa River Dosimetry System TRDS-2009d; final report for milestone 22. Urals Research Center for Radiation Medicine and University of Utah; Chelyabinsk and Salt Lake City: 2009. [Google Scholar]
- 14.Degteva MO, Tolstykh EI, MI V, Shagina NB, Anspaugh LR, Napier BA. Comparative analysis of the possible impact of different sources of environmental and medical exposure for members of the Techa River Cohorts; final report for milestone 14. Urals Research Center for Radiation Medicine and University of Utah; Chelyabinsk and Salt Lake City: 2007. [Google Scholar]
- 15.Degteva MO, Tolstykh EI, Vorobiova MI, Shagina NB, Shishkina EA, Bougrov NG. Techa River Dosimetry System: Current status and future. Radiat Safety Prob. 2006;1:66–80. [Google Scholar]
- 16.Degteva MO, Shagina NB, Tolstykh EI, Bougrov NG, Zalyapin VI, Anspaugh LR, et al. An approach to reduction of uncertainties in internal doses reconstructed for the Techa River population. Radiat Prot Dosim. 2007;127(1–4):480–5. doi: 10.1093/rpd/ncm410. [DOI] [PubMed] [Google Scholar]
- 17.Tolstykh EI, Degteva MO, Peremyslova LM, Shagina NB, Shishkina EA, Krivoshchapov VA, et al. Reconstruction of long-lived radionuclide intakes for Techa Riverside residents: Strontium-90. Health Phys. 2011;101(1):28–47. doi: 10.1097/HP.0b013e318206d0ff. [DOI] [PubMed] [Google Scholar]
- 18.Tolstykh EI, Degteva MO, Vorobiova MI, Peremyslova LM, Shagina NB, Anspaugh LR, et al. Reconstruction of long-lived radionuclide intakes for Techa Riverside residents. Radiat Safety Prob. 2006;1:68–79. [Google Scholar]
- 19.Preston D, Lubin J, Pierce D, McConney M. Epicure user's guide. HiroSoft International Corporation; Seattle, WA: 1993. [Google Scholar]
- 20.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159(6):787–98. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat Res. 2007;167(4):396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 22.Degteva MO, Shagina NB, Vorobiova MI, Golikov VY, Barkovsky AN, Kozyrev AV, et al. Reconstruction of individual medical doses for members of the Extended Techa River Cohort; final report for milestone 19. Urals Research Center for Radiation Medicine and University of Utah; Chelyabinsk and Salt Lake City: 2007. [Google Scholar]
- 23.The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Preston DL, Krestinina LY, Sokolnikov ME, Ron E, Davis FG, Ostroumova EV, et al. How much can we say about site-specific cancer radiation risks? Radiat Res. 2010;174(6):816–24. doi: 10.1667/RR2024.1. [DOI] [PubMed] [Google Scholar]
- 25.Shkolnikov VM, McKee M, Vallin J, Aksel E, Leon D, Chenet L, et al. Cancer mortality in Russia and Ukraine: Validity, competing risks and cohort effects. Int J Epidemiol. 1999;28(1):19–29. doi: 10.1093/ije/28.1.19. [DOI] [PubMed] [Google Scholar]
- 26.Balonov M, Alexakhin R, Bouville A, Liljinzin JO. Report from the Techa River Dosimetry review workshop held on 8–10 December 2003 at the State Research Centre Institute of Biophysics, Moscow, Russia. Health Phys. 2006;90(2):97–113. doi: 10.1097/01.hp.0000175628.64637.8c. [DOI] [PubMed] [Google Scholar]