Abstract
We have proposed a model of motor lateralization, in which the left and right hemispheres are specialized for different aspects of motor control: the left hemisphere for predicting and accounting for limb dynamics and the right hemisphere for stabilizing limb position through impedance control mechanisms. Our previous studies, demonstrating different motor deficits in the ipsilesional arm of stroke patients with left or right hemisphere damage, provided a critical test of our model. However, motor deficits after stroke are most prominent on the contralesional side. Post-stroke rehabilitation has also, naturally, focused on improving contralesional arm impairment and function. Understanding whether contralesional motor deficits differ depending on the hemisphere of damage is, therefore, of vital importance for assessing the impact of brain damage on function and also for designing rehabilitation interventions specific to laterality of damage. We, therefore, asked whether motor deficits in the contralesional arm of unilateral stroke patients reflect hemisphere-dependent control mechanisms. Because our model of lateralization predicts that contralesional deficits will differ depending on the hemisphere of damage, this study also served as an essential assessment of our model. Stroke patients with mild to moderate hemiparesis in either the left or right arm because of contralateral stroke and healthy control subjects performed targeted multi-joint reaching movements in different directions. As predicted, our results indicated a double dissociation; although left hemisphere damage was associated with greater errors in trajectory curvature and movement direction, errors in movement extent were greatest after right hemisphere damage. Thus, our results provide the first demonstration of hemisphere specific motor control deficits in the contralesional arm of stroke patients. Our results also suggest that it is critical to consider the differential deficits induced by right or left hemisphere lesions to enhance post-stroke rehabilitation interventions.
Keywords: lateralization, stroke, motor control, reaching movements, impairment
Introduction
A large body of research has now established that the two cerebral hemispheres show a considerable degree of lateralization or a specialization for controlling different aspects of behaviour. Although such neural lateralization has been characterized primarily through studies of perceptual and cognitive processes, behavioural and neuroimaging studies have raised the possibility that the right and left hemispheres play different roles in the motor control of either arm. Based on our work in young healthy individuals, we have proposed a model of motor lateralization, in which each hemisphere has become specialized for different aspects of motor control, such that the ‘dominant/left’ hemisphere is critical for predicting limb and task dynamics, and the opposite, ‘non-dominant/right’ hemisphere is critical for specifying steady-state limb positions through impedance control mechanisms (see Sainburg, 2010 for a review). Our recent work in patients with unilateral brain damage (Schaefer et al., 2007, 2009a, b; Mutha et al., 2010, 2011a, b; Schaefer et al., 2012) and findings from other previous studies (Haaland and Harrington, 1989; Harrington and Haaland, 1991; Bernspang and Fisher, 1995; Winstein and Pohl, 1995; Haaland et al., 2004) have provided a confirmation for hemispheric specialization for movement control. For example, our study examining movement coordination in right-handed stroke patients (Schaefer et al., 2009b) showed a clear double dissociation between hemisphere status (healthy/hemisphere damage) and arm (right/left) for different features of movement. Patients with left hemisphere damage, but not right hemisphere damage, showed errors in direction and linearity of reaching movements that were associated with poor coordination of intersegmental dynamics. In contrast, patients with right hemisphere damage made well-coordinated and fairly straight movements, but they showed large and highly variable final position errors. In more recent studies, we have significantly expanded on these initial results by demonstrating differential deficits in motor adaptation and error correction mechanisms in left and right hemisphere damage stroke patients (Schaefer et al., 2009a; Mutha et al., 2011b; Schaefer et al., 2012). These studies have consistently revealed a deficit in predictive control after left hemisphere damage and final position control after right hemisphere damage, in line with the predictions of our model.
Our stroke studies were initiated as a critical test of this framework of hemispheric specialization for movement. We reasoned that if a hemisphere contributes its specialization to the movements of both arms, then motor deficits after damage to that hemisphere should be evident even if the ipsilesional arm in stroke patients is used to perform the task. Our studies, therefore, almost always required subjects to use their ipsilesional arm. However, given the crossed organization of the motor system, motor deficits after stroke are most prominent on the contralesional side. Therefore, most studies in stroke patients have, unsurprisingly, been dedicated to understanding the nature of these contralesional motor deficits. These studies have shown that although weakness and spasticity are common with contralesional hemiparesis (Bobath, 1990), discoordination is also a major problem, particularly during point-to-point reaching tasks. For example, Beer et al. (2000, 2004) demonstrated systematic direction errors and poor interjoint coordination with the contralesional arm in a 16-direction centre-out reaching task. In this task, all four directions comprised a quadrant in task space, and deficits were largest in the two quadrants where intersegmental coordination requirements were greatest. Similarly, Levin (1996) and Cirstea and Levin (2000) showed that when stroke patients made horizontal plane reaching movements with the paretic arm, their movements were characterized by high variability and poor synchrony between elbow and shoulder joint motions. In light of these significant contralesional deficits, motor rehabilitation after stroke has also focused on improving the functioning of the contralesional arm. In fact, newer therapeutic approaches, such as constraint-induced movement therapy (Taub et al., 1993; Mark and Taub, 2004), emphasize forced and repetitive use of the contralesional arm while also preventing use of the ipsilesional arm as a means to improve contralesional arm performance.
Despite such strong emphasis on understanding contralesional deficits and improving contralesional arm function, previous studies have not been coupled with the growing body of work that addresses hemispheric specificity for movement control mechanisms. One potential reason for this could be the concern that spasticity, weakness and variability in degree of impairment in the contralesional limb could mask any performance asymmetries. Nevertheless, understanding whether neural lateralization results in contralesional deficits that differ depending on the hemisphere of damage is of critical importance for assessing the impact of brain damage on function and also for designing rehabilitation protocols specific to the impaired limb. In this study, we ask whether the motor deficits in the contralesional arm of unilateral stroke patients reflect hemisphere-specific control mechanisms. Our model of lateralized control predicts clear differences in contralesional motor deficits depending on the laterality of stroke; therefore, this study also serves as a critical test of our model. We overcome the potential limitation of contralesional spasticity and reduced motor abilities by examining patients with only mild to moderate hemiparesis, defined by a score >45 (of a maximum possible 66) on the Fugl-Meyer Assessment of Upper Extremity Function.
Materials and methods
The Institutional Review Boards of the New Mexico Veteran Affairs Healthcare System and Hershey Medical Centre approved the study protocol. Before participation, all subjects gave informed consent according to the Declaration of Helsinki.
Participants
Eighteen unilateral stroke patients (nine with left hemisphere damage, nine with right hemisphere damage; one patient with right hemisphere damage tested at Hershey Medical Centre, others at New Mexico Veteran Affairs Healthcare System) and 20 healthy control subjects (10 left healthy control subjects, 10 right healthy control subjects; all tested at New Mexico Veteran Affairs Healthcare System) participated in this study. All control subjects were right-handed, and the stroke patients were right-handed before the incidence of stroke. Handedness was determined using the 10-item version of the Edinburgh Inventory (Oldfield, 1971). All stroke patients were examined at least 6 months after stroke. All the subjects were screened and excluded based on history of: (i) substance abuse and/or serious psychiatric diagnosis (e.g. psychosis); (ii) non-stroke neurological diseases for the stroke patients and all neurological diagnoses for the control subjects; and (iii) peripheral movement restrictions, such as neuropathy or orthopaedic disorders. Measures of hemiparesis (Fugl-Meyer et al., 1975), grip strength (Heaton et al., 2004), auditory comprehension (Kertesz, 1982), limb apraxia (Haaland and Flaherty, 1984) and visuospatial perception (Judgement of Line Orientation, Benton et al., 1994) were used to characterize the degree of impairment in stroke patients across different domains. A modified line cancellation test was administered to all subjects to test for visual neglect (Albert, 1973). Patients with two or more errors (of the 21 possible) in the contralesional hemispace were classified as having visual neglect, based on the fact that none of the control subjects made more than one error in either the left or the right hemispace.
Table 1 summarizes the characteristics of each subject group. We restricted our patient population to only those who had mild to moderate hemiparesis, as indicated by a Fugl-Meyer score of >45 (of a maximum possible 66) (Murphy et al., 2010). This was done to ensure that subjects could still perform the task with their contralesional arm, yet contralesional hemiparesis was not so extreme that it masked asymmetries between the arms. Further, we intentionally matched our patients with left and right hemisphere damage on the Fugl-Meyer score to rule out the possibility that any group differences in arm reaching could be attributed to a difference in the degree of hemiparesis. The four groups were not significantly different in age [F(3,34) = 0.61, P = 0.61] or education [F(3,34) = 2.53, P = 0.07]. Our groups were also fairly well balanced in terms of number of male and female participants [Table 1; χ2(3,38) = 3.65; P = 0.30], suggesting that sex differences should be accounted for when comparing the left and right hemisphere damage groups, which was the more critical comparison for the current study. However, we should state that our current sample sizes precluded our ability to examine sex as a factor in our analyses. Subjects with left and right hemisphere damage did not significantly differ in number of years post-stroke (P = 0.28), lesion volume (P = 0.94) or contralesional grip strength (P = 0.18). Patients with left hemisphere damage were more apraxic than patients with right hemisphere damage (P = 0.01), consistent with the observations of several previous studies (De Renzi et al., 1980; Haaland and Flaherty, 1984; Ochipa and Gonzalez Rothi, 2000). Auditory language comprehension was also significantly weaker in the group with left hemisphere damage (P = 0.04). However, we made sure that all subjects understood the experimental task they were asked to perform, and task performance in general indicated that they did so. None of the participants had visual neglect, and visuospatial perception on Judgement of Line Orientation test was not significantly different between the two stroke groups (P = 0.994).
Table 1.
Summary of participant information
| Variable | Healthy control subjects |
Hemisphere damaged |
||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| n | 10 | 10 | 9 | 9 |
| Number of male subjects | 8 | 8 | 6 | 4 |
| Age (years) | 63.6 ± 6.3 | 64.2 ± 9.5 | 68.6 ± 11.3 | 63.2 ± 10.6 |
| Education (years) | 16.0 ± 1.8 | 14.9 ± 1.8 | 14.2 ± 3.1 | 13.1 ± 2.5 |
| Years post-strokea | NA | NA | 4.8 ± 3.5 | 7.8 ± 7.5 |
| Lesion volume (cm3)b | NA | NA | 133.7 ± 97.8 | 130.8 ± 68.5 |
| Fugl-Meyer motor scorec | NA | NA | 57.3 ± 6.3 | 57.3 ± 6.5 |
| Visuospatial perceptiond | 25.6 ± 4.38 | 28.3 ± 3.56 | 20.78 ± 2.99 | 20.75 ± 1.85 e |
| Language comprehensionf | 80.0 ± 0.0 | 79.2 ± 2.5 | 57.7 ± 28.03 | 79.1 ± 2.7 |
| Limb apraxiag | 13.7 ± 1.1 | 13.4 ± 1.3 | 10.1 ± 3.2 | 13.5 ± 1.0 |
| Contralesional grip strengthh | 50.3 ± 5.9 | 49.2 ± 8.2 | 25.4 ± 17.3 | 36.4 ± 15.9 |
Values are mean ± SD.
a Years post-stroke are calculated as time elapsed between incidence of stroke and day of data collection.
b Lesion volume is computed from MRI and CT scans using a computer algorithm.
c Maximum score on the total upper-extremity Fugl-Meyer motor score is 66.
d Visuospatial perception was assessed using the Judgement of Line Orientation test.
e One patient with right hemisphere damage was not administered the Judgement of Line Orientation test.
f Language comprehension was assessed using the Western Aphasia Battery.
g Limb apraxia was designated as mean number correct out of 15 items using a validated apraxia battery.
h Grip strength from dynamometer are expressed as standardized t-scores.
NA = not applicable.
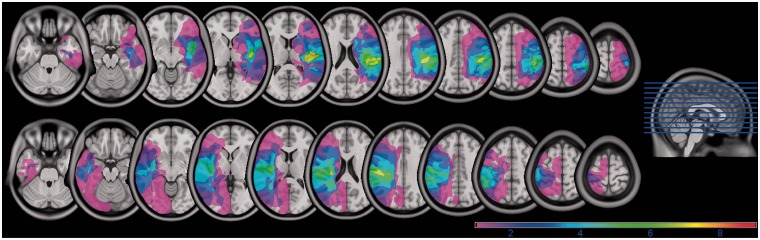
High-resolution T1-weighted MRI scans were obtained from stroke patients and were then normalized to a standard template in Montreal Neurological Institute space using unified segmentation and normalization routines in SPM8 (Ashburner and Friston, 2005) and custom MATLAB scripts. Lesions were then reconstructed on the anatomical images in Adobe Photoshop, and the traced lesions were converted back into volumes using custom MATLAB code. Volumes from multiple patients within a group (left or right hemisphere damage) were then overlaid in MRIcron (Rorden and Brett, 2000) to create overlap images showing areas of damage common to all patients within a group. Figure 1 shows the superimposed lesion locations for all subjects within each stroke group. Colours of the shaded region denote the percentage of subjects in each group with damage in the corresponding area. It should be noted that the lesions are confined to either the left or the right hemisphere. Importantly, all patients with left and right hemisphere damage had damage in at least one region of the sensorimotor motor system (Brodmann areas 4, 6, 3, 1, 2 and/or internal capsule). Lesion volume was not significantly different between the two stroke groups (P = 0.94), and intrahemispheric lesion location was similar between the two groups.
Figure 1.
Overlap images showing locations of lesions between groups with right and left hemisphere damage (colour scale of magenta to red shows increasing overlap). Lesions are confined to either left or right hemisphere.
Experimental set-up
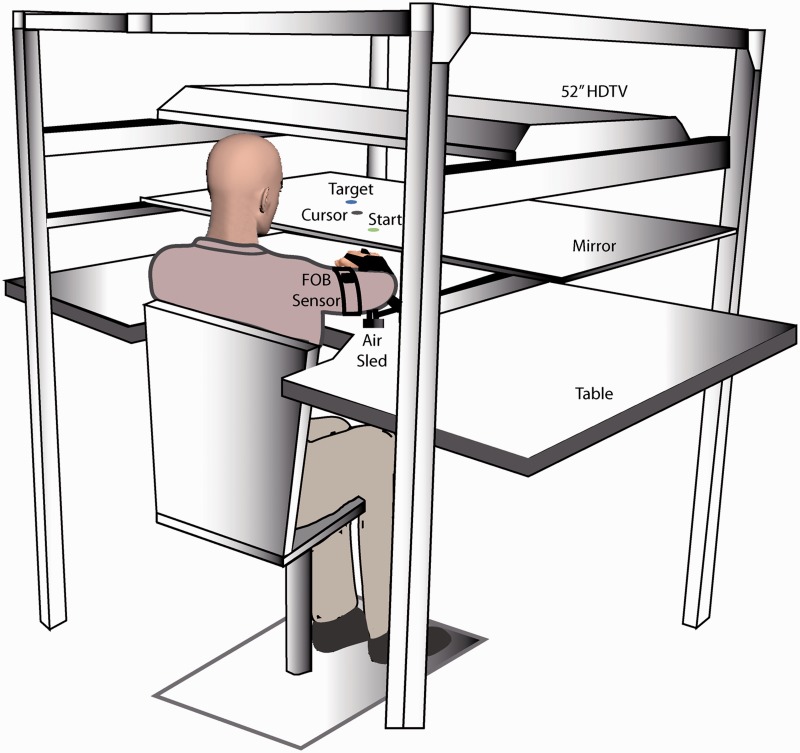
The experimental set-up is shown in Fig. 2. Subjects sat facing a table with either their left or their right arm supported over a horizontal surface by an air-jet system to eliminate the effects of gravity and reduce friction. A start circle, targets and the subject’s fingertip (represented by an on-screen cursor) were displayed on a mirror using a high-definition television positioned horizontally above the mirror. The mirror blocked the direct vision of the subject’s arm, but reflected the visual display to give the illusion that the display was in the same horizontal plane as the fingertip. Position and orientation of the forearm and upper arm segments were sampled using a Flock of Birds (Ascension Technology®) system at 130 Hz. The positions of the index finger tip, lateral epicondyle of the humerus and the acromion, directly posterior to the acromioclavicular joint were digitized using a stylus that was rigidly attached to a 6-degree of freedom Flock of Birds sensor. As sensor data were received, the 3D position of the aforementioned landmarks was computed using custom software, with the x–y plane parallel to the tabletop. We used the computed x–y coordinates of the fingertip to define the projected cursor position.
Figure 2.
Schematic of the experimental set-up. Subjects sat facing a mirror on which the start position and targets were projected using a high definition television, and they rested their arms in an air-sled system placed on a glass tabletop. FOB = Flock of Birds system.
Experimental task
Stroke patients performed the task using their contralesional arm, and the control subjects used their left or right arm depending on their group—right- or left healthy control subjects, respectively. Three targets were presented at a distance of 16 cm from a fixed start position. The start circle was presented at a distance of 40 cm from the front edge of the table and 15 cm from the midline of body in the right or left hemispace depending on whether the participants use their right or left arm, respectively, to perform the task. The targets were oriented 50°, 90° and 130° relative to the horizontal edge of the table and were presented in the left hemispace for participants performing the task with their left arm (medial, central and lateral targets, respectively) or in the right hemispace for participants performing the task with their right arm (lateral, central and medial targets, respectively). Before the start of each trial, the cursor and the start circle were displayed on the screen. To initiate the trial, the subject brought the cursor into the start circle and after a 300 ms delay, one of the three targets appeared on the screen along with an audio-visual ‘Go’ signal, which cued the subjects to initiate a single rapid movement towards the target. Feedback of the fingertip position was removed at this point, and subjects were asked to reach the target with a minimum speed requirement of 0.5 m/s. The speed criterion was used to emphasize consistent performance and was not used as a basis for excluding trials during data analysis. If subjects satisfied the speed criterion, they received ‘points’ based on the location of the index finger at the end of movement relative to the centre of the target; points were also used for motivational purposes only. Visual feedback about final position of the finger and the entire hand trajectory was displayed at the end of every trial. Subjects were asked to return the cursor into the start position to begin a new trial. The target and start position were visible throughout the entire trial, allowing certainty about the visually based target position and distance. The three targets were pseudo-randomly presented over a session of 99 trials, such that no target was presented consecutively.
Data analysis
Finger, elbow and shoulder positions were calculated from sensor’s position and orientation. The joint angles were then calculated, low-pass filtered at 8 Hz using a third order dual-pass Butterworth filter and differentiated to yield tangential velocity and acceleration. The first nine trials were considered as practice trials and were not included in the analysis. All remaining trials were included in the analysis, except in an extremely rare case when a subject failed to initiate a movement in response to the ‘Go’ signal. Movement start was identified by first determining the peak in the tangential velocity profile and then searching backwards to find the first minimum <5% of the peak. Movement end was determined as the first minimum <5% of peak tangential velocity by searching forwards from the time of peak velocity to the end of movement.
Dependent measures
Dependent measures of interest in this study were peak velocity, distance error, final position error, movement duration, absolute initial direction error and absolute direction error at movement end. Peak velocity was the maximum tangential velocity during movement. Distance error was defined as the difference between the target distance and the straight-line distance between the starting and final position of the fingertip, regardless of the path taken. Note that this error was signed if the target was overshot, the distance error was positive, whereas if the target was undershot, the distance error was negative. Final position error was calculated as the distance between the target and finger position at movement end. Movement duration was defined as the time from movement onset to movement end. Absolute initial direction error was calculated relative to the line connecting the start position and the target, and was defined as the angular deviation between the ‘target line’ and the line from the starting location of the hand to the hand location at peak velocity. Absolute direction error at movement end was calculated as the angular deviation between the ‘target line’ and the line from the starting location of hand to the hand location at movement end. As the hand trajectory is convex (curved), the hand-path curvature was calculated as the minor axis divided by the major axis of the hand path. The major axis was defined as the largest distance between any two points in the hand path, whereas the minor axis was defined as the largest distance perpendicular to the major axis (Bagesteiro and Sainburg, 2002; Schaefer et al., 2009b).
Statistical analysis
Performance of patients with left hemisphere damage (contralesional arm: right) was compared with that of the control group that performed the task with their right hand. Performance of patients with right hemisphere damage (contralesional arm: left) was compared with that of the control group that performed the task with their left hand. The individual dependent measures were analysed using three-way mixed model ANOVA, with arm (left or right arm) and group (hemisphere-damaged or healthy control group) as between-subject factors and the target (lateral, center and medial) as the within-subject factor. The effect of target was considered only when it interacted with group and arm, as the primary aim of this study was to determine whether laterality of lesion influences the kinematic measures. When there were significant main or interaction effects, post hoc analyses were performed using Tukey’s honestly significant difference test, which corrects for multiple comparisons (Kutner et al., 2004). Statistical significance levels were set to 0.05. All statistical analyses were carried out using JMP® statistical software.
Results
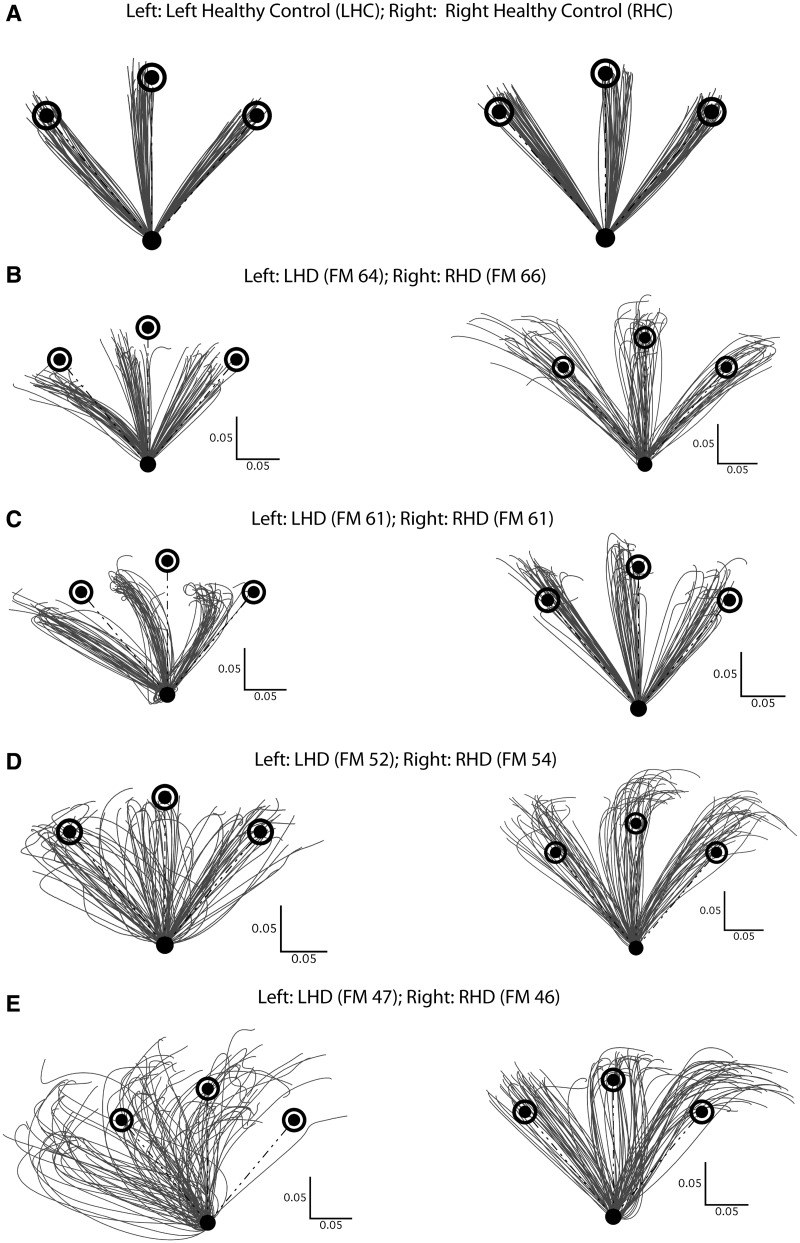
The hand-paths of a representative subject from each control group are shown in Fig. 3A. Regardless of the hand used, the hand-paths of control subjects tended to be fairly straight and directed towards the target and terminated close to the target. Although it was not the focus of this study, we did not observe any major differences between the movement patterns of left- and right healthy control subjects, consistent with our recent reports of a reduction in interlimb differences in reaching coordination in healthy older subjects relative to healthy younger subjects (Przybyla et al., 2011; Wang et al., 2011). Figure 3B–E shows contralesional hand-paths for representative patients with left or right hemisphere damage, separated by the level of dysfunction as quantified by the Fugl-Meyer score. The differences between the hand-paths of patients and healthy control subjects are apparent in Fig. 3. In general, stroke patients made movements that were more variable and less accurate than those of healthy control subjects. Importantly, there were substantial differences in the movements of patients with left and right hemisphere damage. For instance, as shown in Fig. 3B, the patient with left hemisphere damage showed systematic and variable direction errors for all three targets, whereas the patients with right hemisphere damage made straighter movements in the direction of the targets, but consistently overshot them. These systematic differences between patients with left and right hemisphere damage persisted as impairment level increased (Fig. 3B–E). Even at the highest level of impairment (Fig. 3E), the directions of the movements of patients with right hemisphere damage were more clustered, when compared with their counterparts with left hemisphere damage.
Figure 3.
(A) Comparison of hand-paths between representative left healthy (LHC) and right healthy control (RHC) subjects. (B–E) Comparison of hand-paths between patients with left (LHD) and right hemisphere damage (RHD) across severity of hemiparesis assessed using the Fugl-Meyer score. Each right and left hemisphere damage pair has similar Fugl-Meyer (FM) scores; Fugl-Meyer scores decrease from B to E, indicating increasing degree of hemiparesis.
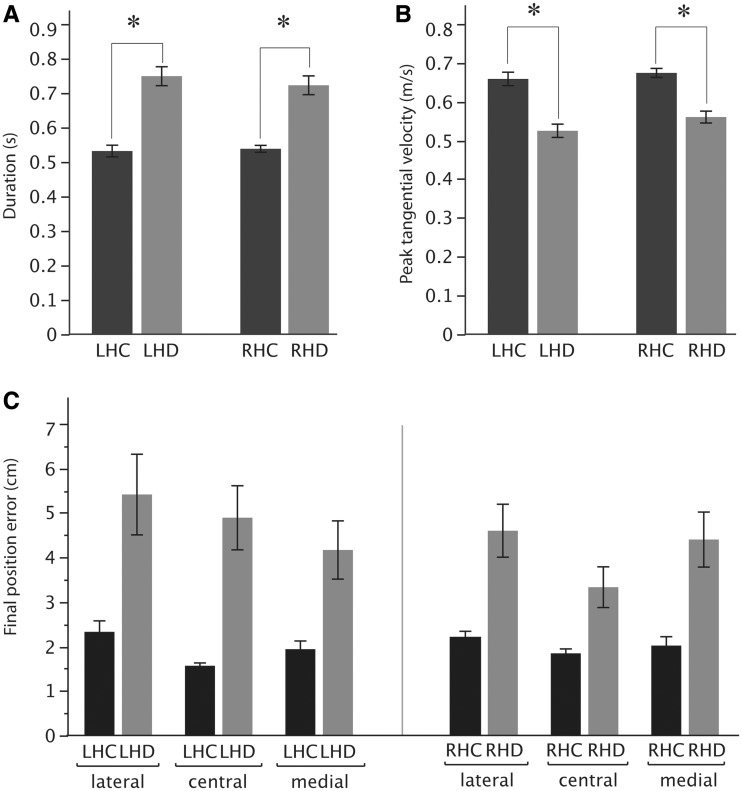
Figure 4 compares these findings and other kinematic parameters across all subjects in each group. For statistical comparison, we performed a three-way ANOVA with group (healthy control group, hemisphere damaged group), arm (left, right) and target (lateral, center, medial) as factors. This analysis did not include Fugl-Meyer impairment level as a factor because the test of our primary hypothesis required comparison between the performance of stroke patients and that of control subjects, who could not be classified based on Fugl-Meyer impairment level. In general, movement duration was greater in the stroke than the control group [F(1,34) = 34.97, P < 0.0001], and this effect did not vary as function of arm, or an interaction between arm and group, or a three-way interaction between arm, group and target (range of P-value: 0.065–0.77). Similarly, peak tangential velocity was significantly lower in the stroke groups than the control groups [Fig. 4B; ANOVA: F(1,34) = 36.17, P < 0.0001] again, without variation as a function of arm, or an interaction between arm and group, or a three-way interaction between arm, group and target (P > 0.08 in all cases).
Figure 4.
(A) Mean duration and (B) mean peak tangential velocity for right and left arm of control subjects (LHC, RHC) (black) and the contralesional arm of patients with left hemisphere damage (LHD, grey) and right hemisphere damage (RHD, grey). (C) Final position error for the same groups for the three different targets (lateral, central, medial). Error bars indicate standard error of the mean. *Significant (P < 0.05) group differences.
However, our ANOVA demonstrated that there was a significant three-way interaction of arm, group and target for final position error [F(2,34) = 3.33, P = 0.04]. This interaction can be seen in Fig. 4C. Although both patient groups showed substantially higher errors than their respective control groups (P < 0.0001), the left hemisphere damage group’s errors depended systematically on movement direction, becoming larger from the medial to lateral target, whereas the right hemisphere damage group’s errors did not. Regardless of this interaction, the overall amplitude of final position errors for patients with left hemisphere damage and right hemisphere damage was not significantly different (P = 0.27) nor was there a difference between the right and left control groups (P = 0.99).
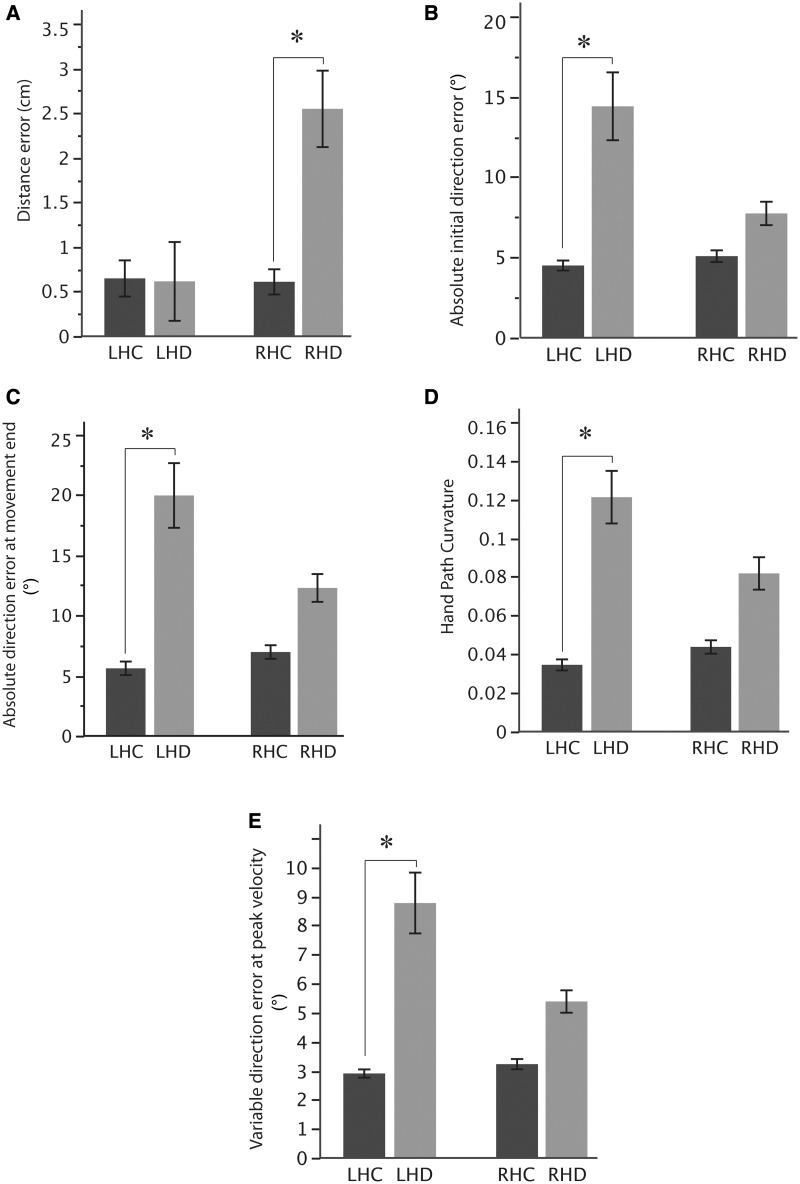
As suggested by the three-way interaction noted previously, the final position errors of right and left hemisphere damage groups were because of different factors: patients with right hemisphere damage tended to overshoot the targets, whereas patients with left hemisphere damage tended to move the correct distance in the wrong direction (Fig. 3B–E). Figure 5A compares our measure of distance error across our four groups. Our ANOVA results revealed a significant interaction between group and arm [F(1,34) = 6.17, P = 0.02]. Post hoc analysis revealed that the stroke patients with right hemisphere damage showed significantly higher distance errors when compared with all other groups (P < 0.01). However, there were no significant differences in distance error between patients with left hemisphere damage and the left healthy control group (P = 0.99). In other words, damage to the right, but not left hemisphere, produced higher distance errors than those of control subjects.
Figure 5.
(A) Mean distance error, (B) mean absolute initial direction error, (C) mean absolute direction error at end of movement, (D) mean hand path curvature and (E) mean variable direction error at peak velocity for the left healthy control (LHC), left hemisphere damage (LHD), right healthy control (RHC) and right hemisphere damage (RHD) groups. Error bars indicate standard error of the mean. *Significant (P < 0.05) group differences.
To assess the deficit in controlling movement direction after left hemisphere damage, we calculated direction error during the early phases of the movement, at peak velocity. These data across our subject groups are shown in Fig. 5B. Our ANOVA results revealed a significant interaction between group and arm [F(1,34) = 4.37, P = 0.04]. Post hoc analysis revealed that patients with left hemisphere damage (P = 0.002), but not patients with right hemisphere damage (P = 0.76), had significantly higher initial direction errors compared with their respective control groups. Thus, damage to the left, but not right hemisphere, resulted in significantly higher initial direction errors.
The hand-paths of the patients with left hemisphere damage in Fig. 3B–D suggested that direction errors of patients with left hemisphere damage continued throughout the course of movement. To more carefully examine this possibility, we calculated direction errors at movement end (Fig. 5C). Again, we observed a significant interaction between group and arm for this measure [F(1,34) = 5.06, P = 0.03], and post hoc analysis indicated that patients with left hemisphere damage produced significantly higher direction errors at the movement end than the left healthy control group (P < 0.0001). However, there were no significant differences in the direction error at the movement end between the right hemisphere damage and right healthy control groups (P = 0.24). One might conclude that the persistence of direction errors early and late in the movement trajectory might indicate that movements of patients with left hemisphere damage were straight. However, the mean direction errors of patients with left hemisphere damage at the end of movement (Fig. 5D) were somewhat higher than at peak velocity (Fig. 5C), indicating that these errors were not corrected during movement. Thus, the movements curved substantially, but this curvature did not reflect directional corrections, as can be seen in the hand-paths in Fig. 3B–D. In fact, there was a significant interaction between group and arm [F(1,34) = 4.39, P = 0.04] for hand-path curvature. Post hoc analysis revealed that patients with left hemisphere damage showed significantly larger movement curvature than the left healthy control participants (P < 0.0001). Although some patients with right hemisphere damage showed some curvature in their movements (Fig. 3D and E), as a group, the hand-path curvature was not significantly different from that of healthy control subjects (P = 0.11). In other words, only left hemisphere damage resulted in increased movement curvature.
Next, we reasoned that a deficit in accurately specifying movement direction after left hemisphere damage might also be evident as higher variability in this measure (Schaefer et al., 2007, 2009). As expected, patients with left hemisphere damage showed much larger variable direction errors compared with all other groups (Fig. 5E). There was a significant interaction between group and arm [F(1,34) = 5.28, P = 0.03], with post hoc analysis showing that the variable direction errors of patients with left hemisphere damage were significantly higher compared with the left healthy control and right hemisphere damage groups (P < 0.02). On the other hand, there were no significant differences in variable direction error between the right hemisphere damage and right healthy control groups (P = 0.37). In other words, damage to left hemisphere, but not right hemisphere, resulted in high variability in movement direction.
In summary, our analyses revealed a double dissociation between the type of error and the hemisphere of damage: right hemisphere damage, but not left hemisphere damage, patients showed substantial deficits in movement distance. In contrast, left hemisphere damage, but not right hemisphere damage, patients showed substantial deficits in constant and variable measures of movement direction. Final position inaccuracies were not dissociated by hemisphere of damage, but seem to be produced by the aforementioned differential deficits.
Effect of impairment level
We next explored the relationship between the extent of the movement deficits after left and right hemisphere damage, and the severity of clinical motor dysfunction, as indicated by the Fugl-Meyer Assessment of Upper Extremity Function score. We divided each of our stroke groups into the following two subgroups: mildly impaired (Fugl-Meyer score of 58–66) and moderately impaired (Fugl-Meyer score of 46–57), similar to previously published cut scores (Murphy et al., 2010). Both stroke groups had five patients in the mildly impaired range and four patients who were moderately impaired. Age was also comparable among the mild (left hemisphere damage: 74.2 ± 8.1, right hemisphere damage: 62.6 ± 13.5) and moderate groups (left hemisphere damage: 61.5 ± 11.5, right hemisphere damage: 64 ± 7.5). The measures from the previous analysis that showed an interaction between arm and group were subjected to a mixed model three-way ANOVA with damaged hemisphere (left or right), Fugl-Meyer impairment level (mild or moderate) and target (lateral, central and medial) as factors.
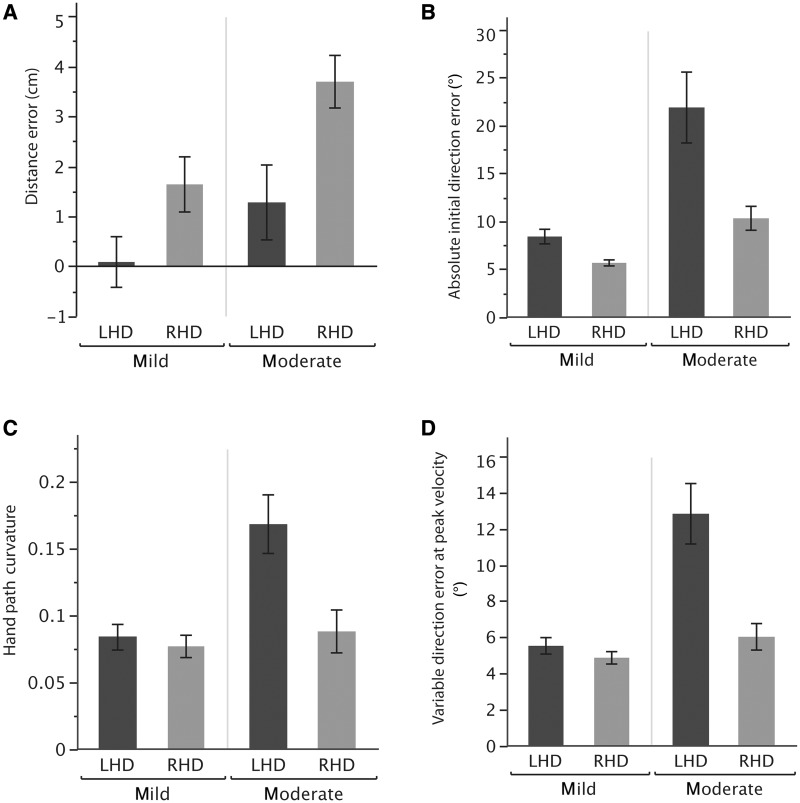
Figure 6 compares our kinematic measures between the two Fugl-Meyer score groups for patients with right and left hemisphere damage. For distance error (Fig. 6A), our ANOVA results showed a significant main effect of Fugl-Meyer impairment level [F(1,42) = 8.48, P < 0.01] and damaged hemisphere [F(1,42) = 12.67, P < 0.001]. However, there was no significant interaction between Fugl-Meyer impairment level and damaged hemisphere [F(1,42) = 0.59, P = 0.44]. Thus, distance error was higher for patients with right hemisphere damage when compared with patients with left hemisphere damage, regardless of their Fugl-Meyer severity.
Figure 6.
(A) Mean distance error, (B) mean absolute initial direction error, (C) mean hand path curvature and (D) mean variable direction error at peak velocity of mildly and moderately impaired patients with left hemisphere damage (LHD, black) and right hemisphere damage (RHD, grey). Error bars indicate standard error of the mean.
In terms of initial direction error, Fig. 6B shows that the difference between left and right hemisphere damage is larger in the moderately impaired group. This conclusion is confirmed by the ANOVA results, which revealed a significant interaction between Fugl-Meyer impairment level and damaged hemisphere [F(1,42) = 5.28, P = 0.03]. In other words, as the level of impairment increased, there was a substantial increase in direction error in patients with left hemisphere damage, but not in patients with right hemisphere damage.
Similar to initial direction error, our hand-path curvature and variable direction error ANOVA results also showed a significant interaction between damaged hemisphere and Fugl-Meyer impairment [curvature: F(1,42) = 6.69, P = 0.01; variable direction error: F(1,42) = 14.37, P < 0.001]. As the level of impairment increased, there was a substantial increase in both of these measures for the group with left hemisphere damage, but not for the group with right hemisphere damage (Fig. 6C and D).
In summary, these results suggest a substantial effect of the severity of impairment on the reaching performance of stroke patients. Although patients with left hemisphere damage showed increasing variability, hand-path curvature and direction error with increasing contralesional hemiparesis, patients with right hemisphere damage did not show variation in these measures based on impairment level. In addition, the specific right hemisphere damage deficits in movement distance also did not vary with impairment level.
Discussion
There is substantial research dedicated to understanding contralesional motor deficits after unilateral stroke and improving contralesional arm performance through rehabilitation. However, none of these studies have examined whether movement deficits in the contralesional arm differ depending on the hemisphere of damage, despite a growing body of work indicating that each hemisphere of the brain might be specialized for controlling different aspects of movement. Our results indicate compelling differences in contralesional arm performance in unilateral stroke patients depending on the hemisphere of damage. These findings support our predictions that left hemisphere, but not right hemisphere, damage produces deficits in movement trajectory, whereas right hemisphere, but not left hemisphere, damage produces deficits in stabilizing the limb at the end of movement. We also show that left hemisphere deficits vary with the severity of impairment. These findings not only broaden the scope of our model of hemisphere-specific control but also have significant implications for understanding the impact of stroke on function and for clinical rehabilitation.
Our results expand the findings of previous studies, which revealed significant coordination deficits in the contralesional arm of stroke patients. In particular, the studies of Beer et al. (2000, 2004) reported a failure to predictively account for dynamic intersegmental interactions when stroke patients performed reaching actions with their paretic arm. Levin (1996) and Cirstea and Levin (2000) largely focused on movement kinematics and reported problems in coordinating the actions of shoulder and elbow joints during contralesional arm motion. Kamper et al. (2002) reported contralesional deficits in movement velocity, smoothness, linearity and direction. Although earlier studies that examined deficits, such as weakness and spasticity, attributed contralesional impairments to reduced agonist (El-Abd et al., 1993; Fellows et al., 1994) and antagonist muscle activation (McLellan et al., 1985; El-Abd et al., 1993), hyperactive reflexes (Mizrahi and Angel, 1979), peripheral disturbances, such as changes in tissue properties (Dietz et al., 1991; Given et al., 1995), or the presence of abnormal synergies (Bourbonnais et al., 1989; Dewald et al., 1995); more recent studies that also address coordination deficits are beginning to identify inadequacies in movement planning (Beer et al., 2000; Kusoffsky et al., 2001) as a potential source of these problems. Our results agree with these studies that a deficit in predictive control mechanisms gives rise to coordination deficits post-stroke. However, our current and previous results provide specificity regarding the neural substrates underlying these deficits by demonstrating that predictive control of movement trajectory features is disturbed only from damage to the left hemisphere. In fact, damage to right hemisphere regions does not impact on the coordination of movement. In contrast, deficits in controlling movement distance arise from damage to the right hemisphere (Schaefer et al., 2007, 2009b). Although Kamper et al. (2002) observed deficits in controlling movement distance with the contralesional limb, they did not identify whether such deficits differed depending on the hemisphere of damage. Our current results show that only patients with right hemisphere damage made substantial distance errors, indicating that mechanisms that ensure termination of a movement and stabilization of the arm at a goal location are lateralized to the right hemisphere.
Nature of motor deficits after left and right hemisphere damage
Before discussing the specific nature of the deficits after left and right hemisphere damage, it should be emphasized that our model of hemispheric specialization (Sainburg, 2002; 2005; 2010) is bi-hemispheric—we posit that each hemisphere contributes different aspects of control to movements of both arms. Such bi-hemispheric control is consistent with observations from previous neuroimaging studies that motor cortical areas of both brain hemispheres are active during unilateral finger movement (Li et al., 1996; Cramer et al., 1999), finger sequencing (Kawashima et al., 1993; Kim et al., 1993) and unilateral arm movements (Nirkko et al., 2001; Winstein et al., 1997). Our previous findings in healthy individuals in which we documented limb-specific advantages for the dominant and non-dominant arms (Sainburg and Kalakanis, 2000; Bagesteiro and Sainburg, 2002, 2003) and our recent work in the ipsilesional arm of stroke patients (Schaefer et al., 2007, 2009b; Mutha et al., 2010, 2011b; Schaefer et al., 2012) have supported this bi-hemispheric model of control. In fact, Schaefer et al. (2007, 2009b) have demonstrated that ipsilesional deficits in motor coordination and learning mirror the functional advantages that we had previously reported in the dominant and non-dominant arms of healthy subjects. For example, left hemisphere damage produced deficits in intersegmental coordination and in direction learning during adaptation to visuomotor rotation, whereas right hemisphere damage produced deficits in final position accuracy and position adaptation. In addition, Desrosiers et al. (1996) and Schaefer et al. (2009b) showed that these deficits are often functionally relevant, and that they correlate with deficits in clinical movement evaluations that include simulated activities of daily living. In a more recent study, Robertson et al. (2012) revealed coordination deficits in both arms of stroke patients with left hemisphere damage, during unconstrained reaching movements. Although left and right brain damaged patient groups had reduced scapula protraction in the contralesional paretic arm, scapula protraction was only reduced in the ipsilesional arms of the group with left hemisphere damage. The authors concluded that left hemisphere damage produced deficits in proximal coordination, a finding that they suggested might be consistent with previous findings of reduced intersegmental coordination in the ipsilesional arm of patients with left hemisphere damage (Schaefer et al., 2009b). Our current findings confirm that these specific ipsilesional deficits also occur in the contralesional hemiparetic arm of right and left hemisphere damage stroke patients.
Although the nature of our previously observed ipsilesional and current contralesional deficits seems similar, two important differences between these studies must be pointed out. First, in the current study, we did not observe a dependence of left hemisphere damage-related trajectory deficits on intersegmental coordination requirements. Although deficits in movement direction or hand-path curvature in patients with left hemisphere damage were always larger than those in left healthy control subjects, our ANOVA results for these measures did not show a significant three-way interaction among group, arm and target. These findings agree with those of Kamper et al. (2002) who showed only a modest dependence of contralesional deficits on movement direction, yet they stand in contrast with our findings in the ipsilesional arm where the magnitude of the deficits almost always increased as intersegmental coordination requirements increased. This raises the interesting question of whether left hemisphere contributions to coordination of ipsilateral arm movements become more critical as the complexity of the movement increases. Although we cannot directly answer this question yet, this suggestion is not far from some observations of functional neuroimaging and lesion studies of finger movements. Harrington and Haaland (1991) previously observed that performance of complex heterogeneous arm posture sequences with the ipsilateral arm was more impaired than that of simple repetitive ones after left hemisphere damage, but not right hemisphere damage. Haaland et al. (2004) also showed greater left hemisphere activation during the performance of complex rather than simple finger sequences, even when the ipsilateral left hand was used to perform the task. Verstynen et al. (2005) extended these findings by showing that increased ipsilateral left hemisphere contributions did not necessarily require sequential actions, but were present during the performance of any complex finger movement. Whether a similar conclusion can be drawn for arm reaching movements remains a subject of future investigation. Second, in contrast to our previous ipsilesional studies, in the current study, final position errors were not significantly different between patients with left and right hemisphere damage. However, it must be stressed that here, left hemisphere damage final position errors were because of deficits in direction control, whereas right hemisphere damage errors resulted from deficits in adequately stopping at the end of movement. Although the most impaired patient with left hemisphere damage in our current study (Fig. 3E) did show some difficulty in stopping at the medial target, this was not observed in other patients with left hemisphere damage (Fig. 3B–D). These patients did not show any systematic overshoot or undershoot, which would be indicative of a deficit in adequately stopping the movement at the target location. Although the movements of the patient with left hemisphere damage in Fig. 3E might give the impression of a distance control problem, this was not the case. Note that the movements directed towards the lateral target ended closer to the centre target than the lateral target as a result of large direction errors. However, the average distance of these movements was fairly well matched to the target distance, confirming that final position errors in patients with left hemisphere damage were largely because of initial direction error, but not distance control deficits. In contrast, for patients with right hemisphere damage, directional deviations, if any, were initiated late in the movement, mostly after the hand had crossed the target (Fig. 3D and E). The direction errors made by patients with right hemisphere damage were in fact small and comparable with control subjects. Thus, the final position errors in the group with right hemisphere damage were largely because of the patients consistently overshooting the target, as can be seen in Fig. 3B–E. These findings in patients with right hemisphere damage are consistent with our previous results (e.g. Schaefer et al., 2009b), but we had previously observed intact accuracy (final position errors comparable with control subjects) in our patients with left hemisphere damage, who tended to correct their movements back to the target position despite initially deviating from the target direction when moving with the ipsilesional arm. We interpreted such corrections in patients with left hemisphere damage as a contribution of the intact right hemisphere to the ipsilesional, left arm through crossed descending pathways. However, in the current study, after left hemisphere damage, corrections of the contralesional right arm were ineffective or absent, leading to large errors at the end of movement. This might result from the fact that the intact right hemisphere has limited direct access to the contralesional right arm, which could limit effective corrections and stabilization of the arm at the desired goal location.
Although we have emphasized that the final position errors after right, but not left hemisphere damage, arise because of a deficit in controlling movement distance, it is interesting to speculate whether our results could be explained on the basis of differential deficit in left and right hemisphere damages solely in estimating or planning movement distance. First, our neuropsychological tests showed that there were no significant differences between stroke groups with left and right hemisphere damage in visuospatial perception (Judgement of Line Orientation Test: P = 0.994), making it unlikely that visuospatial deficits, if any, played a differential role in estimating target distance as shorter or longer in one group over the other. Second, although a certain component of movement distance seems to be preplanned (Gordon and Ghez, 1987a, b), our recent work has shown that achievement of a target distance relies on ‘online’ (during movement) processes that use sensory information to modulate limb impedance (Mutha et al., 2008). We suggest that it is these impedance mechanisms that are disrupted by right hemisphere damage, producing a deficit in accurately stopping at a goal location and thereby affecting the achievement of a target distance. In contrast, movement distance in patients with left hemisphere damage was fairly well matched to the target distance. In fact, distance errors in patients with left hemisphere damage were small, positive on average and, most importantly, comparable with control subjects. Thus, we do not believe that left hemisphere damage affected processes that regulate achievement of movement distance. Nevertheless, further research is necessary to comprehensively examine the contributions of distance planning mechanisms to these differential deficits.
Although we addressed these distinct deficits in patients with left and right hemisphere damage, it is imperative to explain why elderly stroke patients demonstrate such differential deficits, when healthy elderly subjects tend to show a reduction in motor lateralization (Przybyla et al., 2011). Previous brain imaging studies have shown that such a reduction in lateralization with ageing is a consequence of increased neural recruitment bilaterally, rather than a reduced specialization of one hemisphere. For example, Cabeza (2002) showed that for certain neuropsychological functions that are associated with asymmetric patterns of recruitment in young subjects, older subjects recruit more symmetric patterns of cortical activity. Furthermore, these patterns are associated with sustained performance on the neuropsychological tasks, suggesting that bilateral recruitment is likely to be compensatory in nature, in light of reduced unilateral neural capacity. This forms the basis of the HAROLD (hemisphere asymmetry reduction in older adults) model proposed by Cabeza (2002). In line with these results, we have shown that older adults show more symmetric patterns of motor behaviour and interlimb transfer of motor learning (Przybyla et al., 2011; Wang et al., 2011). Taken together, our findings and related imaging findings (Mattay et al., 2002) suggest that as people age, increased symmetry in behaviour is not because of a reduction in specialization of one hemisphere, but rather because of an increase in bilateral hemispheric recruitment. However, Adamo et al. (2009) reported the emergence of proprioceptive wrist matching asymmetries with age, suggesting that some new neural asymmetries may develop, whereas others become diminished with ageing. The current findings in the contralesional arm, together with previous findings in the ipsilesional arm (Schaefer et al., 2007, 2009b) support this view by showing that loss of contribution from one hemisphere, either right or left, will re-establish systematic motor asymmetries. If, on the other hand, hemispheric specialization itself had become reduced, motor asymmetries between patients with left and right hemisphere damage would not have been observed. We, therefore, conclude that our current findings support the HAROLD model, and extend it to motor function.
Differentiating right hemisphere deficits in visuospatial processing from motor control
Right hemisphere damage has previously been shown to produce deficits in cognition and perception, including unilateral neglect (Bottini et al., 2009). It is plausible that the accuracy deficits revealed in the current study for patients with right hemisphere damage could emerge from such perceptual deficits. Pisella et al. (2011) argued that the right hemisphere might be dominant for visuospatial processing, based on several studies examining optic ataxia, neglect and visual agnosia. For example, in a study focused on correlating the critical neural substrates associated with unilateral visual neglect, Vallar and Perani (1986) recruited 110 stroke patients to participate in a circle cancellation task. The results indicated that damage to the right inferior parietal lobe commonly resulted in contralateral visual neglect, which was consistent with later studies documenting visual neglect after right hemisphere damage (Vallar et al., 1993; Mattingley, 1999). In the current study, patients with right hemisphere damage consistently overshot the targets, resulting in large distance errors (Fig. 3B–E). However, none of our patients with right hemisphere damage had visual neglect, which rules out the possibility that these errors are a consequence of neglect. It is also unlikely that these errors are attributable to optic ataxia because the hand-paths of patients with right hemisphere damage were fairly accurate in direction, which is not characteristic of this deficit. In addition, several studies have indicated that errors because of optic ataxia are predominant when patients try to grasp or reach to objects located in their peripheral or extra-foveal visual field (Buxbaum and Coslett, 1997; Dijkerman et al., 2006). However, in the current study, the targets were presented close to the centre of the subject’s visual field and workspace, and no restrictions were imposed on the gaze direction of the subjects. We conclude that the errors associated with difficulty stopping on targets in the current study are unlikely to result from deficits, such as optic ataxia or unilateral visual neglect. Instead, we attribute these errors to a deficit in the specialized role of right hemisphere in controlling limb impedance for stabilizing limb position at the end of movement.
Effect of impairment level on contralesional deficits
Our results show that patients with moderate to mild hemiparesis show motor deficits that vary with the hemisphere of damage, and that these deficits in patients with left hemisphere damage vary with the extent of impairment measured by the Fugl-Meyer score. One of the difficulties in developing a more specific understanding of how movement deficits after stroke are affected by lesion location and impairment level has been the paucity of research that has detailed the relationship between the degree of motor impairment and kinematic, kinetic and/or electrophysiological measures of motor performance. Only a few studies have investigated this association. For example, Kamper et al. (2002) examined the reaching performance of mild to severely paretic chronic stroke patients while they made reaching movements to 75 targets. They reported that deficits in a variety of contralesional performance measures, including velocity, direction error and linearity, strongly correlated with impairment level, as measured by the Chedoke-McMaster Stroke Arm Assessment Scale. However, no previous studies have assessed how this dependence might be modulated by the hemisphere of damage. Our current findings indicate that the trajectory-based deficits produced by patients with left hemisphere damage increase with the severity of Fugl-Meyer impairment, whereas the severity of impairment does not modulate differences in distance error for patients with right hemisphere damage (Fig. 6). It is difficult to explain this asymmetric dependence of performance errors on level of impairment. It may simply be the case that variations in level of impairment have a more graded effect on the ability to coordinate the segments of the arm than on the ability to stop at a given location. It is also notable that previous neural activation studies have shown asymmetries related to side of impairment. For example, Zemke (2003) showed that when stroke patients performed a finger-tapping task with their paretic arm, patients with left hemisphere damage had higher contralesional sensorimotor cortex activation than patients with right hemisphere damage. These findings are somewhat consistent with neural activation studies in healthy subjects, indicating greater ipsilateral activation when performing sequential finger apposition with the non-dominant hand as compared with the dominant hand (Kim et al., 1993). Zemke’s (2003) findings suggest that the greater recruitment of ipsilateral cortex is maintained after right hemisphere damage, but not left hemisphere damage. It is plausible that the contralesional arm of patients with right hemisphere damage receives greater contribution from the intact ipsilateral (left) hemisphere, and this may diminish the effect of impairment level on performance measures, like accuracy. However, it is impossible to conclusively explain the asymmetrical effects of right and left hemisphere damage on the relationship between our performance measures and clinical measures of impairment in the current study.
Implications for rehabilitation
The finding that contralesional deficits differ depending on the side of hemisphere of damage has important implications for the design of clinical rehabilitation intervention. Although approaches, such as constraint-induced movement therapy (Taub et al., 1993; Mark and Taub, 2004), have shown decreased impairment and improved function through forced use of the contralesional arm, these techniques have not differentiated the type or amount of therapy depending on side of damage. Our results suggest that therapy might be designed to address specific deficits that emerge after left or right hemisphere damage. Such a focused approach is possible through the use of new methodologies that exploit advanced robotic and computer-based intervention that allows high-resolution analysis of performance during and after therapy (e.g. Volpe et al., 2001). These tools could be used to design specific tasks and modify movement-related feedback so as to emphasize certain variables over others. For example, after left hemisphere damage, task feedback can be modified to amplify errors perpendicular to the desired trajectory while reducing errors in the direction of the desired movement. Such changes would penalize deviations from the desired movement path while allowing errors in the direction of movement. In contrast, following right hemisphere damage, tasks that penalize final position errors could be designed. Finally, given the presence of ipsilesional deficits that mirror contralesional problems, bilateral training should be a critical component to therapeutic intervention in unilateral stroke (Whitall et al., 2000; Cunningham et al., 2002; Cauraugh et al., 2010; Latimer et al., 2010). Bilateral training is not only important to facilitate remediation in the ipsilesional arm but also because unilateral training may not automatically carry-over to spontaneous bilateral performance, which is the best predictor of better performance on everyday tasks (Haaland et al., 2012). In fact, recent research has indicated that learning novel kinetic and visuomotor environments with a single arm transfers only partially to bilateral movements, even when the same arm experiences the imposed forces under unilateral and bilateral conditions (Nozaki et al., 2006). Thus, we suggest that it is critical to consider the specific deficits induced by right or left hemisphere lesions and consider bilateral training to enhance motor rehabilitation post-stroke.
Funding
National Institutes of Health, National Institute for Child Health and Human Development (R01HD39311and R01HD059783 to R.L.S.); Department of Veterans Affairs Research and Development Medical Merit Review (101BX007080); Rehabilitation Research and Development (B4125R to K.Y.H.).
Acknowledgements
The authors thank Jenna Keller, Melissa Daniels, Jennifer Hogan and Michelle Temple for assistance with data collection; Lee Stapp for help with MRI tracings; Dr Brad Cushnyr for radiological consultation; HealthSouth Rehabilitation Hospital, Lovelace Medical Centre and Hershey Medical Centre for patient referral.
References
- Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act. 2009;17:272–93. doi: 10.1123/japa.17.3.272. [DOI] [PubMed] [Google Scholar]
- Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–64. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–21. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003;90:1503–13. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RF, Dewald JPA, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–70. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JPA, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–19. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan A, Hamsher K, Varney N, Spreen O. New York: Oxford University Press; 1994. Contributions to neuropsychological assessment: a clinical manual. 2nd edn. [Google Scholar]
- Bernspang B, Fisher AG. Differences between persons with right or left cerebral vascular accident on the assessment of motor and process skills. Arch Phys Med Rehabil. 1995;76:1144–51. doi: 10.1016/s0003-9993(95)80124-3. [DOI] [PubMed] [Google Scholar]
- Bobath B. London: Heinemann; 1990. Adult hemiplegia: evaluation and treatment. [Google Scholar]
- Bottini G, Sedda A, Ferrè ER, Invernizzi P, Gandola M, Paulesu E. Productive symptoms in right brain damage. Curr Opin Neurol. 2009;22:589–93. doi: 10.1097/WCO.0b013e328332c71d. [DOI] [PubMed] [Google Scholar]
- Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Coslett HB. Subtypes of optic ataxia: reframing the disconnection account. Neurocase. 1997;3:159–66. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Pscyhol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Lodha N, Naik SK, Summers JJ. Bilateral movement training and stroke motor recovery progress: a structured review and meta-analysis. Hum Mov Sci. 2010;29:853–70. doi: 10.1016/j.humov.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–53. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81:383–7. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychol (Amst) 2002;110:321–37. doi: 10.1016/s0001-6918(02)00040-9. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Motti F, Nichelli P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol. 1980;37:6–10. doi: 10.1001/archneur.1980.00500500036003. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the “unaffected” upper extremity of elderly stroke patients. Stroke. 1996;27:1564–70. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol. 1991;30:767–79. doi: 10.1002/ana.410300605. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, McIntosh RD, Anema HA, de Haan EH, Kappelle LJ, Milner AD. Reaching errors in optic ataxia are linked to eye position rather than head or body position. Neuropsychologia. 2006;44:2766–73. doi: 10.1016/j.neuropsychologia.2005.10.018. [DOI] [PubMed] [Google Scholar]
- El-Abd MA, Ibrahim IK, Dietz V. Impaired activation pattern in antagonist elbow muscles of patients with spastic hemiparesis: contribution to movement disorder. Electromyogr Clin Neurophysiol. 1993;33:247–55. [PubMed] [Google Scholar]
- Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann Neurol. 1994;36:397–407. doi: 10.1002/ana.410360311. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Given JD, Dewald JP, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Nerol Neurosurg Psychiatry. 1995;59:271–9. doi: 10.1136/jnnp.59.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. II. Pulse height control. Exp Brain Res. 1987a;67:241–52. doi: 10.1007/BF00248546. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. III. Compensatory adjustments for initial errors. Exp Brain Res. 1987b;67:253–69. doi: 10.1007/BF00248547. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3:370–84. doi: 10.1016/0278-2626(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain Cogn. 1989;9:158–80. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–58. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Mutha PK, Rinehart JK, Daniels M, Cushnyr B, Adair JC. The relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch Phys Med Rehabil. 2012. Advance access published on May;24 doi: 10.1016/j.apmr.2012.05.011. 2012; doi: 10.1016/j.apmr.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Hemispheric specialization for motor sequencing: abnormalities in levels of programming. Neuropsychologia. 1991;29:147–63. doi: 10.1016/0028-3932(91)90017-3. [DOI] [PubMed] [Google Scholar]
- Heaton R, Miller S, Taylor M, Grant I. demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources Inc; 2004. Revised comprehensive norms for an expanded Halstead-Reitan Battery. [Google Scholar]
- Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83:702–7. doi: 10.1053/apmr.2002.32446. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, et al. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993;623:33–40. doi: 10.1016/0006-8993(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Kertesz A. New York: The Psychological Corporation; 1982. Western aphasia battery. [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Uğurbil K, et al. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–17. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Kusoffsky A, Apel I, Hirschfeld H. Reaching-lifting-placing task during standing after stroke: coordination among ground forces, ankle muscle activity, and hand movement. Arch Phys Med Rehabil. 2001;82:650–60. doi: 10.1053/apmr.2001.22611. [DOI] [PubMed] [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J, Li W. New York, NY: McGraw-Hill/Irwin; 2004. Applied linear statistical models. 5th edn. [Google Scholar]
- Latimer CP, Keeling J, Lin B, Henderson M, Hale LA. The impact of bilateral therapy on upper limb function after chronic stroke: a systematic review. Disabil Rehabil. 2010;32:1221–31. doi: 10.3109/09638280903483877. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–93. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Li A, Yetkin FZ, Cox R, Haughton VM. Ipsilateral hemisphere activation during motor and sensory tasks. AJNR Am J Neuroradiol. 1996;17:651–5. [PMC free article] [PubMed] [Google Scholar]
- Mark VW, Taub E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor Neurol Neurosci. 2004;22:317–36. [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Mattingley JB. Attention, consciousness, and the damaged brain: insights from parietal neglect and extinction. Psyche. 1999;5:14. [Google Scholar]
- McLellan DL, Hassan N, Hodgson JA. Tracking tasks in the assessment of spasticity. In: Delwaide PJ, Young RR, editors. Clinical neurophysiology in spasticity. Amsterdam: Elsevier; 1985. pp. 131–9. [Google Scholar]
- Mizrahi EM, Angel RW. Impairment of voluntary movement by spasticity. Ann Neurol. 1979;5:594–5. doi: 10.1002/ana.410050620. [DOI] [PubMed] [Google Scholar]
- Murphy MA, Willen C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2010;25:71–80. doi: 10.1177/1545968310370748. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res. 2008;1246:54–69. doi: 10.1016/j.brainres.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48:3855–67. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci. 2011a;31:6972–81. doi: 10.1523/JNEUROSCI.6432-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. Brain. 2011b;134:3647–61. doi: 10.1093/brain/awr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirkko AC, Ozdoba C, Redmond SM, Burki M, Schroth G, Hess CW, et al. Different ipsilateral representations for distal and proximal movements in the sensorimotor cortex: activation and deactivation patterns. Neuroimage. 2001;13:825–35. doi: 10.1006/nimg.2000.0739. [DOI] [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci. 2006;9:1364–6. doi: 10.1038/nn1785. [DOI] [PubMed] [Google Scholar]
- Ochipa C, Gonzalez Rothi LJ. Limb apraxia. Semin Neurol. 2000;20:471–8. doi: 10.1055/s-2000-13180. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pisella L, Alahyane N, Blangero A, Thery F, Blanc S, Pelisson D. Right-hemispheric dominance for visual remapping in humans. Phil Trans R Soc B Biol Sci. 2011;366:572–85. doi: 10.1098/rstb.2010.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A, Haaland KY, Bagesteiro LB, Sainburg RL. Motor asymmetry reduction in older adults. Neurosci Lett. 2011;489:99–104. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JV, Roche N, Roby-Brami A. Influence of the side of brain damage on postural upper-limb control including the scapula in stroke patients. Exp Brain Res. 2012;218:141–55. doi: 10.1007/s00221-012-3014-y. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–58. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005;33:206–13. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. vision and goal directed movement. Champaign, IL: Human Kinetics; 2010. Lateralization of goal-directed movement. [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83:2661–75. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–58. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res. 2009a;1298:78–91. doi: 10.1016/j.brainres.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009b;47:2953–66. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Mutha PK, Haaland KY, Sainburg RL. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb Cortex. 2012;22:1407–19. doi: 10.1093/cercor/bhr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW III, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- Vallar G, Antonucci G, Guariglia C, Pizzamiglio L. Deficits of position sense, unilateral neglect and optokinetic stimulation. Neuropsychologia. 1993;31:1191–200. doi: 10.1016/0028-3932(93)90067-a. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24:609–22. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–22. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N. Is robot-aided sensorimotor training in stroke rehabilitation a realistic option? Curr Opin Neurol. 2001;14:745–52. doi: 10.1097/00019052-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Wang J, Przybyla A, Wuebbenhorst K, Haaland KY, Sainburg RL. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp Brain Res. 2011;210:283–90. doi: 10.1007/s00221-011-2631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–5. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol. 1997;77:1581–94. doi: 10.1152/jn.1997.77.3.1581. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–74. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Zemke AC. Motor cortex organization after stroke is related to side of stroke and level of recovery. Stroke. 2003;34:23–8. doi: 10.1161/01.STR.0000065827.35634.5E. [DOI] [PubMed] [Google Scholar]