Abstract
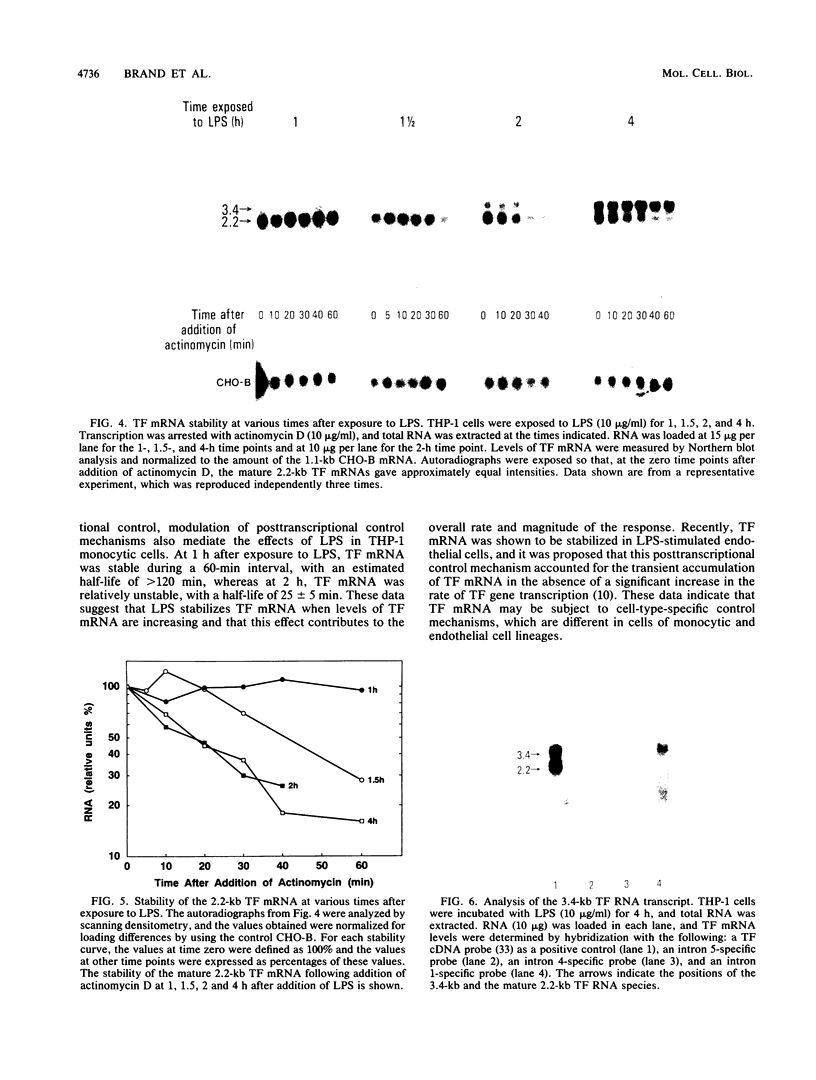
Tissue factor (TF) is transiently expressed in human monocytes exposed to the inflammatory agonist bacterial lipopolysaccharide (LPS). Since TF is the major cellular initiator of the coagulation protease cascades, it is inferred that its expression within the vasculature is strictly regulated. In this study, we investigated mechanisms which control TF mRNA expression in the human monocytic cell line THP-1. LPS induced a rapid and transient accumulation of the mature 2.2-kb TF mRNA, which was maximal at 2 h. After stimulation, the rate of transcription of the TF gene was increased (3.3 +/- 1.3)fold. In addition, we observed a significant change in TF mRNA stability: at 1 h after LPS stimulation, TF mRNA was stable during a 60-min period and had a half-life of greater than 120 min, whereas at 2 h, the half-life had declined to 25 +/- 5 min. Furthermore, a larger (3.4-kb) TF RNA species was induced in these cells; the size of this species and data from selective hybridizations with intron-specific probes are consistent with the presence of an unspliced copy of intron 1. These results demonstrate that the LPS-induced accumulation of TF mRNA levels in these monocytic cells is accomplished by both transcriptional and posttranscriptional control mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Rehemtulla A., Fowler B. J., Edgington T. S., Mackman N. Conservation of tissue factor primary sequence among three mammalian species. Gene. 1991 Feb 15;98(2):265–269. doi: 10.1016/0378-1119(91)90184-d. [DOI] [PubMed] [Google Scholar]
- Bach R. R. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23(4):339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- Bauer J., Ganter U., Geiger T., Jacobshagen U., Hirano T., Matsuda T., Kishimoto T., Andus T., Acs G., Gerok W. Regulation of interleukin-6 expression in cultured human blood monocytes and monocyte-derived macrophages. Blood. 1988 Oct;72(4):1134–1140. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett S. K., Weaver W. M., Westall J. A., Larsen A., Kronheim S., Wilson C. B. Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988 May 15;140(10):3473–3481. [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. M., Bach R., Rosenberg R. D., Konigsberg W. H. Tumor necrosis factor enhances expression of tissue factor mRNA in endothelial cells. Thromb Res. 1989 Feb 1;53(3):231–241. doi: 10.1016/0049-3848(89)90098-4. [DOI] [PubMed] [Google Scholar]
- Crossman D. C., Carr D. P., Tuddenham E. G., Pearson J. D., McVey J. H. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem. 1990 Jun 15;265(17):9782–9787. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. Macrophage procoagulants. Prog Hemost Thromb. 1984;7:183–209. [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Fan S. T., Edgington T. S. Clonal analysis of mechanisms of murine T helper cell collaboration with effector cells of macrophage lineage. J Immunol. 1988 Sep 15;141(6):1819–1827. [PubMed] [Google Scholar]
- Fenton M. J., Clark B. D., Collins K. L., Webb A. C., Rich A., Auron P. E. Transcriptional regulation of the human prointerleukin 1 beta gene. J Immunol. 1987 Jun 1;138(11):3972–3979. [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gregory S. A., Kornbluth R. S., Helin H., Remold H. G., Edgington T. S. Monocyte procoagulant inducing factor: a lymphokine involved in the T cell-instructed monocyte procoagulant response to antigen. J Immunol. 1986 Nov 15;137(10):3231–3239. [PubMed] [Google Scholar]
- Gregory S. A., Morrissey J. H., Edgington T. S. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989 Jun;9(6):2752–2755. doi: 10.1128/mcb.9.6.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hartzell S., Ryder K., Lanahan A., Lau L. F., Nathan D. A growth factor-responsive gene of murine BALB/c 3T3 cells encodes a protein homologous to human tissue factor. Mol Cell Biol. 1989 Jun;9(6):2567–2573. doi: 10.1128/mcb.9.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Sariban E., Kufe D. Transcriptional and posttranscriptional regulation of CSF-1 gene expression in human monocytes. Mol Cell Biol. 1988 Sep;8(9):3951–3954. doi: 10.1128/mcb.8.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Fowler B. J., Edgington T. S., Morrissey J. H. Functional analysis of the human tissue factor promoter and induction by serum. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2254–2258. doi: 10.1073/pnas.87.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Morrissey J. H., Fowler B., Edgington T. S. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989 Feb 21;28(4):1755–1762. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Medcalf R. L., Van den Berg E., Schleuning W. D. Glucocorticoid-modulated gene expression of tissue- and urinary-type plasminogen activator and plasminogen activator inhibitor 1 and 2. J Cell Biol. 1988 Mar;106(3):971–978. doi: 10.1083/jcb.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H., Fair D. S., Edgington T. S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988 Nov 1;52(3):247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Fakhrai H., Edgington T. S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987 Jul 3;50(1):129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Niemetz J., Morrison D. C. Lipid A as the biologically active moiety in bacterial endotoxin (LPS)-initiated generation of procoagulant activity by peripheral blood leukocytes. Blood. 1977 Jun;49(6):947–956. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Ryan J., Geczy C. Coagulation and the expression of cell-mediated immunity. Immunol Cell Biol. 1987 Apr;65(Pt 2):127–139. doi: 10.1038/icb.1987.14. [DOI] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Luebbers R., Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest. 1988 May;81(5):1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Podor T. J., Loskutoff D. J. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-beta, lipopolysaccharide, and tumor necrosis factor-alpha. J Biol Chem. 1989 Jun 25;264(18):10396–10401. [PubMed] [Google Scholar]
- Scarpati E. M., Sadler J. E. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989 Dec 5;264(34):20705–20713. [PubMed] [Google Scholar]
- Scarpati E. M., Wen D., Broze G. J., Jr, Miletich J. P., Flandermeyer R. R., Siegel N. R., Sadler J. E. Human tissue factor: cDNA sequence and chromosome localization of the gene. Biochemistry. 1987 Aug 25;26(17):5234–5238. doi: 10.1021/bi00391a004. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Ruf W., Morrissey J. H., Hinshaw L., Catlett R., Blick K., Edgington T. S. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991 Mar;33(3):127–134. [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]








