Abstract
It is notoriously difficult to name odours. Without the benefit of non-olfactory information, even common household smells elude our ability to name them. The neuroscientific basis for this olfactory language ‘deficit’ is poorly understood, and even basic models to explain how odour inputs gain access to transmodal representations required for naming have not been put forward. This study used patients with primary progressive aphasia, a clinical dementia syndrome characterized by primary deficits in language, to investigate the interactions between olfactory inputs and lexical access by assessing behavioural performance of olfactory knowledge and its relationship to brain atrophy. We specifically hypothesized that the temporal pole would play a key role in linking odour object representations to transmodal networks, given its anatomical proximity to olfactory and visual object processing areas. Behaviourally, patients with primary progressive aphasia with non-semantic subtypes were severely impaired on an odour naming task, in comparison with an age-matched control group. However, with the availability of picture cues or word cues, odour matching performance approached control levels, demonstrating an inability to retrieve but not to recognize the name and nature of the odorant. The magnitude of cortical thinning in the temporal pole was found to correlate with reductions in odour familiarity and odour matching to visual cues, whereas the inferior frontal gyrus correlated with both odour naming and matching. Volumetric changes in the mediodorsal thalamus correlated with the proportion of categorical mismatch errors, indicating a possible role of this region in error-signal monitoring to optimize recognition of associations linked to the odour. A complementary analysis of patients with the semantic subtype of primary progressive aphasia, which is associated with marked temporopolar atrophy, revealed much more pronounced impairments of odour naming and matching. In identifying the critical role of the temporal pole and inferior frontal gyrus in transmodal linking and verbalization of olfactory objects, our findings provide a new neurobiological foundation for understanding why even common odours are hard to name.
Keywords: olfaction, odour object knowledge, recognition, naming, language, semantics, temporal pole, inferior frontal gyrus, primary progressive aphasia, human brain, MRI
Introduction
Among the more enigmatic phenomena in human sensory perception is a ‘physiological’ olfactory anomia—a naming deficit for smells. Perfectly healthy individuals have great difficulty naming and identifying odours that are highly familiar to them and show improvement only when provided with relevant non-olfactory cues (Cain, 1979; Cain et al., 1998). Odours might evoke a strong sense of overall recognition (familiarity), but in contrast with ‘tip of the tongue’ states where the first letter of the name of a person, place, or object can be generated (Brown and Mcneill, 1966), the first letter of an odour’s name is rarely retrieved. Such findings suggest that a processing failure occurs even before the name retrieval stage (Jonsson and Olsson, 2003). Although the cause of this naming failure is disputed, inefficient perceptual discrimination, cognitive or affective interference and competition, and a breakdown of lexical-semantic integration have been proposed as plausible mechanisms (Herz and Engen, 1996; Lorig, 1999; Yeshurun and Sobel, 2010).
Across different sensory modalities, object naming is likely to share a similar neurocognitive framework: sensory inputs activate object representations in sensory-specific association cortex, which, through transmodal nodes, activate knowledge-based representations in the language network to support word selection (Mesulam, 2000). As used here, the term ‘transmodal’ refers to brain areas that support cross-modal sensory integration. With regard to the olfactory modality, increasing evidence from animals and humans indicates that perceptual representation of odour objects and categories are encoded in the piriform cortex (Gottfried, 2010). However, the route by which piriform representations of odour objects gain access to the language network is poorly understood. The fact that odour naming is so impoverished even in healthy individuals raises the possibility that the olfactory system is relatively isolated from cortical areas mediating object naming and recognition.
Combining neuropsychological and cortical MRI assessments from patients with neurodegenerative language impairment, we attempted to localize the neural substrates underlying odour naming and recognition. Specifically, we tested olfactory perceptual functions (Fig. 1) in patients with primary progressive aphasia (PPA), a clinical syndrome characterized by progressive decline of language skills initially in the absence of other cognitive or sensory-perceptual deficits (Mesulam, 2001; Gorno-Tempini et al., 2004; Mesulam et al., 2008). Because PPA is associated with widespread cortical atrophy in the left perisylvian language network (Mesulam, 2001), this group of patients constitutes a suitable ‘lesion model’ to investigate olfactory-language interactions, specifically by correlating measures of behavioural performance with measures of cortical integrity. Such procedures have been used previously for clarifying cortical relationships with non-olfactory language measures (Mion et al., 2010; Sapolsky et al., 2010; Rogalski et al., 2011b).
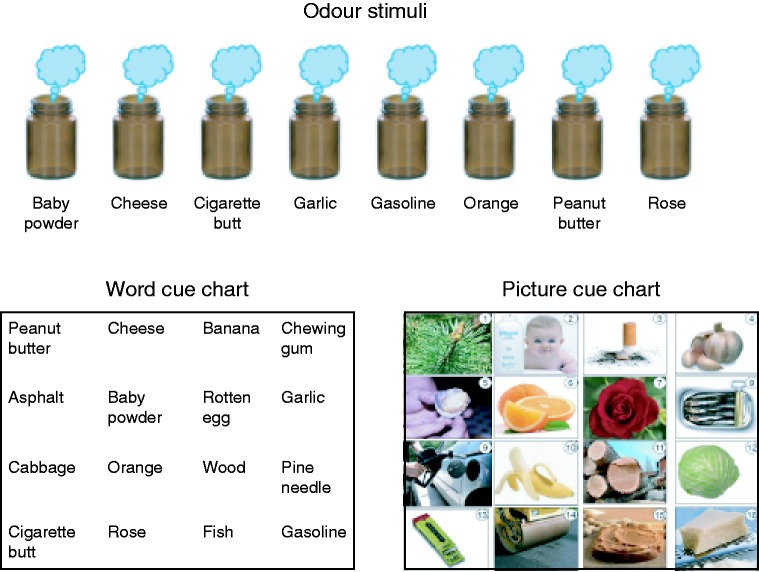
Figure 1.
Odour stimuli (top) and visual cue charts (bottom) used to assess uncued odour naming and cued odour matching.
Our main objective was to identify the cognitive and neural mechanisms involved in translating odour inputs into recognizable and nameable objects. Several brain areas can be hypothesized to play complementary roles in these processes, including the temporal pole, the inferior frontal gyrus and the mediodorsal thalamus. The temporal pole is of particular interest; it is virtually adjacent to the piriform cortex (Fig. 2). Neuroanatomical tracer studies in monkeys have shown that olfactory associative inputs from the piriform cortex project directly to the dorsomedial, agranular sector of the temporal pole, which also receives limbic and paralimbic inputs from amygdala and orbitofrontal cortex (Moran et al., 1987). In turn, visual and auditory associative inputs terminate on more differentiated granular sectors of the ventrolateral and dorsolateral temporal pole, respectively (Markowitsch et al., 1985; Moran et al., 1987; Kondo et al., 2003). This confluence of multisensory processing streams within the temporopolar cortex would provide an expedient route (Mesulam et al., 2009a; Schwartz et al., 2009; Hurley et al., 2012; Mesulam et al., 2012) by which odour object information engages transmodal components of the language network. Severe atrophy in this region, typically arising from semantic dementia, leads to associative agnosia and a loss of multimodal semantic knowledge (Warrington, 1975; Hodges et al., 1992; Markowitsch, 1995; Snowden et al., 2004; Brambati et al., 2006; Patterson et al., 2007; Mesulam et al., 2009a). Lesions in the vicinity of the temporal pole have been shown to impair both object naming and object recognition (Tsapkini et al., 2011), and can induce food preference changes and hyperorality in monkeys, perhaps reflecting a disconnection between food-related odour representations and knowledge-based associations in heteromodal cortical areas (Horel et al., 1975; Kling et al., 1993).
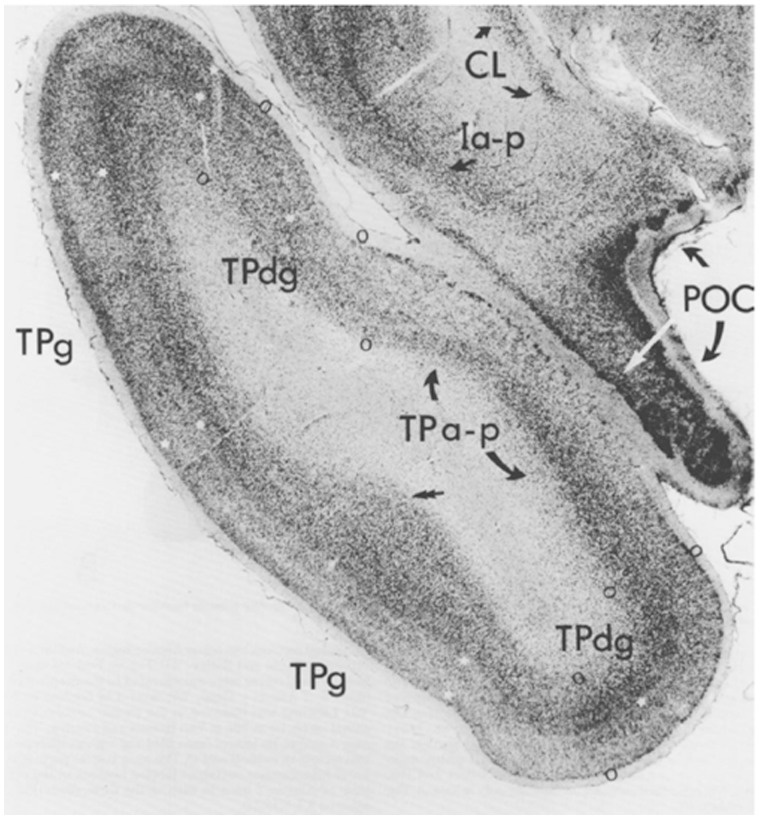
Figure 2.
Architectonics of the temporal pole and adjacent olfactory cortex. This photomicrograph shows agranular/peri-allocortical (TPa-p), dysgranular/peri-isocortical (TPdg), and granular/isocortical (TPg) zones of temporopolar cortex (boundaries demarcated by open circles). The piriform olfactory allocortex (POC) immediately overlies TPa-p, which itself is flanked by TPdg. White stars indicate granular layers 2 and 4 in TPdg and TPg. CL = claustrum; Ia-p = insula, agranular/periallocortical. Reproduced from Moran et al. (1987), with permission.
As a major component of the language network, the inferior frontal gyrus has been implicated in tasks requiring verbal knowledge, for example, synonym matching and retrieval of abstract concepts (Binder et al., 2009; Liakakis et al., 2011), and functional connectivity analyses suggest that top-down feedback from inferior frontal gyrus to the temporal pole helps maintain language network function (Warren et al., 2009). Human olfactory imaging studies indicate that the inferior frontal gyrus is specifically engaged during cognitive processing of familiar odours (Royet et al., 1999, 2001; Savic and Berglund, 2004), and is involved in maintaining information about highly nameable odours in working memory (Zelano et al., 2009). These findings suggest a possible role for the inferior frontal gyrus in olfactory verbalization.
With regard to the mediodorsal thalamus, direct olfactory inputs from piriform cortex have been documented anatomically and electrophysiologically (Yarita et al., 1980; Price and Slotnick, 1983; Moran et al., 1987; Russchen et al., 1987), and dense projections from the anterior temporal pole onto the mediodorsal thalamus (Russchen et al., 1987) could provide opportunity for integrating odour information with representations in other sensory domains. Our prior imaging work has found that attention to odour modulates the connectivity strength between piriform cortex and mediodorsal thalamus (Plailly et al., 2008), and that the mediodorsal thalamus generates a prediction error signal during delivery of unexpected (versus expected) odour stimuli (Zelano et al., 2011). These results are congruent with recent neuropsychological findings (Tham et al., 2009).
The present study primarily centred on patients with non-semantic PPA, including logopenic and agrammatic variants. These patients show reduced word-finding, fluency and syntax, but relatively preserved language comprehension and object knowledge (Gorno-Tempini et al., 2011). A systematic testing battery, including olfactory perceptual ratings, odour naming, picture-odour and word-odour matching, and picture-word matching, enabled us to correlate these scores with cortical thickness measurements, and to distinguish between perceptual, semantic and verbalization contributions to olfactory naming impairments. We hypothesized that patients with non-semantic PPA (versus control subjects) would be highly impaired in odour naming because olfactory information might have an intrinsically tenuous access to lexical representations available for verbalization, such that odour naming would be more vulnerable to perturbations arising from neurodegenerative changes in the language network. However, because olfactory perceptual and semantic information should be largely preserved in non-semantic PPA, we predicted that the availability of picture or word cues should largely rescue the olfactory naming impairment. Finally, we hypothesized that if the temporal pole links olfactory objects to transmodal representations subserving recognition, then cortical thickness in this region would correlate most strongly with olfactory test measures of recognition.
Note that although the semantic variant of PPA is strongly associated with temporal pole atrophy (Gorno-Tempini et al., 2004; Mesulam et al., 2012), we mainly focused on non-semantic PPA. Cortical atrophy in non-semantic PPA is distributed in several areas, including the left temporoparietal region (logopenic variant) and inferior frontal gyrus (agrammatic variant) (Gorno-Tempini et al., 2004; Mesulam et al., 2012), but there is also involvement of the temporal pole and anterior temporal lobe in patients with non-semantic PPA (Rogalski et al., 2011b). In contrast, the profound atrophy in semantic PPA would likely yield minimal variability in temporal pole thinning between patients with semantic PPA, and also extensively disrupt all olfactory-associative functions, weakening the ability to detect significant correlations to behaviour. Nevertheless, we did conduct a complementary analysis in patients with semantic PPA, to validate the role of the temporal pole in linking odour representations to the language network, and to contrast our findings with the non-semantic PPA data set. Based on prior work indicating that patients with semantic dementia are broadly impaired on olfactory-related language tasks (Luzzi et al., 2007; Rami et al., 2007; Piwnica-Worms et al., 2010), we anticipated that the semantic PPA group would show marked odour naming and recognition deficits (Gorno-Tempini et al., 2004; Mesulam et al., 2009a).
Materials and methods
Participants
The diagnosis of PPA was made on the basis of progressive language impairment as the principal presenting feature and fulfilment of established ancillary criteria (Mesulam, 2001). Aphasia was established through the Western Aphasia Battery (Shewan and Kertesz, 1980; Kertesz, 1982), and clinical progression of aphasia was established through history taken from the patient, medical records, and from at least one other informant. Patients with non-semantic PPA were differentiated from those with semantic PPA as per previously published criteria (Gorno-Tempini et al., 2011). To be considered for participation, participants also had to be right-handed according to the Edinburgh scale (Oldfield, 1971). No participant reported any other neurological or psychiatric disorder, except for one patient with mild depression.
Three patients were excluded because of self-reported chronic smell problems, inability to perform the olfactory tasks due to complete muteness, or inability to comprehend task instructions, resulting in a final sample of 12 patients with non-semantic PPA, eight patients with semantic PPA and 12 control subjects. Table 1 provides group demographic and cognitive data, and Table 2 provides individual clinical, cognitive and demographic data for patients with non-semantic PPA. Duration of disease at the time of testing varied from 1 to 4.5 years for patients with non-semantic PPA and 2 to 7.5 years for patients with semantic PPA. Note that data from two different control groups were used. Olfactory behavioural results from the patients with PPA were compared with results from the control group of 12 participants mentioned above. However, group-based results showing regional distributions of MRI cortical thinning in the patients with PPA were compared with those from an independent group of 27 healthy participants who did not undergo olfactory testing (see below). This latter group has been previously used as a reference population in published studies of PPA (Rogalski et al., 2011a, b; Mesulam et al., 2013), and for clarity will be referred to as the MRI control reference group.
Table 1.
Behavioural data and summary statistics of olfactory and cognitive tests (scores, mean ± SD) for patients with non-semantic PPA and for age-matched control subjects
| PPA-NS score | PPA-NS range | CTL score | CTL range | P-value | |
|---|---|---|---|---|---|
| Age | 66.33 (9.69) | 54–79 | 61.67 (7.48) | 51–73 | 0.200 |
| Gender (F/M) | 7/5 | 7/5 | |||
| Education | 16.17 (1.95) | 13–18 | 16.33 (2.39) | 14–20 | 0.853 |
| Odour intensity | 3.53 (0.56) | 2.75–4.38 | 3.82 (0.58) | 2.63–4.63 | 0.230 |
| Odour familiarity | 3.42 (0.79) | 2.13–4.75 | 3.97 (0.63) | 2.75–5.00 | 0.074 |
| Odour naming | 1.83 (1.53) | 0–4 | 4.67 (1.40) | 2–7 | <0.001 |
| Picture-odour matching | 5.50 (1.88) | 2–8 | 6.67 (0.89) | 5–8 | 0.065 |
| Word-odour matching | 5.58 (1.56) | 3–8 | 6.58 (1.08) | 4–8 | 0.082 |
| Odour mismatching within category | −0.83 (1.80) | −3–1 | 0.25 (2.14) | −2–4 | 0.193 |
| Picture chart naming | 14.25 (1.81) | 11–16 | 15.58 (0.51) | 15–16 | 0.023 |
| Word chart reading | 14.33 (4.66) | 0–16 | 15.83 (0.39) | 15–16 | 0.278 |
| BNT | 43.08 (14.11) | 19–59 | 56.17 (3.30) | 51–60 | 0.005 |
| MMSE | 22.83 (7.95) | 5–30 | 29.25 (0.97) | 27–30 | 0.011 |
Note that ‘odour mismatch within category’ is calculated as the number of within-category errors minus between-category errors. Higher scores on this measure thus indicate that odour perceptual categorization is better preserved.
CTL = control subjects; PPA-NS = non-semantic PPA.
Table 2.
Demographic and performance data for individual patients with non-semantic PPA
| Patient (L/G/U) | Age | Gender | Education (years) | PPVT | Odour naming | Picture-odour matching | Word-odour matching | BNT | MMSE |
|---|---|---|---|---|---|---|---|---|---|
| 1(L)a | 61 | M | 16 | 32 | 1 | 5 | 6 | 19 | 5 |
| 2(L)a | 77 | F | 16 | 34 | 0 | 2 | 5 | 30 | 19 |
| 3(L)a | 57 | F | 18 | 36 | 4 | 8 | 7 | 54 | 30 |
| 4(G)a | 79 | F | 14 | 34 | 2 | 8 | 6 | 54 | 30 |
| 5(U)a | 78 | F | 14 | 35 | 4 | 8 | 5 | 28 | 29 |
| 6(G)a | 69 | M | 18 | 35 | 0 | 4 | 5 | 41 | 30 |
| 7(G) | 54 | M | 17 | 33 | 3 | 7 | 8 | 57 | 27 |
| 8(G) | 79 | M | 13 | 34 | 3 | 4 | 4 | 27 | 12 |
| 9(G)a | 57 | F | 18 | 34 | 0 | 5 | 3 | 41 | 20 |
| 10(G)a | 65 | M | 18 | 35 | 1 | 5 | 4 | 59 | 20 |
| 11(G)a | 57 | F | 14 | 33 | 3 | 5 | 6 | 49 | 26 |
| 12(G)a | 63 | F | 18 | 35 | 1 | 5 | 8 | 58 | 26 |
G = agrammatic subtype; L = logopenic subtype; PPVT = Peabody Picture Vocabulary Test; U = unclassifiable.
a Patient was included in the correlation analysis.
Study protocol standards
Patients were recruited for participation in the study from our PPA Research Programme, funded by the National Institute on Deafness and Other Communication Disorders, and tested at the Cognitive Neurology and Alzheimer’s Disease Centre at Northwestern University Feinberg School of Medicine. Age-matched healthy control subjects were recruited among volunteering subjects at the Cognitive Neurology and Alzheimer’s Disease Centre. Informed consent was obtained from all control subjects and from all patients with PPA (or their caregivers) to take part in the study, which was approved by the Northwestern University Institutional Review Board.
Cognitive testing
To characterize the cognitive impairment in the PPA group, scores on the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and Boston Naming Test (BNT) (Kaplan et al., 1983) were assessed. For the MMSE, the patient performs a variety of cognitive tasks engaging memory, orientation and language, with a maximum score of 30. For the BNT, the patient is asked to name the objects depicted in 60 line drawings, which decrease in frequency and increase in difficulty from the beginning to end of the test. The BNT engages visual naming systems and has been shown to be sensitive to language dysfunction in PPA (Gorno-Tempini et al., 2011).
Olfactory stimuli
Odour stimuli consisted of eight common smells that varied in pleasantness and edibility: peanut butter, orange, rose, baby powder, cheese, garlic, gasoline and cigarette ash (Fig. 1). On the basis of pilot testing, each odour was correctly named by at least 5 of 10 healthy young adults (aged 19–32 years) and was not systematically misidentified as other test items or response alternatives in picture-odour and word-odour matching tests. All odours were obtained from natural samples except for rose and orange, which were obtained from essential oils.
Word and picture stimuli
Word and picture cues were presented in the form of charts for the odour-matching tests (Fig. 1). These charts consisted of the eight words (or pictures) corresponding to the eight odours, plus another eight foil items whose corresponding odours also varied in pleasantness and edibility: chewing gum, banana, pine needle, wood, cabbage, fish, rotten egg and asphalt. This yielded 16 response options, with targets and foils varying along major valence and edibility dimensions (Zarzo, 2008). Items were arranged in the charts randomly in a 4 × 4 matrix. Note that other assessments such as the Smell Identification Test, a widely used four-choice test to assess word-cued odour identification, provide a single score to summarize olfactory ability (Doty et al., 1984). However, our aim to uncover mechanisms underlying olfactory naming impairment in PPA (e.g. perceptual, semantic, verbalization) motivated a customized assessment where the retrieval support was manipulated (naming, picture-odour matching, or word-odour matching), resulting in three scores.
Procedure
The olfactory test battery proceeded in several steps. First, patients participated in a test of odour naming. On each trial, one of the eight odours was presented in a randomized order, and the patient was instructed to sniff the stimulus and try to name it. Perceptual ratings of odour intensity, pleasantness, familiarity and edibility were also obtained at this time, using scales ranging from 1 (low) to 5 (high). Participants were permitted to smell a given odour more than once during the trial, as needed, while performing ratings, and after completing the ratings. This procedure was designed to optimize naming performance and to minimize potential confounds related to working memory or task demands. Participants were reminded to provide specific names (avoiding superordinate terms such as ‘pleasant’ or ‘food’ if possible) but were not given cues or corrective feedback during the naming portion of the test. Furthermore, participants were encouraged to make a guess in cases where they did not spontaneously provide an odour name.
Following the odour naming test, patients were introduced to the 16-item word and picture charts (order counterbalanced across subjects) and then asked to read the words and name the pictures to assess word reading and visual object naming. In case of errors, the experimenter provided the correct word and an error was noted. After the assessment of word reading and object naming, odour matching was formally assessed with the word chart or picture chart, in which participants selected a response by naming (or pointing to) the item best corresponding to the odour. The relative order of the picture and word conditions was randomized, as was the odour presentation order in each condition. Chance-level performance for the odour matching tests was 6%, or 1 out of 16. Participants then performed a control task where they were verbally cued to point to eight different objects in the picture chart, to ensure intact semantic matching of visual objects to their names. Picture objects were chosen that were not represented in the odour set, in order to minimize effects of odour priming potentially arising from the main tests.
Odour naming and matching responses were coded as either hits (1) or misses (0). Because the eight odorants varied along the dimensions of pleasantness and edibility (comprising four perceptual categories: pleasant edible; pleasant inedible; unpleasant edible; unpleasant inedible), we were also able to quantify the proportion of within-category and between-category matching errors. Based on a total of 16 items within the picture and word charts, there were three within-category foils and 12 between-category foils for a given odorant, resulting in a within-category by-chance error rate of 20%, i.e. 3/(3 + 12). Thus, incorrectly matching rose odour to the picture of baby powder would be scored as a within-category error (both are perceived as high in pleasantness and low in edibility), whereas incorrectly matching rose odour to the picture of peanut butter would be scored as a between-category error (since the items differ in edibility). Critically, this measure (number of between-category errors subtracted from number of within-category errors) is independent of odour matching accuracy rates, and enabled us to assess the degree to which patients were able to update their choices on the basis of error signalling; more between-category than within-category mismatches would suggest that patients had greater difficulty in updating their choices effectively.
In a final step, participants underwent a three-way forced-choice (‘triangle’) odour discrimination test. The goal of this task was to identify the ‘odd one out’ in sets of three odour bottles, with chance performance at 33% (Bartoshuk, 1978; Doty and Laing, 2003). Participants were asked to distinguish between phenethyl alcohol (with a pleasant rose-like smell) and vanillin, and between phenethyl alcohol and isovaleric acid (with an unpleasant sweat-like smell). Odours were presented at iso-intensity and a total of four trials were obtained per participant.
Magnetic resonance imaging
Structural MRI scans were acquired and reconstructed with the FreeSurfer image analysis suite (version 4.5.0) as previously described (Mesulam et al., 2009a). Cortical thickness maps of non-semantic and semantic PPA groups were statistically contrasted against the averaged map from an MRI control reference group of 27 healthy volunteers who did not take part in the olfactory experiment (as discussed above). Differences in cortical thickness between groups were calculated by conducting a general linear model on every vertex along the cortical surface. False Discovery Rate (FDR) was applied at 0.05 to adjust for multiple comparisons and to detect areas of peak cortical thinning (i.e. atrophy) in PPA groups compared with control subjects (Genovese et al., 2002).
Based on the structure of our olfactory task, we selected five regions of interest that corresponded to core language, visual associative, and olfactory networks (Figs 2 and 3). Note that control participants did not undergo MRI scanning, given that cortical thickness measures in this group were likely to be relatively preserved and therefore minimally informative in comparison with the patient group. Data analysis was restricted to left hemispheric regions of interest due to the left lateralization of language function and the distinctly left-sided cortical atrophy in most patients with PPA. The language regions of interest spanned areas commonly affected in PPA, including inferior frontal gyrus (both pars opercularis and pars triangularis), involved in retrieval and selection of lexical-semantic information (Thompson-Schill et al., 1997; Wagner et al., 2001; Moss et al., 2005), supramarginal gyrus, disruption of which results in phonological (Rohrer et al., 2010) and grammatical (Rogalski et al., 2011a) impairment, and the temporal pole. The visual associative region of interest included the full rostral-caudal extent of the fusiform gyrus, implicated in storage of lexical and visual object representations (Kanwisher et al., 1997; Haxby et al., 2001; McCandliss et al., 2003), and activated during picture object categorization (Ishai et al., 1999), modality-independent lexical processing (DeLeon et al., 2007) and word–picture integration (Martin, 2007; Binder et al., 2009). Finally, the olfactory regions of interest included piriform cortex, which is involved in odour quality coding, object recognition and olfactory perceptual learning (Gottfried, 2010; Wilson and Sullivan, 2011), and mediodorsal thalamus, which receives direct inputs from piriform cortex (Yarita et al., 1980; Russchen et al., 1987), is engaged during olfactory attentional processing (Plailly et al., 2008) and generates an error signal upon delivery of unpredicted versus predicted odours (Zelano et al., 2011).
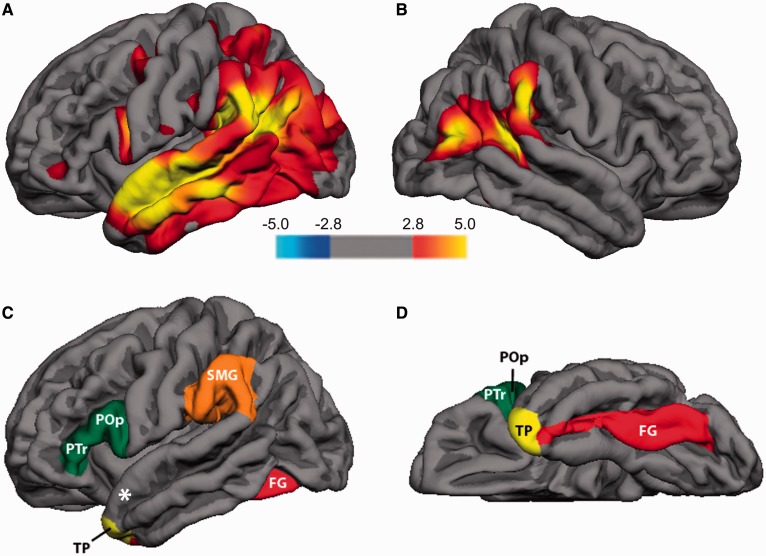
Figure 3.
Cortical atrophy patterns in non-semantic PPA patients compared to an MRI control reference group that was not part of the olfactory study. Red and yellow areas designate significant sites of thinning (atrophy) in patients compared to control subjects, depicted on left hemisphere (A) and right hemisphere (B) lateral views. Significance was set at FDR 0.01 and displayed as −log10(p) values on the flame scale. The cortical regions of interest used for correlation analyses are shown on images of the lateral (C) and ventral (D) left hemisphere. These included temporal pole (TP), fusiform gyrus (FG), supramarginal gyrus (SMG), and inferior frontal gyrus (including pars triangularis, PT, and pars opercularis, POp). Not visible in figure are piriform cortex (PCx) and mediodoursal thalamus, though white ‘*’ indicates approximate PCx location within dorsomedial aspect of the anterior medial temporal lobe.
All of the regions of interest except the piriform cortex were defined according to the conventional parcellations implemented in the software package FreeSurfer (Fischl and Dale, 2000; Fischl et al., 2002) (Fig. 3). FreeSurfer defines the temporal pole from the anterior portion of the temporal lobe (rostrally) to the entorhinal cortex (caudally), and medially from the medial aspect of the temporal lobe and laterally to the superior temporal sulcus (anteriorly) or the inferior temporal sulcus posteriorly (Desikan et al., 2006). FreeSurfer does not have a region of interest for the piriform cortex, and therefore we created a region of interest centred on a seed coordinate where odour-evoked piriform activity tends to be maximal, as based on previous functional MRI research (Howard et al., 2009; Zelano et al., 2011), and expanding 16 concentric circles in grey matter from this coordinate, using Freesurfer (Fig. 3).
Cortical thickness measurements and their correlations with behavioural measures
To localize the brain areas subserving olfactory cognitive processing, we tested a series of correlations between cortical thickness and olfactory perceptual measures. Notably, the amount and distribution of atrophy varies widely across individual patients. Thus, to the extent that specific cortical regions are critical for different tasks, correlations between olfactory perceptual performance and cortical thickness measures should provide a robust method of specifying the functional anatomy of olfactory-language interactions (Rogalski et al., 2011a). Subject-specific ratings of odour intensity and familiarity, odour naming, and odour matching (combined across picture-odour and word-odour matching) were each entered into these analyses, enabling us to survey the different component processes that factor into odour naming and matching. In that the set of odour stimuli systematically varied across categories of edibility and valence, we were also able to enter a proportional measure of within-category and between-category matching errors (as described above) into the correlation analysis.
Statistical analysis
Behavioural data were statistically analysed using SPSS 17 software. Group-level comparisons were carried out using ANOVAs and hierarchical regression analysis. Within-group analyses of associations between variables were carried out using correlation-based statistics. Spearman correlations (two-tailed) were used to identify associations.
Results
Non-semantic primary progressive aphasia
Patient demographics
Control subjects and patients with non-semantic PPA were matched for age [F(1,22) = 1.745, P = 0.200] and education [F(1,22) = 0.035, P = 0.853]. As expected, the patients performed worse than control subjects on the BNT [F(1,22) = 9.787, P = 0.005] and the MMSE [F(1,22) = 7.699, P = 0.011]. While all patients with non-semantic PPA had preserved word comprehension, they presented primarily ‘logopenic’ or ‘agrammatic’ symptoms, except for one patient who was regarded as ‘unclassifiable’ (Mesulam et al., 2012). These subgroups did not differ from each other in relevant demographic or performance measures (all P-values > 0.1; Table 2), and were therefore collapsed into non-semantic PPA in further analyses.
Cortical thinning
Ten patients with non-semantic PPA completed MRI scanning (two of the patients had medical contraindications). Patients with non-semantic PPA showed evidence of cortical atrophy localized in the left lateral temporal cortex, bilateral temporoparietal junction and left inferior frontal gyrus (Fig. 3). Cortical thickness in the temporal pole, inferior frontal gyrus, supramarginal gyrus and fusiform gyrus was significantly lower in the non-semantic PPA group compared with the MRI control reference group (all P values < 0.05), and the cortical volume of mediodorsal thalamus was also lower in non-semantic PPA (P < 0.05). Only the piriform cortex showed no difference in thickness between non-semantic PPA and the MRI control reference group (P = 0.411).
Baseline olfactory perception
Subjective mean ratings of odour intensity, pleasantness and edibility revealed no differences between patients with non-semantic PPA and control subjects (all P-values > 0.20), suggesting that difficulties perceiving these basic olfactory features was unlikely to explain groupwise effects on odour naming and matching. There was a non-significant trend for patients to rate the odours as less familiar than the control subjects [F(1,22) = 3.512, P = 0.074], and odour discrimination was marginally impaired in the patient group [F(1,22) = 4.622, P = 0.043]. Given that the discrimination test requires participants to retain information about three items in mind, this latter impairment may partially reflect working memory deficits in olfaction (Lau et al., 2004).
Word and picture matching
Accuracy for reading the 16 words did not differ across groups [mean non-semantic PPA: 14.3; mean control group: 15.8; F(1,22) = 1.236, P = 0.278]. Note that one patient was unable to perform the reading task, and instead the experimenter read the words aloud while pointing to the corresponding words in the chart. This patient was, however, later able to match odours using the word chart (scoring 6 of 8), indicating no impairment in understanding the meanings of the words. Although most of the 16 pictures were identified in both control (mean 15.6) and patient (mean 14.3) groups, naming was slightly impaired in patients [F(1,22) = 5.991, P = 0.023]. However, all patients were able to match eight verbal word cues to picture objects with perfect accuracy. These results confirmed that multimodal knowledge for the test items was intact in these patients with non-semantic PPA, and that they performed only slightly worse than control subjects in reading and naming test-chart items.
Odour naming
Patients with non-semantic PPA showed severely impaired performance on the odour naming task (Fig. 4): accuracy was 23 ± 19% (mean ± SD) for the patient group compared with 58 ± 17% for the control group, a highly significant effect [F(1,22) = 22.871, P < 0.001]. Performance on this task did not correlate with sex, age or education (all P-values > 0.1) in the overall sample and in patient and control groups when analysed separately (all P-values > 0.1). Importantly, odour naming was not correlated with olfactory discrimination in either non-semantic PPA or control participants (P > 0.6), suggesting that lower group-level accuracy on the discrimination test did not systematically influence naming performance in non-semantic PPA.
Figure 4.
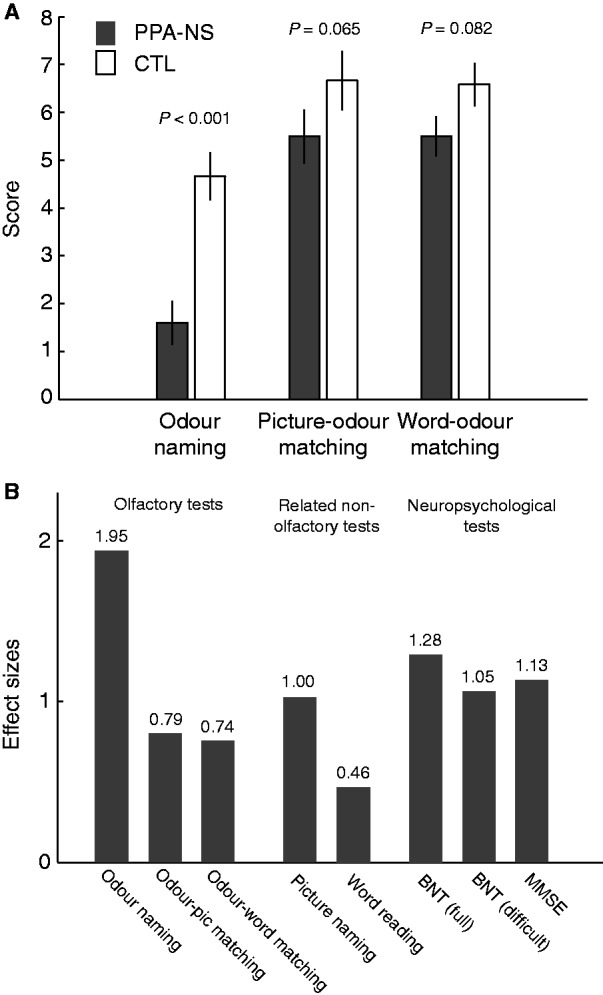
Performance on behavioural tests in non-semantic PPA (PPA-NS) and control (CTL) subjects. (A) Olfactory perceptual performance (mean ± SEM) for patients with non-semantic PPA and control subjects on odour naming and in picture-cued and word-cued odour matching tasks. (B) Effect sizes for the olfactory tests, related non-olfactory tests, and the neuropsychological measures. In all tests, control subjects perform nominally better than patients with non-semantic PPA, such that the height of bars denotes the standardized measures of impairment in non-semantic PPA.
Odour matching
Odour identification in patients with non-semantic PPA substantially improved with the availability of word cues (70 ± 20%) and picture cues (69 ± 24%), with performance approaching control levels [word cues: 82 ± 14%, group difference effect: F(1,22) = 3.314, P = 0.082; picture cues: 83 ± 11%, group difference effect: F(1,22) = 3.769, P = 0.065; Fig. 4]. In support of the above results, a repeated-measures ANOVA with factors of group (patients with non-semantic PPA and control subjects) and odour task (naming, picture-odour matching, and word-odour matching) showed a main effect of group [F(1,22) = 12.603, P = 0.002], a main effect of odour task [F(1,22) = 73.373, P < 0.001] and an interaction of group and odour task [F(1,22) = 5.828, P = 0.008]. The interaction effect constitutes evidence that relative to odour naming, the patient group benefited significantly more than control subjects from access to either picture cues (P = 0.002) or word cues (P = 0.011; Fig. 4). The picture-odour and word-odour matching assessments yielded equal performance levels for both non-semantic PPA patient and control groups (P-values > 0.70). Because an olfactory sensory impairment would have prevented patients from making such effective use of picture or word cues, these findings suggest instead that impaired access to odour names underlies the deficit in the odour naming test. To assess whether the limited performance range in the odour naming test prevented us from differentiating at the individual level, we correlated odour naming scores with odour matching scores (combined across matching tasks) in patient and control groups. These scores were positively correlated among patients, as well as among control subjects (P-values < 0.05), suggesting that both the naming and matching tests accurately differentiate individuals of varying impairment.
As a method to estimate the relative magnitude of the olfactory naming impairment in non-semantic PPA, we calculated the Cohen’s d effect sizes for odour naming and odour matching, BNT and MMSE tests by dividing the group mean differences (control and patients with non-semantic PPA) by the pooled standard deviation (SD) (Cohen, 1992). According to Cohen’s nomenclature, effect sizes ∼0.5 are considered moderate, and >0.8 are considered large (Cohen, 1992). Although all tests yielded moderate to large effects (Fig. 4), odour naming in patients with non-semantic PPA was notably more impaired (∼50% worse, on average) than that of picture naming on the BNT. To the extent that naming odours is more difficult than naming pictures, we also addressed the possibility that task performance per se might have accounted for the effect-size differences between the odour naming test and the BNT. Specifically, because the picture items on the BNT are ordered in progressive difficulty, we recalculated the BNT effect-size based only on the last 10 (most difficult) pictures. Although difficulty increased as expected (BNT performance in control subjects dropped from 95.3% in the full test to 72.3% in the difficult subset), the effect-size did not increase, but decreased slightly from 1.29 to 1.05.
Finally, to establish the specific association between group status (non-semantic PPA versus control subjects) and odour naming, we designed a hierarchical regression model that controlled for demographic variables (age, gender and education), olfactory performance (odour discrimination, word-odour matching and picture-odour matching), and visual object naming and reading (including the BNT, as well as naming pictures and reading words from our charts; Table 3). Results show that after each of these variables was taken into account, group status selectively contributed to the performance pattern on the odour naming task (P = 0.001). These findings converge on a robust association between non-semantic PPA and odour naming that is unlikely to be attributed to generalized naming, object recognition deficits or basic olfactory processing deficits, and instead suggests that odour naming ability is highly vulnerable to perturbations arising from neurodegenerative changes in the language network.
Table 3.
Hierarchical regression model for predicting performance on the odour naming task (control subjects and patients with non-semantic PPA)
| R2 change | R2 | β | P | ||
|---|---|---|---|---|---|
| 1 | Demographics | 0.124 | 0.124 | ||
| Age | −0.325 | 0.144 | |||
| Gender (1 = M, 2 = F) | −0.050 | 0.818 | |||
| Education | −0.107 | 0.694 | |||
| 2 | Olfactory tasks | 0.450 | 0.573 | ||
| Discrimination | −0.071 | 0.755 | |||
| Picture-odour matching | 0.642 | 0.003 | |||
| Word-odour matching | 0.171 | 0.428 | |||
| 3 | Visual tasks | 0.090 | 0.663 | ||
| Picture naming | 0.239 | 0.314 | |||
| Word reading | 0.242 | 0.371 | |||
| BNT | −0.276 | 0.372 | |||
| 4 | PPA status | 0.199 | 0.862 | −0.633 | 0.001 |
The β weights are the standardized regression coefficients at each step.
Within-category errors were calculated as a fraction of the total number of odour-matching errors (collapsed across picture and word cues). In total, the frequency of within-category mismatches (out of all mismatches) exceeded that of chance (20%) in both non-semantic PPA (25 within-category errors out of a total of 60 mismatches = 46%) and control participants (18 within-category errors out of a total of 33 mismatches = 54%).
Magnetic resonance imaging correlation analysis
Based on the olfactory perceptual and cognitive profiles observed in non-semantic PPA, the next step was to map these behavioural variables onto their cortical and subcortical substrates. On a patient-by-patient basis MRI measurements of cortical thickness from olfactory (piriform cortex), lexical-semantic (inferior frontal gyrus, supramarginal gyrus, temporal pole), and visual associative (fusiform gyrus) regions of interest were regressed against the behavioural test measures, including: (i) mean ratings of odour familiarity, which engages olfactory semantic memory and object recognition, but not explicit object identification or overt word production; (ii) odour naming, which requires integrating olfactory information with semantic representations, explicit name retrieval, and verbal production; (iii) odour matching (average of word-cued and picture-cued tasks), which places demands on odour-visual integration but not naming; (iv) the number of within- versus between-category errors in the odour matching tasks (collapsed across word-cued and picture-cued tasks); and (v) mean ratings of odour intensity, as an olfactory control, given that it should minimally engage knowledge-based odour memory and language systems. Insofar as patients with impaired olfactory sensitivity would perceive odours as less intense (e.g. Murphy, 1993), this last task was also included to assess whether impaired olfaction at the peripheral sensory level could otherwise account for the naming-related correlations observed with the other perceptual tests.
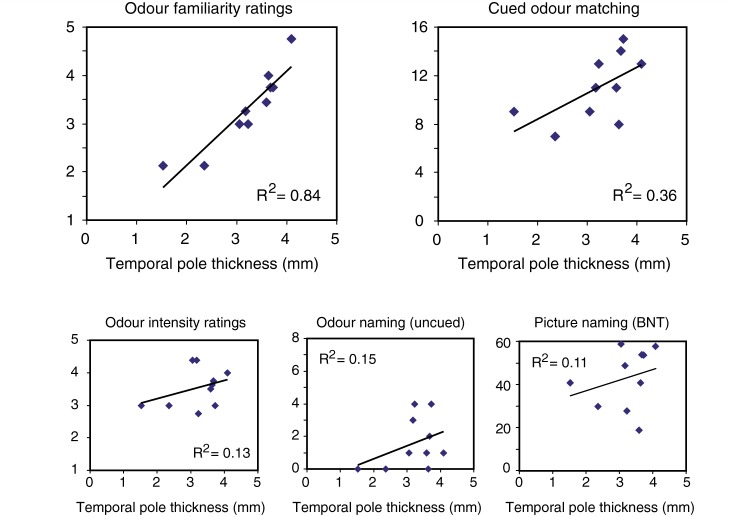
Results of the correlation analysis revealed distinct associations between specific behavioural tasks and cortical regions in the non-semantic PPA group. In the temporal pole, cortical thickness was significantly correlated with odour familiarity (r = 0.94) and odour matching (r = 0.70), but were not strongly associated with the other olfactory measures (Fig. 5). Thus, more pronounced volume loss (cortical thinning) in the temporal pole was associated with a greater tendency to evaluate the set of eight odours as unfamiliar (unrecognizable), and with a greater inability to utilize visually-based semantic information to guide odour object recognition (matching).
Figure 5.
Correlations between olfactory cognitive measures and MRI cortical thickness (mm) in the temporal pole.
Different correlation profiles were observed within the other regions of interest (Table 3). Other than the temporal pole, no other region was associated with odour familiarity. Cortical thickness measures in the fusiform gyrus and inferior frontal gyrus also correlated with odour matching, whereas the inferior frontal gyrus was the only region correlating with odour naming performance. The cortical volume of the mediodorsal thalamus correlated exclusively with error types on the odour matching tasks, such that patients with smaller thalamic volumes exhibited a higher proportion of between-category matching errors, implying a greater inability to refine performance on the basis of error information. Notably, thickness measures of supramarginal gyrus and piriform cortex were not strongly correlated with any behavioural measure. The lack of mean-level cortical thinning in piriform cortex indicated that this region is relatively unaffected in non-semantic PPA. Odour intensity ratings did not correlate significantly with any of the specified regions, despite a wide variation in the subject sample. Follow-up analyses further revealed that age had no influence on any observed associations between cortical thickness and behavioural variables.
Comparison with semantic primary progressive aphasia
To the extent that the temporal pole may mediate access of odour representations to transmodal object representations, we reasoned that olfactory naming and matching deficits would be prominent in patients with semantic PPA, whose atrophy is severe in this brain region. Therefore, in a complementary analysis, we characterized MRI cortical thinning and olfactory behavioural performance in a group of eight patients with semantic PPA (Table 5). The pattern of cortical thinning in the semantic PPA group (compared with the MRI control reference group) was similar to that of non-semantic PPA, but with the addition of pronounced cortical thinning bilaterally in the temporal pole, and in the left orbitofrontal cortex (Fig. 6).
Table 5.
Demographic and performance data for individual patients with semantic PPA
| Patient | Age | Gender | Education (years) | PPVT | Odour naming | Picture-odour matching | Word-odour matching | BNT | MMSE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 12 | 25 | 0 | 3 | 5 | 12 | 23 |
| 2 | 53 | M | 12 | 11 | 0 | 2 | 1 | 3 | 12 |
| 3 | 54 | F | 16 | 15 | 1 | 3 | 4 | 5 | 22 |
| 4 | 61 | F | 13 | 10 | 0 | 2 | 2 | 4 | 24 |
| 5 | 65 | M | 18 | 11 | 0 | 1 | 1 | 3 | 22 |
| 6 | 56 | F | 12 | 8 | 0 | 2 | – | 2 | 17 |
| 7 | 58 | F | 18 | – | 0 | 1 | – | 1 | 2 |
| 8 | 70 | M | 18 | 11 | 0 | – | – | 3 | 15 |
PPVT = Peabody Picture Vocabulary Test.
Missing data are indicated by a dash.
Figure 6.
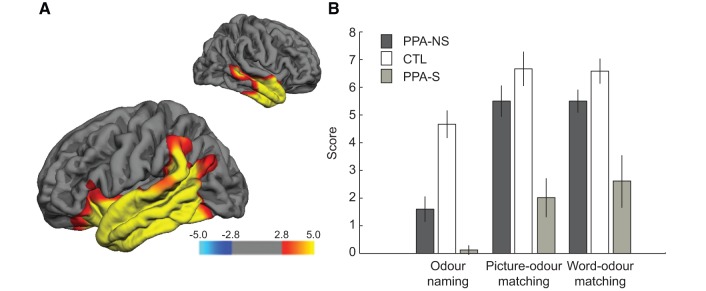
Imaging and behavioral data in patients with semantic PPA. (A) Cortical atrophy patterns in non-semantic PPA patients compared to an MRI control reference group. Red and yellow areas designate significant sites of thinning (atrophy) in patients compared to control subjects, depicted on left hemisphere and right hemisphere lateral views. Significance was set at FDR 0.01 and displayed as −log10(p) values on the flame scale. (B) Group-level behavioral performance on uncued odour naming, as well as picture-cued and word-cued odour matching (mean ± SE), is plotted beside the data from the non-semantic PPA patients and control subjects (from Fig. 4A) for comparison.
In comparison with control subjects, patients with semantic PPA exhibited marked impairments in visual object naming as assessed by the BNT (P < 0.001). Patients were impaired in both odour naming (P < 0.001) and odour matching (P < 0.001), similar to previous reports in semantic dementia and frontotemporal lobar degeneration (Luzzi et al., 2007; Rami et al., 2007; Piwnica-Worms et al., 2010), as well as odour familiarity ratings (P = 0.001). As expected, semantic PPA performance on most of these measures was significantly lower even when compared with non-semantic PPA (naming: P = 0.006; odour-cued matching: P < 0.001; familiarity: P = 0.131), even though both PPA groups performed at equal levels on the odour discrimination task (P = 0.771). Along these lines, patients with semantic PPA showed minimal improvement upon access to word or picture cues, with significantly smaller gains from odour naming to odour matching in comparison with the patients with non-semantic PPA (interaction effect of group-by-odour task; P = 0.007). Finally, the proportion of within-category mismatches approached the 20% chance-level (15 within-category errors out of a total of 58 mismatches = 26%), a profile strikingly different from non-semantic PPA. Together these results reinforce the notion that the temporal pole mediates access of odour representations to transmodal object representations in the language network. Note that because the patients with semantic PPA showed generalized deficits in both olfactory and non-olfactory cognitive tasks, presumably as a consequence of severe and uniform atrophy in the anterior temporal lobes, parametric brain-behaviour correlations for semantic PPA were generally uninterpretable. That temporal pole cortical thickness in semantic PPA was 50% of temporal pole cortical thickness in non-semantic PPA, with a standard deviation across patients of 36% compared with that of non-semantic PPA, strongly suggests that the semantic PPA group had profound temporopolar atrophy that varied little across patients. Such striking differences between semantic and non-semantic PPA were not observed in any other region of interest.
Discussion
The naming of common everyday odours without the benefit of contextual cues can be extremely challenging (Cain, 1979). That olfactory anomia is manifest even in young healthy individuals raises important questions about why odour objects are so hard to verbalize. Here, we investigated the neurocognitive mechanisms underlying olfactory naming, utilizing PPA as an effective ‘lesion model’ of language function. Compared with an age-matched group, patients with non-semantic PPA demonstrated a profound impairment in retrieving the name of an odour even when they could recognize the word or picture representing that odour in a multiple-choice task. The same patients had no comparable difficulty in retrieving the name of pictures of the corresponding objects, thus reflecting the selective difficulty of using olfactory cues to verbalize the corresponding lexical label.
Taken together, our findings indicate that in non-semantic PPA, olfactory afferent pathways and lexical-semantic representations are both intact, pointing instead towards a fundamental disconnection syndrome that limits access of olfactory representations to the language network. As a consequence of the neuronal loss in PPA, the remaining connections can still support the informatically less challenging task of olfactory lexical recognition (matching), but not the more taxing task of olfactory lexical retrieval (naming). An analogous process, attributed to a weakening of the putative pre-lexical ‘lemma’, has been observed in the visual object naming performance of non-semantic PPA variants (Hurley et al., 2012).
Because patients with PPA typically display greater anomia and word-finding problems for lower-frequency visual and verbal items (Patterson et al., 2006), it could be argued that the use of uncommon or atypical olfactory stimuli resulted in poorer performance on the odour naming tasks, compared with the picture naming task (i.e. BNT). However, confining our analysis to the 10 lowest-frequency items on the BNT resulted in no increase in effect-size (Fig. 4B). Furthermore, all odours were based on common, easily recognizable objects and were rated as highly familiar by the control participants (Table 1). This pattern of results suggests that odour naming—a task that is already effortful in healthy individuals—is particularly vulnerable to further disruption as a consequence of early neurodegenerative changes in the language network.
Concurrent MRI measurements of cortical thickness in the patient group enabled us to identify some of the key structures underpinning olfactory processing from odour input to naming and recognition. Based on previous anatomical work, we hypothesized that an odour–language interface may exist in the temporal pole, a region of sensory multisensory convergence (Markowitsch et al., 1985; Moran et al., 1987) that may serve as an associative link to heteromodal object representations (Mesulam et al., 2009a; Schwartz et al., 2009; Hurley et al., 2012; Mesulam et al., 2013). The temporal pole has access to odour information relatively early in the olfactory processing stream (cf. Fig. 2), receiving direct short-fibre projections from piriform cortex, and at the same time receiving visual and auditory associative information (Moran et al., 1987; Russchen et al., 1987), making it an ideal bridge between olfactory and transmodal networks.
Patient- and region-specific measures of cortical atrophy in non-semantic PPA revealed that temporal pole thickness was robustly correlated with both odour familiarity scores (r = 0.94) and odour matching (r = 0.70). Importantly, all of the odours used in our study corresponded with common everyday smells that the patients with PPA would have likely experienced many times in the past. Therefore, the tendency to rate these odours as familiar, i.e. to recognize these odours as familiar objects, depends on linking olfactory cortical representations (e.g. peanut butter smell) to transmodal knowledge-based representations (e.g. can be eaten with bread and jam; sticks to the roof of your mouth; former US President Jimmy Carter’s farm crop). It follows that patients with extensive temporal lobe atrophy, such as those with semantic PPA, should have correspondingly greater impairments in odour familiarity, naming, and matching (Fig. 6). Our results thus support the idea that the temporal pole constitutes an associative node where representations of odour objects (as well as other objects) gain access to their experientially stored associations. With greater atrophy in temporal pole, olfactory information becomes increasingly disconnected from stored transmodal representations, making it difficult to determine whether the odours are recognizable and familiar objects (Luzzi et al., 2007; Plailly et al., 2007; Rami et al., 2007; Piwnica-Worms et al., 2010). Ultimately, whether the temporal pole is acting like a throughput gateway to link sensory representations with concepts distributed elsewhere in the brain (Mesulam et al., 2013), or has a more active role as a storage hub that integrates information across different sensory inputs (Patterson et al., 2007), is presently unclear, though in either case it appears that the temporal pole makes a critical contribution to the instantiation of knowledge-based olfactory representations.
A breakdown in olfactory associations could arise from direct degradation of upstream odour representations in piriform cortex (Zatorre et al., 1992; Jones-Gotman et al., 1997). This is unlikely, given that patients with non-semantic PPA improved their olfactory performance significantly more than control subjects upon access to visual cues, which would be difficult to achieve with an olfactory-sensory impairment. It should also be noted that cortical thinning in the piriform cortex was not detected in non-semantic PPA relative to control subjects, and that piriform cortex cortical thickness did not significantly correlate with performance on any of the olfactory tasks (Table 4). Alternatively, it is possible that degradation at the level of non-olfactory associations could also compromise performance on olfactory tasks. However, as noted above, the ability of patients with non-semantic PPA to correctly name and identify the picture objects suggests that multimodal representations of these specific items were essentially intact even though odour naming was impaired.
Table 4.
Correlation matrix for all included olfactory behavioural measures and cortical areas in non-semantic PPA
| PPA-NS | Odour naming | Odour matching | Odour familiarity | Mismatch errors (within – between) | Odour intensity |
|---|---|---|---|---|---|
| PCx | −0.524 | −0.373 | 0.257 | −0.238 | 0.154 |
| TP | 0.418 | 0.697* | 0.936*** | 0.077 | 0.160 |
| FG | 0.436 | 0.795** | 0.398 | 0.347 | −0.018 |
| IFG | 0.835** | 0.765** | 0.018 | 0.437 | −0.185 |
| MDT | 0.131 | 0.236 | 0.031 | 0.705* | −0.519 |
| SMG | 0.374 | 0.502 | −0.080 | 0.135 | −0.123 |
FG = fusiform gyrus; IFG = inferior frontal gyrus; MDT = mediodorsal thalamus; PCx = piriform cortex; PPA-NS = non-semantic PPA; SMG = supramarginal gyrus; TP = temporal pole.
*P < 0.05; **P < 0.01; ***P < 0.001 (Spearman correlations, two-tailed).
Interestingly, odour matching performance in the non-semantic PPA group correlated with thickness measures not only in temporal pole, but also in fusiform gyrus and inferior frontal gyrus. These findings suggest recruitment of the object recognition and language networks when multimodal associations of an odour need to be accessed. The need to bring together olfactory and visual inputs and to link transmodal representations would be optimized with the participation of the temporal pole, based on its anatomical connectivity as depicted in Fig. 2 (Moran et al., 1987). In this scheme, the medial dorsal thalamus might help constrain the set of viable options by biasing the system away from unlikely (out of category) matches, which would be broadly compatible with its involvement in olfactory attention and prediction error signalling (Plailly et al., 2008; Tham et al., 2011). With olfactory input arriving from piriform cortex and higher-order associative input arriving from temporal pole, the medial dorsal thalamus would be in an ideal position to compare these inputs and generate an error signal for guiding successful odour recognition. This concept of top-down predictions refining perception of bottom-up projections has been proposed to explain information processing in the visual system (Rao and Ballard, 1999; Spratling, 2008; Summerfield and Egner, 2009). In semantic PPA, the observed breakdown of odour categories might thus be explained by their severe temporal pole atrophy, which would effectively abolish communication with the medial dorsal thalamus.
Amongst all of the cortical regions of interest, the inferior frontal gyrus was implicated in odour naming (r = 0.84). Insofar as the inferior frontal gyrus plays an important role in retrieval of lexical-semantic information and in selection of competing semantic alternatives, we propose that an under-specification of the mapping of odours to lexical-semantic representations places greater processing demands on inferior frontal gyrus at the verbalization stage (Herz and Engen, 1996). Congruent with this proposal, human imaging data suggest that a working-memory buffer specifically for nameable odours elicits the participation of the inferior frontal gyrus, where lexical-semantic information can be maintained in a phonological loop in advance of articulating veridical odour names (Savic and Berglund, 2004; Zelano et al., 2009). That being said, it is important to note that the cortical findings were based on a modest patient sample size; thus, some of the non-significant correlations in Table 4 might reflect type II (false negative) error and should be interpreted with caution.
Assuming that the temporal pole helps to bridge the olfactory and language systems, it is worth speculating why odour object identification and naming in healthy individuals is so impoverished, particularly in comparison with visual object naming. In the olfactory system, odour information is minimally elaborated before arriving at the temporal pole, with only three intervening synapses: from olfactory receptor neurons to olfactory bulb, olfactory bulb to piriform cortex, piriform cortex to temporal pole. Thus, the temporal pole would present relatively coarse and unprocessed odour object information to the lexical-semantic network, leading to mapping imprecision and object mismatches. By comparison, in the visual system, information is substantially trafficked through multiple unimodal areas prior to reaching the temporal pole. Because of these additional synapses in unimodal cortices, visual object representations have greater associative depth and robustness to facilitate interactions with the language network.
This difference in the extent of unimodal areas available for object processing—many in the visual system, few in the olfactory system—helps explain why odour identification is so much more vulnerable to perceptual ambiguity. An associative perceptual problem, such as naming the picture of a wet dog, can be solved through a large number of alternative pathways (i.e. has more redundancy), compared with the analogous problem of naming the smell of a wet dog. This processing dilemma may reflect the evolutionary legacy of olfaction, well-designed to support limbic-based adaptive behaviour, but poorly constructed to partner with perisylvian language regions that evolved much later. The results presented here provide a starting point for future studies aiming to understand not only how the brain translates odour inputs into odour names, but also more generally, how limbic and paralimbic areas interface with cortical centres to modulate perception, language and other cognitive functions.
Funding
This work was supported by a postdoctoral fellowship to J.K.O. from the Swedish Research Council, NIH grants to M.M.M. from the National Institute on Deafness and Other Communication Disorders (DC008552) and the National Institute on Aging (AG13854; Alzheimer’s Disease Centre), and NIH grants to J.A.G. from the National Institute on Deafness and Other Communication Disorders (DC010014; DC007653), grant to E.R. from the National Centre for Research Resources (5KL2RR025740).
Acknowledgements
We thank Christina Wieneke and Joseph Boyle for their contributions in the administrative aspects of the study, Rob Hurley for valuable discussions, and Adam Martersteck for assistance with data analysis.
Glossary
Abbreviations
- BNT
Boston Naming Test
- MMSE
Mini-Mental State Examination
- PPA
primary progressive aphasia
References
- Bartoshuk LM. Psychophysics of taste. Am J Clin Nutr. 1978;31:1068–77. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–53. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Brown R, Mcneill D. Tip of tongue phenomenon. J Verbal Learn Verbal Behav. 1966;5:325–37. [Google Scholar]
- Cain WS. Know with the nose—keys to odor identification. Science. 1979;203:467–70. doi: 10.1126/science.760202. [DOI] [PubMed] [Google Scholar]
- Cain WS, de Wijk R, Lulejian C, Schiet F, See LC. Odor identification: perceptual and semantic dimensions. Chem Senses. 1998;23:309–26. doi: 10.1093/chemse/23.3.309. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Laing DG. Psychophysical measurement of human olfactory function, including odorant mixture assessment. In: Doty RL, editor. Handbook of olfaction and gustation. 2nd edn. New York: Marcel Dekker, Inc; 2003. pp. 338–81. [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University-of-Pennsylvania smell identification test—a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test. 4th edn. Toronto, Ontario: Pearson Canada Assessment, Inc.; 2006. [Google Scholar]
- Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain [Article] Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-mental state-practical method for grading cognitive state of patients for clinician. J Psychiatric Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–41. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Herz RS, Engen T. Odor memory: review and analysis. Psychonomic Bull Rev. 1996;3:300–13. doi: 10.3758/BF03210754. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia—progressive fluent aphasia with temporal-lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Horel JA, Keating EG, Misantone LJ. Partial Kluver-Bucy syndrome produced by destroying temporal neocortex or amygdala. Brain Res. 1975;94:347–59. doi: 10.1016/0006-8993(75)90067-0. [DOI] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12:932–38. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, Mesulam MM. Neural mechanisms of object naming and word comprehension in promary progressive aphasia. J Neurosci. 2012;32:4848–55. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten HL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA. 1999;96:9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Gotman M, Zatorre RJ, Cendes F, Olivier A, Andermann F, McMackin D, et al. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120:1845–56. doi: 10.1093/brain/120.10.1845. [DOI] [PubMed] [Google Scholar]
- Jonsson FU, Olsson MJ. Olfactory metacognition. Chem Senses. 2003;28:651–8. doi: 10.1093/chemse/bjg058. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Kertesz A. Western aphasia battery. San Antonio, TX: Psychological Corp; 1982. [Google Scholar]
- Kling AS, Tachiki K, Lloyd R. Neurochemical correlates of the Kluver-Bucy syndrome by in-vivo microdialysis in monkey. Behav Brain Res. 1993;56:161–70. doi: 10.1016/0166-4328(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Lau S, O'Mahony M, Rousseau B. Are three-sample tasks less sensitive than two-sample tasks? Memory effects in the testing of taste discrimination. Percep Psychophys. 2004;66:464–74. doi: 10.3758/bf03194894. [DOI] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behav Brain Res. 2011;225:341–7. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Lorig T. On the similarity of odor and language perception. Neurosci Biobehav Rev. 1999;23:391–8. doi: 10.1016/s0149-7634(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–31. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Res Rev. 1995;21:117–27. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primates temporal pole—a study of rhesus-monkeys, squirrel-monkeys, and marmosets. J Comp Neurol. 1985;242:425–58. doi: 10.1002/cne.902420310. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–9. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam MM, editor. Principles of behavioral and cognitive neurology. 2nd edn. Oxford: Oxford University Press; 2000. [Google Scholar]
- Mesulam MM, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009a;132:2553–65. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley RS, Rademaker A, Weintraub S, Rogalski ER. Words and objects in the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013 doi: 10.1093/brain/aws336. doi: 10.1093/brain/aws336. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009b;66:1545–51. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–53. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–68. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus-monkey. J Comp Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, et al. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–35. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. Nutrition and chemosensory perception in the elderly. Crit Rev Food Sci Nutr. 1993;33:3–15. doi: 10.1080/10408399309527607. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness—Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;812:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Patterson K, Lambon Ralph MA, Jefferies E, Woollams A, Jones R, Hodges JR, et al. “Presemantic” cognition in semantic dementia: six deficits in search of an explanation. J Cogn Neurosci. 2006;18:169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex. 2010;46:761–8. doi: 10.1016/j.cortex.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 2008;28:5257–67. doi: 10.1523/JNEUROSCI.5607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J, Radnovich AJ, Sabri M, Royet JP, Kareken DA. Involvement of the left anterior insula and frontopolar gyrus in odor discrimination. Hum Brain Mapp. 2007;28:363–372. doi: 10.1002/hbm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Slotnick BM. Dual olfactory representation in the rat thalamus—an anatomical and electro-physiological study. J Comp Neurol. 1983;215:63–77. doi: 10.1002/cne.902150106. [DOI] [PubMed] [Google Scholar]
- Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. J Neurol. 2007;254:431–5. doi: 10.1007/s00415-006-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RPN, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011a;31:3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011b;76:1804–10. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49:984–93. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, et al. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13:506–19. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, et al. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, macaca-fascicularis. J Comp Neurol. 1987;256:175–210. doi: 10.1002/cne.902560202. [DOI] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, et al. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–66. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Berglund H. Passive perception of odors and semantic circuits. Hum Brain Mapp. 2004;21:271–8. doi: 10.1002/hbm.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan CM, Kertesz A. Reliability and validity characteristics of the western aphasia battery (wab) J Speech Hear Disord. 1980;45:308–24. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127:860–72. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Spratling MW. Reconciling predictive coding and biased competition models of cortical function. Front Comput Neurosci. 2008;2:1–8. doi: 10.3389/neuro.10.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Egner T. Expectation (and attention) in visual cognition. Trends Cogn Sci. 2009;13:403–9. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tham WW, Stevenson RJ, Miller LA. The functional role of the medio dorsal thalamic nucleus in olfaction. Brain Res Rev. 2009;62:109–26. doi: 10.1016/j.brainresrev.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Tham WW, Stevenson RJ, Miller LA. The role of the mediodorsal thalamic nucleus in human olfaction. Neurocase. 2011;17:148–59. doi: 10.1080/13554794.2010.504728. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain. 2011;134:3094–105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–19. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarita H, Iino M, Tanabe T, Kogure S, Takagi SF. Transthalamic olfactory pathway to orbitofrontal cortex in the monkey. J Neurophysiol. 1980;43:69–85. doi: 10.1152/jn.1980.43.1.69. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 2010;61:219–41. doi: 10.1146/annurev.psych.60.110707.163639. [DOI] [PubMed] [Google Scholar]
- Zarzo M. Psychologic dimensions in the perception of everyday odors: pleasantness and edibility. J Sens Stud. 2008;23:354–76. [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–40. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- Zelano C, Mohanty A, Gottfried JA. Olfactory predictive codes and stimulus templates in piriform cortex. Neuron. 2011;72:178–87. doi: 10.1016/j.neuron.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Montag J, Khan R, Sobel N. A specialized odor memory buffer in primary olfactory cortex. Plos One. 2009;4:e4965. doi: 10.1371/journal.pone.0004965. [DOI] [PMC free article] [PubMed] [Google Scholar]