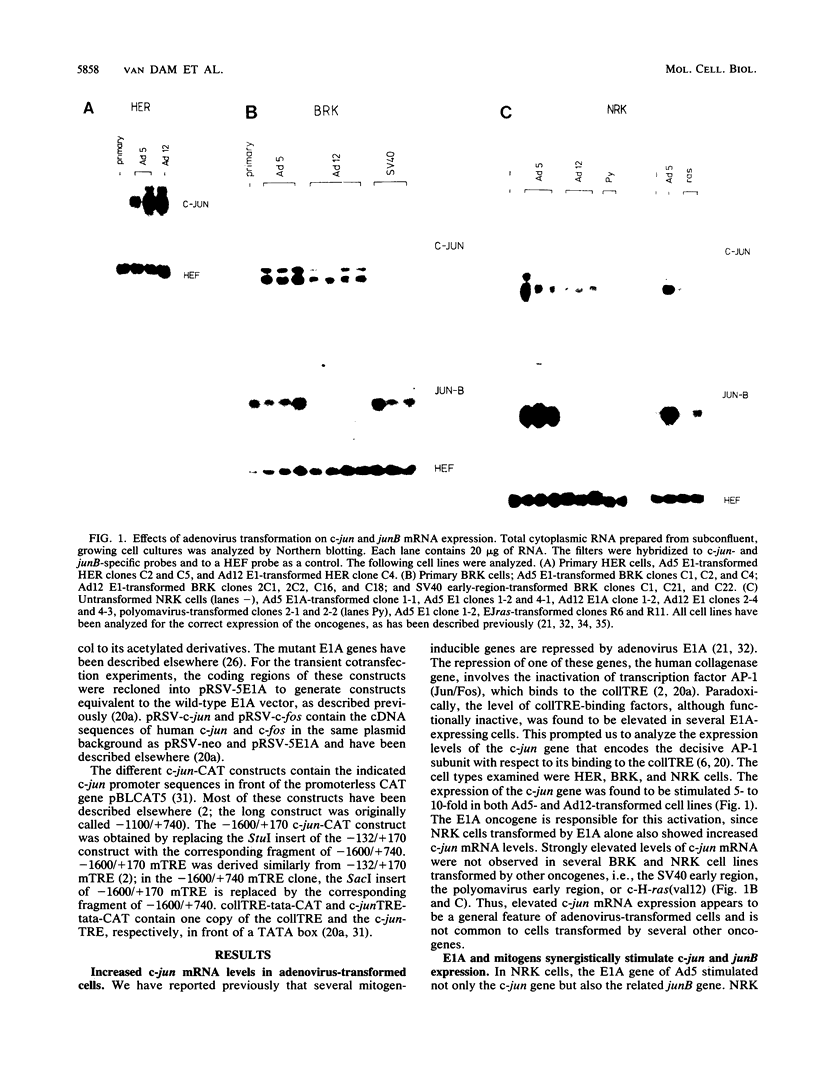
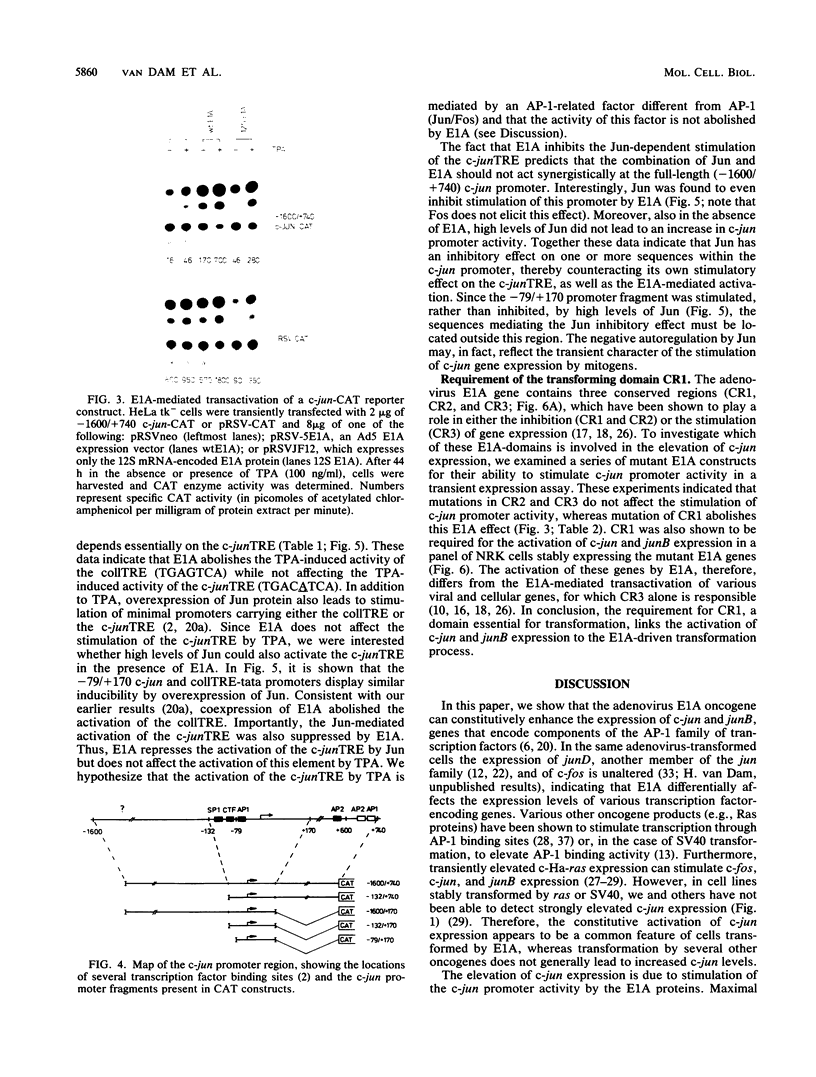
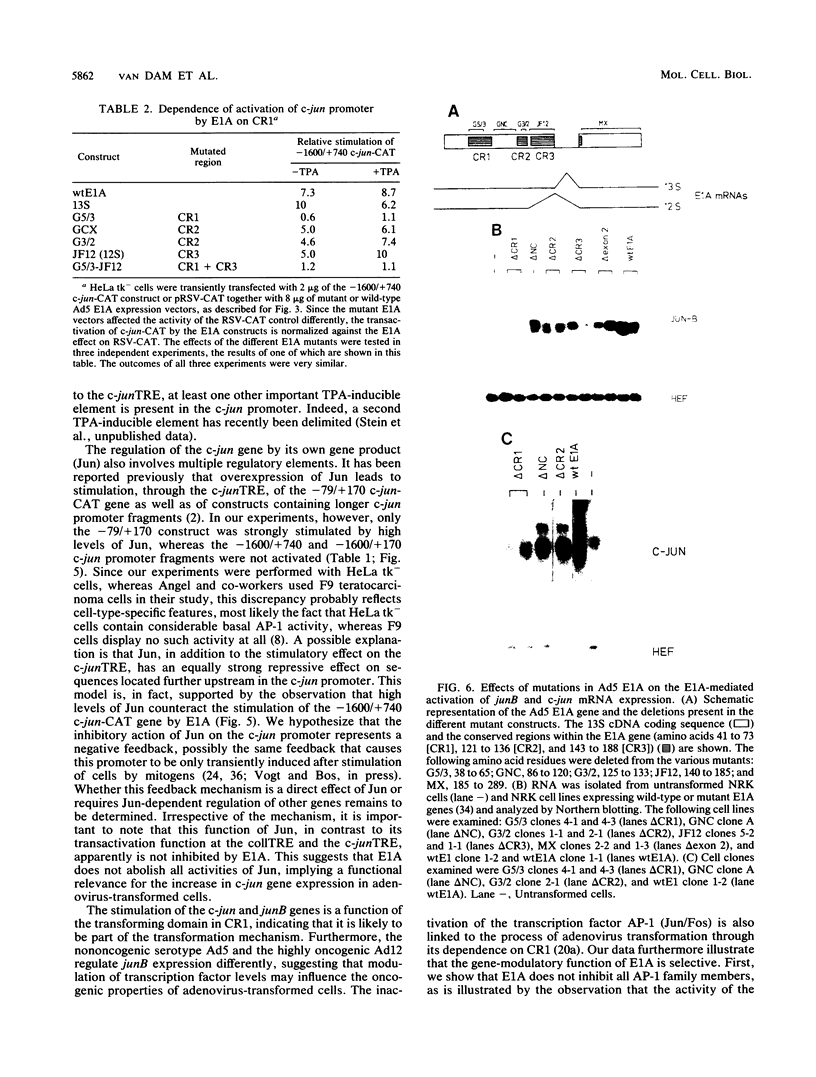
Abstract
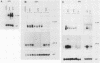
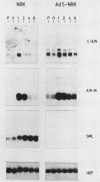
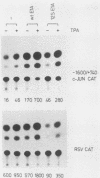
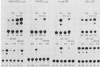
The adenovirus early region 1A (E1A) oncogene interferes with the expression level and activity of the AP-1 transcription factor family. E1A abolished the transactivating function of AP-1 (Jun/Fos), which binds to the 12-O-tetradecanoylphorbol-13-acetate-responsive element of the collagenase gene (collTRE). In contrast, the activity of another member of the AP-1 family that binds to the c-junTRE was not repressed. The mRNA expression of the c-jun gene was, in fact, strongly elevated in various cell types expressing the E1A gene of either adenovirus type 5 (Ad5) or Ad12. The regulation of the junB gene by adenovirus E1A, on the other hand, depended both on the cell type and on the transforming adenovirus serotype. The fact that E1A-induced alterations in the repertoire of AP-1 transcription factors depend on its transforming domain in conserved region 1 suggests that the effects are relevant for the transformation process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M., Pusztai R., Symington J. S. An adenovirus E1A protein domain activates transcription in vivo and in vitro in the absence of protein synthesis. Cell. 1988 Jun 17;53(6):921–926. doi: 10.1016/s0092-8674(88)90429-1. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Offringa R., Gebel S., van Dam H., Timmers M., Smits A., Zwart R., Stein B., Bos J. L., van der Eb A., Herrlich P. A novel function of the transforming domain of E1a: repression of AP-1 activity. Cell. 1990 Aug 10;62(3):527–538. doi: 10.1016/0092-8674(90)90017-9. [DOI] [PubMed] [Google Scholar]
- Offringa R., Smits A. M., Houweling A., Bos J. L., van der Eb A. J. Similar effects of adenovirus E1A and glucocorticoid hormones on the expression of the metalloprotease stromelysin. Nucleic Acids Res. 1988 Dec 9;16(23):10973–10984. doi: 10.1093/nar/16.23.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hirai S., Yaniv M. Constitutive synthesis of activator protein 1 transcription factor after viral transformation of mouse fibroblasts. Proc Natl Acad Sci U S A. 1988 May;85(10):3401–3405. doi: 10.1073/pnas.85.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Kamps M., Verma I. M. fos-associated cellular p39 is related to nuclear transcription factor AP-1. Cell. 1988 Aug 12;54(4):553–560. doi: 10.1016/0092-8674(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Gebel S., Stein B., Ponta H., Rahmsdorf H. J., Herrlich P. Nuclear oncoproteins determine the genetic program in response to external stimuli. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):779–787. doi: 10.1101/sqb.1988.053.01.088. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Mäkelä T. P., Keski-Oja J., Alitalo K. The cellular response to induction of the p21 c-Ha-ras oncoprotein includes stimulation of jun gene expression. EMBO J. 1989 Mar;8(3):815–822. doi: 10.1002/j.1460-2075.1989.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers H. T., De Wit D., Bos J. L., Van der Eb A. J. E1A products of adenoviruses reduce the expression of cellular proliferation-associated genes. Oncogene Res. 1988;3(1):67–76. [PubMed] [Google Scholar]
- Timmers H. T., van Dam H., Pronk G. J., Bos J. L., Van der Eb A. J. Adenovirus E1A represses transcription of the cellular JE gene. J Virol. 1989 Mar;63(3):1470–1473. doi: 10.1128/jvi.63.3.1470-1473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. The oncogene jun and nuclear signalling. Trends Biochem Sci. 1989 May;14(5):172–175. doi: 10.1016/0968-0004(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. E1A control of gene expression is mediated by sequences 5' to the transcriptional starts of the early viral genes. Mol Cell Biol. 1983 Jul;3(7):1222–1234. doi: 10.1128/mcb.3.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Smits A. M., Bos J. L., Jones N. C., van der Eb A. J. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene. 1989 Oct;4(10):1207–1212. [PubMed] [Google Scholar]
- van der Lubbe J. L., Abrahams P. J., van Drunen C. M., van der Eb A. J. Enhanced induction of SV40 replication from transformed rat cells by fusion with UV-irradiated normal and repair-deficient human fibroblasts. Mutat Res. 1986 Mar;165(2):47–56. doi: 10.1016/0167-8817(86)90059-3. [DOI] [PubMed] [Google Scholar]