Abstract
The aim of the present study was to determine whether the sensitivity of thymocytes to X-ray radiation depends on their proliferative states and whether radiation impairs the maturation of donor-derived thymocytes in recipient thymus. We assigned 8-week-old C57BL/6J mice into three treatment groups: 1) untreated; 2) X-ray radiation; 3) X-ray radiation plus bone marrow transplantation with donor bone marrow cells from transgenic mice expressing enhanced green fluorescent protein (GFP) on a universal promoter. After 4 weeks, the size of the thymus, the number and proliferation of thymocytes and ratios of different stage thymocytes were analyzed by immunohistochemistry and flow cytometry. The results showed that: 1) CD4+CD8+ thymocytes were more sensitive to X-ray radiation-induced cell death than other thymocytes; 2) the proliferative capacity of CD4+CD8+ thymocytes was higher than that of other thymocytes; 3) the size of the thymus, the number of thymocytes and ratios of thymocytes of different stages in irradiated mice recovered to the normal level of untreated mice by bone marrow transplantation; 4) the ratio of GFP-positive CD4+CD8+ thymocytes increased significantly, whereas the ratio of GFP-positive CD4+ or CD8+ thymocytes decreased significantly. These results indicate that the degree of sensitivity of thymocytes to X-ray radiation depends on their proliferative states and radiation impairs the maturation of donor-derived CD4+CD8+ thymocytes in recipient thymus.
Keywords: thymus, radiation, CD4+CD8+ thymocytes, sensitivity, donor cells
INTRODUCTION
T-cell development in the thymus is a complex process. CD4– CD8– double-negative (DN) thymocytes develop from early thymic progenitors located in the subcapsular zone (SCZ), and then advance to CD4+CD8+ double-positive (DP) cells as they meander through the cortex. DP thymocytes in the cortex undergo a critical developmental checkpoint called positive selection and a small fraction of DP thymocytes successfully complete positive selection, travel to the medulla and become CD4+ or CD8+ single-positive (SP) thymocytes. In the medulla, negative selection is required for eliminating self-reactive thymocytes. Mature thymocytes in the medulla egress from the thymus to the blood stream and then migrate to the peripheral lymphoid organs[1],[2].
It is known that ionizing radiation causes organ atrophy and inhibition of the immune system and the underlying mechanism involves radiation-induced apoptosis in immunocytes[3]. Furthermore, in the thymus, non-immune structural cells such as thymic epithelial cells, which are recognized as one of the important components of the microenvironment for T-cell development, are also damaged.
Immature DP thymocytes, a major component of the thymus, are intrinsically sensitive to p53-dependent or independent apoptosis induced by radiation[4]–[7]. Previous studies mainly focused on radiation-induced apoptosis and its underlying mechanism. However, whether DN and SP thymocytes could completely escape from radiation-induced cell death and what contributes to the different cell fates of DN, DP and SP thymocytes remains to be elucidated.
Once lymphoid precursors enter the thymus from the blood stream, they come into contact with thymic epithelial cells that guide their maturation into functionally competent T cells[8]. Some studies have proven that radiation could induce microenvironment damage, which plays an active role in carcinogenesis[9]. Total body radiation has been widely used clinically to eliminate malignant cells or to inhibit immune response for bone marrow transplantation (BMT). Therefore, whether donor-derived precursors could develop normally in the thymus of irradiated recipient is drawing increasing attention.
Here, we designed this animal experiment to determine whether the sensitivity of thymocytes to X-ray radiation (XR) depends on their proliferative states and whether radiation impairs the maturation of donor-derived thymocytes in recipient thymus.
MATERIALS AND METHODS
Mice
C57BL/6J (WT) mice (≤8 weeks) and transgenic mice expressing enhanced GFP were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA) and Riken BioResource Center (Tsukuba, Ibaraki, Japan), respectively. The mice were maintained in the Experimental Animal Center of Nanjing Medical University. All experimental procedures were carried out in accordance with the United States National Institutes of Health Guidelines for Care and Use of Laboratory Animals. C57BL/6J (WT) mice were assigned into three treatment groups: 1) untreated control; 2) XR; 3) XR+BMT (transplanted with bone marrow cells from transgenic mice expressing EGFP).
XR and BMT
The genotypes of donor and recipient mice used in this study are EGFP transgenic mice and C57BL/6J wildtype mice, respectively. At 8 weeks of age, recipient mice first received 9.5 Gy single-dose total body irradiation by a Varian 600CD linear accelerator. Within 24 h, 5×106 donor cells suspended in 100 µL α-minimal essential medium (α-MEM, Invitrogen, Carlsbad, CA, USA) were administered via the tail vein as previously described[10]. Four weeks after transplantation, recipient mice were euthanized and the respective thymus tissues were prepared for further analysis.
BrdU incorporation assay
One dose of 3 mg BrdU (Sigma, St. Louis, MO, USA) was intraperitoneally (i.p.) injected into mice. Bone marrow (BM) samples were collected 3 d later for further analysis.
Flow cytometry
Cell surface labeling: Single-cell suspensions of thymocytes stained with FITC-CD4, PE-CD8α or APC-CD8α (eBioscience, San Diego, CA, USA) were analyzed by flow cytometry. All labeling was performed in a sodium potassium buffer containing glucose and 1% BSA for 30 min at 4°C. After centrifugation, the cells were washed with phosphate buffered saline (PBS), fixed with 2% formalin and subsequently analyzed on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
BrdU staining: Single-cell suspension of thymocytes was stained using the above fluorescence labeling antibodies targeting CD4 and CD8, and then fixed with 95% alcohol for 10 min in 4°C. The fixed cells were washed and incubated with a mouse anti-BrdU antibody (Millipore, Billerica, MA, USA) for 30 min. The cells were then washed again and incubated with an APC-conjugated goat anti-mouse IgG (Biolegend, San Diego, CA, USA) following the manufacturer's instructions.
Cell cycle analysis
Totally, 1×106 mouse thymus cells were stained using the cocktail of the above fluorescence labeling antibodies targeting CD4 and CD8 and fixed by 70% ethanol for 1 h at -20°C. After washing with PBS, the cells were stained with 300 µL propidium iodide (PI) staining solution (BD Biosciences) for 30 min on ice. Finally, the sample was kept in the dark for analysis. All data of flow cytometry were analyzed with the FlowJo Version 7.6.1 software (TreeStar Inc., Ashland, OR, USA).
Polymerase chain reaction (PCR)
DNA was extracted from the thymus and amplified by PCR. PCR was performed with the following protocol: 95°C for 5 min×1 cycle; 94°C for 30 sec, 52°C for 45 s, 72°C for 1 min×35 cycles; 72°C for 2 min×1 cycle; stored at 4°C. The following primers were used: EGFP with 5′-GCC ACA AGT TCA GCG TGT CCG-3′ (forward primer) and 5′-GTT GGG GTC TTT GCT CAG GGC G-3′ (reverse primer). Agarose gel electrophoresis was performed to solve the PCR products of EGFP (565 bp).
Immunohistochemistry
Immunohistochemical staining for Ki67 and BrdU was performed using an affinity-purified rabbit anti-mouse Ki67 antibody (Abcam, Cambridge, UK) and a mouse anti-BrdU antibody (Millipore), respectively, as described previously[11]. Briefly, dewaxed and rehydrated paraffin-embedded sections were incubated with hydrogen peroxide in methanol (1:10) to block endogenous peroxidase activity and then washed with Tris-buffered saline (TBS, pH 7.6). The sections were then incubated with the primary antibody overnight at room temperature. After rinsing with TBS for 15 min, the sections were incubated with the secondary antibody (biotinylated goat anti-rabbit IgG or biotinylated goat anti-mouse IgG; Sigma, Louis, MO, USA). The sections were then washed and incubated with the Vectastain Elite ABC reagent (Vector Laboratories, Burlington, Ontario, Canada) for 45 min before addition of 2.5 mg/mL 3,3-diaminobenzidine (DAB). Finally, the stained sections were counterstained with hematoxylin and eosin (H&E) staining[12]. Images were acquired with a Leica microscope (Leica DM4000B, Solms, German) equipped with Leica software.
Statistical analysis
Experiments were independently performed for at least 3 times. Student's t-test and one-way ANOVA with Fisher's least significant difference (LSD) post hoc test were used to analyze the statistical significance among thymic parameters. A Pearson correlation analysis was performed to investigate the relationship between the percentages of thymocytes maintained at G2/M phase before radiation and the percentages of radiation-induced cell death 4 weeks after radiation in different-stage populations. Statistical analysis was performed using SPSS 16.0 (SPSS Institute, Chicago, IL, USA) and the data in tables are expressed as mean±SD. The data in figures were analyzed by GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) and were expressed as mean±SD. P < 0.05 was considered statistically significant.
RESULTS
Thymocytes at different stages exhibit different radiosensitivities
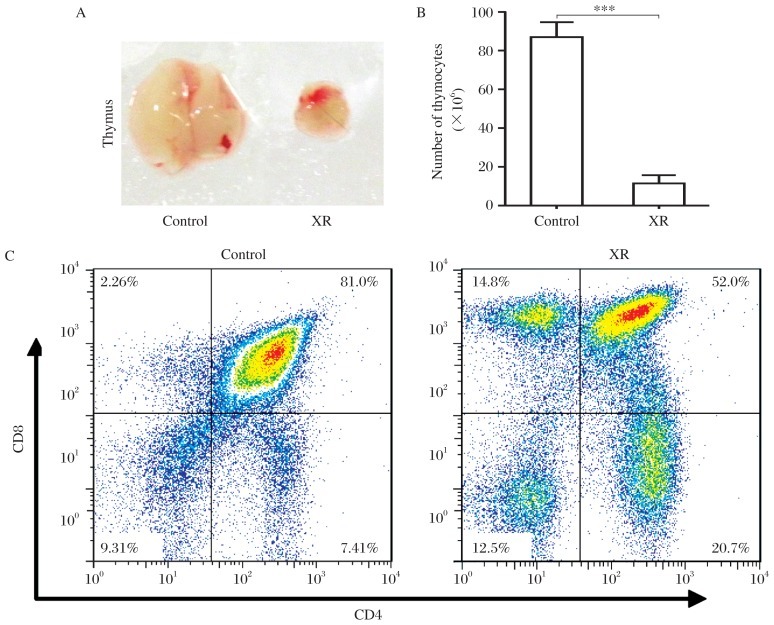
To determine the change in the thymus after radiation, we first evaluated the size and cellularity of the thymus in untreated and irradiated mice. The results showed that, at 4 weeks after irradiation with 9.5 Gy dose, the size of the thymus and the number of thymocytes decreased significantly in the irradiated mice compared with untreated mice (Fig. 1A and 1B). Flow cytometric analysis revealed that the ratio of DP thymocytes decreased significantly, whereas that of CD4–CD8– (DN), CD4+CD8– (CD4-SP) and CD4–CD8+ (CD8-SP) thymocytes increased significantly in the irradiated mice compared with untreated mice (Fig. 1C). However, the numbers of DP, DN, CD4-SP and CD8-SP thymocytes decreased significantly in the irradiated mice to 7.9%, 23.6%, 40.2% and 80.3% of untreated mice, respectively (Table 1). These results revealed that all different stage thymocytes could be affected when exposed to irradiation, and DP thymocytes were the most severely affected while other thymocytes were less affected.
Fig. 1. Different sensitivities to X-ray-induced cell death in thymocytes at different stages.
A: Representative gross graph of the thymus from 12-week-old non-irradiated mice (untreated control) and X-ray irradiated mice (XR) with a dose of 9.5 Gy; B: Bar graph shows the total number of thymocytes in each group (mean±SD; ***P < 0.001; n = 5 each). C: Representative figures of flow cytometric analysis of CD4 and CD8 expression in thymocytes in the untreated and irradiated mice 4 weeks after irradiation.
Table 1. Effects of radiation on thymocytes of different stages.
| Group | Constituent ratio (%) |
Cellularity (×106) |
Cell death (%) (1-XR/Untreated) ×100% | ||
| Untreated | XR | Untreated | XR | ||
| CD4–CD8– | 08.21±2.08 | 14.17±1.88* | 07.07±1.55 | 1.67±0.85** | 76.38 |
| CD4+CD8+ | 82.63±2.10 | 49.87±1.91*** | 71.95±7.97 | 5.70±2.08*** | 92.08 |
| CD4+CD8– | 06.65±0.69 | 20.53±1.46*** | 05.75±0.07 | 2.31±0.69** | 59.83 |
| CD4–CD8+ | 002.5±0.50 | 015.4±0.60*** | 02.19±0.59 | 1.76±0.66 | 19.63 |
*P < 0.05, **P < 0.01, ***P < 0.001 compared with the untreated group. XR: irradiation.
(mean±SD, n = 5)
Radiosensitivity of thymocytes at different stage depends on their proliferative states
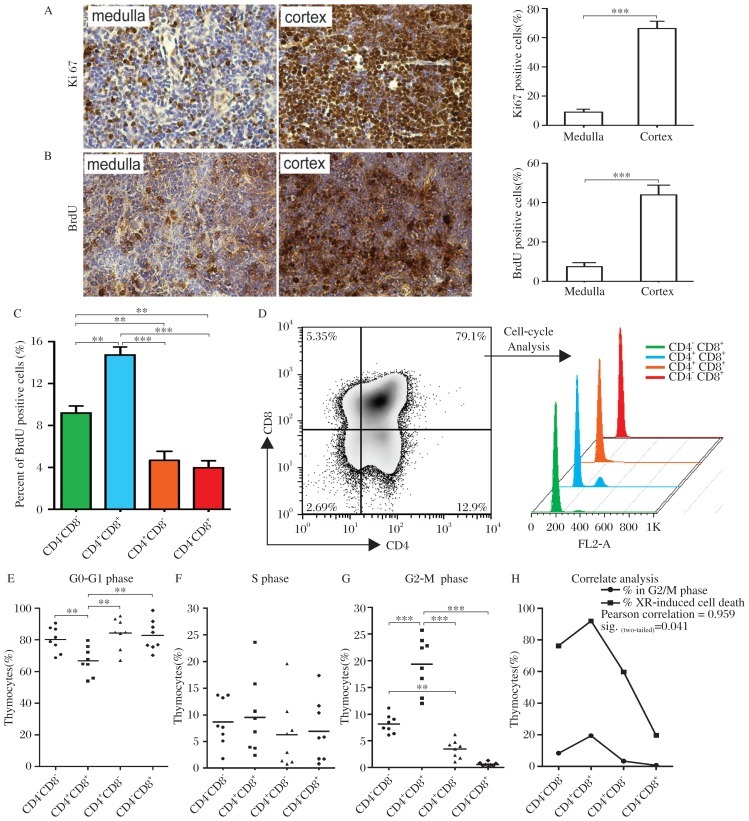
Previous studies have reported that DNA double-strand breaks constitute the most dangerous type of DNA damage induced by ionizing radiation, and genomic instability induced by perturbed recombination in cancer cells make them sensitive to ionizing irradiation[13]. Therefore, we hypothesized that the degree of radiosensitivity of thymocytes at different stages depends on their proliferative states. To test this hypothesis, the proliferation of thymocytes from C57BL/6J mice (≤8 week) before radiation was detected. The results of Ki67 immunohistochemistry revealed that the percentage of Ki67 positive cells was higher in the cortex than that in the medulla (Fig. 2A). To further confirm this result, we performed a 3-d BrdU incorporation assay to evaluate the proliferation of thymocytes. The results of BrdU incorporation assay also confirmed that the percentage of BrdU-positive cells was higher in the cortex than that in the medulla (Fig. 2B). In view of the fact that immature DN and DP thymocytes are localized principally in the SCZ and cortical regions, respectively, and mature SP thymocytes reside within the inner thymic medulla[8], we further analyzed the percentage of BrdU-positive thymocytes in different developmental stages using flow cytometry. The results showed that the percentages of BrdU-positive thymocytes in DN, DP, CD4-SP and CD8-SP populations were 9.2%, 14.7%, 4.7% and 4.0%, respectively. The percentages of BrdU-positive thymocytes in DN and DP populations were higher than those in CD4-SP and CD8-SP populations (Fig. 2C). When cell cycle was analyzed, an obvious G2/M peak was detected in DP thymocytes, but not in the other 3 populations (Fig. 2D). Statistical analysis revealed that 1) the percentage of thymocytes at G0/G1 phase in DP population was significantly lower than that in DN, CD4-SP and CD8-SP thymocytes (Fig. 2E); 2) the percentage of thymocytes at S phase was not significantly altered in DN, DP, CD4-SP and CD8-SP populations (Fig. 2F); 3) the percentage of thymocytes at G2/M phase in DP population was significantly higher than that in DN, CD4-SP and CD8-SP populations; 4) the percentage of thymocytes at G2/M phase in CD4-SP population was significantly higher than that in DN and CD8-SP populations (Fig. 2G). In addition, we performed a correlation analysis to investigate the relationship between the percentages of thymocytes maintained at G2/M phase before radiation and the percentages of radiation-induced cell death 4 weeks after radiation in different-stage populations. As shown in Fig. 2H, there was a significant correlation between the two variables, suggesting that the degree of radiosensitivity of thymocytes depends on their proliferative states.
Fig. 2. The degree of radiosensitivity of thymocytes depends on their proliferative states.
A: Representative tissue sections of the thymus stained immunohistochemically for Ki67. The right panel is the percentage of Ki67 positive cells in the cortex and the medulla of the thymus from 8-week-old C57BL/6J (WT) mice (mean±SD; ***P < 0.001; n = 3 each); B: Thymocyte proliferation was tested by BrdU incorporation assay for 3 days and by immunohistochemistry staining for BrdU. The right panel is the percentage of BrdU positive cells in the cortex and the medulla of the thymus (mean±SD; ***P < 0.001; n = 3 each). C: The percentage of BrdU positive cells in thymocytes detected by flow cytometry at different stages is presented (mean±SD; **P < 0.01, ***P < 0.001; n = 6 each). D: Thymocytes were triple-stained with CD4, CD8 and propidium iodide (PI) to reveal the cell-cycle status of thymocytes of different stages. Representative cell-cycle states of thymocytes of different stages from one thymus are demonstrated on the right panel. The percentages of G0/G1 phase (E), S phase (F) and G2/M phase (G) in thymocytes of different stages from 8 independent mice were statistically analyzed (mean±SD; *P < 0.05, **P < 0.01, ***P < 0.001; n = 8 each). H: Correlation analysis was performed between the percentages of G2/M phase thymocytes and the percentages of radiation-induced cell death in each thymocytes of different stages (Pearson correlation = 0.959*).
Donor cells contribute to thymus regeneration in transplant recipients
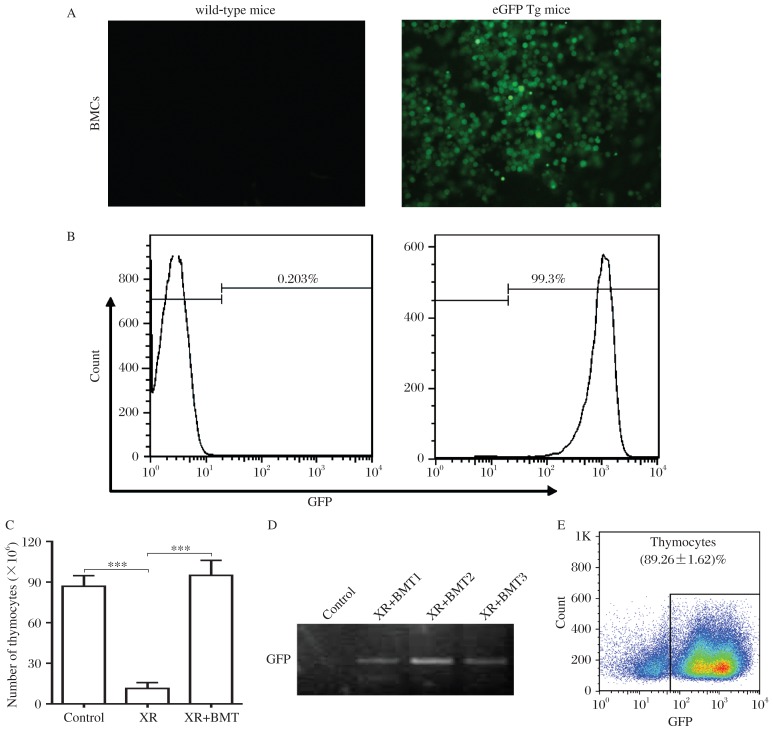
To assess the role of BM cell transplantation in thymus regeneration in irradiated recipients, 5×106 BM cells harvested from EGFP transgenic mice were transplanted into the irradiated mice. After 4 weeks, the size of the thymus, the number of thymocytes and the percentage of donor-derived GFP-positive thymocytes were analyzed. Fluorescence microscopy and flow cytometry for GFP expression revealed that 99.3% of donor BM cells were GFP-positive (Fig. 3A and 3B). At 4 weeks after the transplantation, the size of the thymus and the number of thymocytes in the irradiated mice with BMT recovered to the normal level of untreated mice (Fig. 3C). Furthermore, the presence of GFP DNA was confirmed by PCR in thymocytes harvested from recipient mice (Fig. 3D) and flow cytometry demonstrated that 89.26±1.62% thymocytes were GFP positive in recipient mice (Fig. 3E). Taken together, these results demonstrated that donor BM cells contributed to thymus regeneration in transplant recipients.
Fig. 3. Donor cells contributed to thymus regeneration in transplant recipients.
A: Representative fluorescence micrographs for GFP expression (original magnification×400) in BM cells (BMCs) from C57BL/6J wildtype (WT) mice and EGFP transgenic (EGFP Tg) mice. B: Flow cytometric analysis of GFP expression in BMCs from WT mice and EGFP Tg mice. C: The bar graph showed the total numbers of thymocytes from control, XR and the irradiated mice with BMT (mean±SD; ***P < 0.001, n = 5 each). D: GFP gene expression in the thymuses from the control and XR+BMT mice. E: Flow cytometric analysis for the percentage of GFP-positive thymocytes in the thymus from the irradiated mice with BMT at 4 weeks after transplantation. Data are expressed as mean±SD (n = 5).
Radiation impairs the maturation of donor cells in recipient thymus
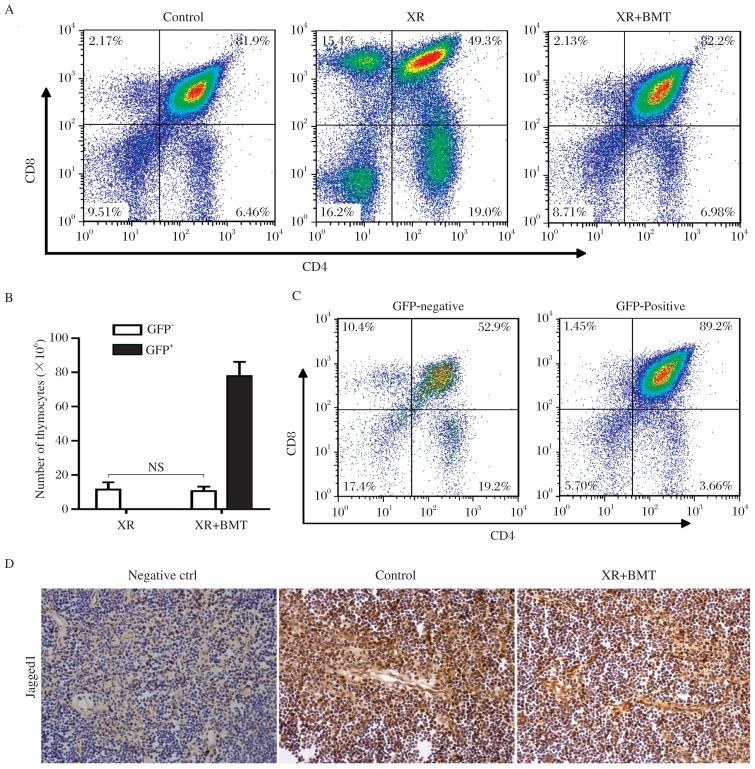
To assess the effect of BM cell transplantation on the reconstruction of thymocytes of different stages in irradiated recipients, DN, DP, CD4-SP and CD8-SP thymocytes were compared between the irradiated mice with BMTand control mice or XR mice. The results showed that the ratios of DN, DP, CD4-SP and CD8-SP thymocytes recovered to normal level in the irradiated mice with BMT (Fig. 4A, Table 2). Compared with XR mice, the ratio of DP thymocytes significantly increased, whereas the ratios of DN and SP thymocytes significantly decreased in the irradiated mice with BMT (Fig. 4A, Table 2). To further assess the contribution of donor BM cells in the reconstruction of thymocytes of different stages in irradiated recipients, total thymocytes and thymocytes of different stages derived from recipient and donor were identified using flow cytometric analysis. The results showed that the number of GFP-negative thymocytes from the irradiated mice with BMT was comparable with that in the irradiated mice (Fig. 4B). The ratios of DN, DP, CD4-SP and CD8-SP thymocytes in GFP-negative population in the irradiated mice with BMT were comparable with those in total thymocytes from the irradiated mice (Fig. 4C, left panel). These results confirmed that the reconstruction of irradiated thymus depended on donor BM cells. In GFP-positive population, the ratio of DN thymocytes did not decrease, whereas the ratio of DP thymocytes was increased significantly. In contrast, the ratios of CD4-SP and CD8-SP thymocytes decreased significantly in the irradiated mice with BMT compared with those in total thymocytes from the control mice (Fig. 4C, right panel, Table 3). These results suggest that radiation impairs the maturation of donor-derived DP thymocytes in recipient thymus.
Fig. 4. Radiation impairs the maturation of donor cells in recipient thymus.
A: Representative flow cytometric analyses for the CD4/CD8 double-stained thymocytes from mice at 4 weeks after transplantation. B: The numbers of GFP-negative or GFP-positive thymocytes in the irradiated mice and the irradiated mice with bone marrow transplantation (BMT), respectively (mean±SD; n = 5 each; NS: not significant). C: Representative flow cytometric analyses for the CD4/CD8 double-stained thymocytes in GFP-negative thymocytes and GFP-positive thymocytes from the irradiated mice with BMT. D: Representative tissue sections of thymus stained immunohistochemically for Jagged1 expression in thymic cortical epithelia in the untreated mice and the irradiated mice with BMT.
Table 2. Role of bone marrow transplantation (BMT) in the regeneration of thymocytes of different stages in irradiated mice.
| Cell type | Constituent ratio (%) |
Cellularity (×106) |
||||
| Untreated | XR | XR+BMT | Untreated | XR | XR+BMT | |
| CD4–CD8– | 8.21±2.08 | 14.17±1.88* | 6.96±2.43# | 07.07±1.55 | 1.67±0.85** | 6.48±1.91# |
| CD4+CD8+ | 82.63±2.10 | 49.87±1.91*** | 83.03±0.72### | 71.95±7.97 | 5.70±2.08*** | 78.91±9.79### |
| CD4+CD8– | 06.65±0.69 | 20.53±1.46*** | 07.77±1.95### | 05.75±0.07 | 2.31±0.69** | 06.45±2.48# |
| CD4–CD8+ | 02.50±0.50 | 15.40±0.60*** | 02.25±0.16### | 02.19±0.59 | 1.76±0.66 | 02.65±0.38 |
*P < 0.05, **P < 0.01, ***P < 0.001 compared with the untreated group; #P < 0.05, ###P < 0.001 compared with the irradiated (XR) group.
(mean±SD, n = 5)
Table 3. Maturation of donor cells in the thymus of irradiated mice.
| Cell type | Constituent ratio (%) |
|
| Untreated | GFP+ (XR+BMT) | |
| CD4–CD8– | 08.21±2.08 | 05.92±0.56 |
| CD4+CD8+ | 82.63±2.10 | 89.20±1.10** |
| CD4+CD8– | 06.65±0.69 | 03.58±0.18** |
| CD4–CD8+ | 02.50±0.49 | 0 1.54±0.27* |
*P < 0.05, **P < 0.01 compared with the control group. BMT: bone marrow transplantation; XR: X-ray irradiation.
(mean±SD, n = 5)
To determine whether the impaired maturation of donor-derived DP thymocytes in recipient thymus is associated with the down-regulation of Notch signaling, thymic tissue sections were stained immunohistochemically for Jagged1, a Notch ligand. The results showed that Jagged1 expression was detected in the cortical epithelia and was reduced in the irradiated thymus with BMT compared to untreated thymus (Fig. 4D). This result suggests that reduced expression of Jagged1 may play a significant role in radiation-impaired maturation of donor-derived DP thymocytes in recipient thymus.
DISCUSSION
Previous studies have reported that radiation induces acute thymic atrophy and reduces the total number of thymocytes[18],[19], especially DP thymocytes[4]. Our results are consistent with these findings. In addition to DP thymocytes, other thymocytes of different stages also cannot escape from radiation-induced cell death, especially DN thymocytes, which suffered a 76.38% loss. Previous studies have mainly focused on the great sensitivity of DP thymocytes to radiation[4],[20]. However, what contributes to the different sensitivity of thymocytes of different stages to radiation-induced cell death is unclear. The viewpoint that proliferative cancer cells are sensitive to radiation damage has been widely accepted in cancer therapy. Therefore, we raised the questions whether different proliferation exists among thymocytes of different stages and whether the degree of radiosensitivity of thymocytes of different stages correlates with their proliferation rates. Our results demonstrated the following facts: 1) Different proliferation exists in thymocytes of different stages; 2) There was a significant correlation between the percentage of thymocytes maintained at G2/M phase and the percentage of radiation-induced cell death in the thymus. These results provide strong evidence that the degree of radiosensitivity of thymocytes depends on their proliferative states and would shed some light on elucidating the mechanism of radiation-induced damage on thymocytes.
In the present study, irradiated mice were transplanted with donor BM cells from GFP transgenic mice. At 4 weeks after transplantation, the size and cellularity of the thymus and a totally rescued percentage of thymocytes of different stages were detected. We found that the total number of GFP-negative thymocytes and percentages of thymocytes of different stages in GFP-negative thymocytes are comparable between the irradiated mice and the irradiate mice with BMT, suggesting that reconstruction of the thymus from the irradiated mice with BMT is completely due to donor-derived BM cells. However, our results demonstrated that the percentage of GFP-positive DP thymocytes increased significantly, whereas the percentages of GFP-positive CD4-SP and CD8-SP thymocytes decreased significantly in the irradiated mice with BMT compared with untreated mice. These results indicated that the maturation of donor-cells in the thymus of irradiated mice was not completed, with a dysfunctional transition from DP thymocytes to SP thymocytes. The maturation of T-cell precursors is a non-cell autonomous process, and the interactions of cells with the thymic microenvironment are required for T-cell development[21]–[23]. The notch signaling pathway is critical to the process of thymocytes maturation, including positive[14],[15] and negative[16] selection of DP thymocytes. Notch activation requires binding of Notch ligand, such as Jagged1, which is expressed by a cortical epithelial subset[17]. Our results demonstrated that Jagged1 expression was significantly down-regulated in thymic cortical epithelia in the irradiated mice with BMT, suggesting that the transition dysfunction of donor-derived DP thymocytes is possibly related to the damaged thymic microenvironment mediated by Notch signaling.
Post-BMT immunodeficiency causes significant morbidity and mortality in BMT recipients[24]–[26]. Although neutropenia-related infections predominate in the immediate post-transplantation period, infections after myeloid engraftment are generally due to bacterial, fungal, and viral pathogens that are controlled by the adaptive immune system[27]. Therefore, it is very important to promote the generation of functional SP T lymphocytes in the functional recovery of recipient immune system. In our study, despite the engraftment of donor BM cells into recipient thymus and the recovery of partial thymocytes at different stages, the generation of functional T lymphocytes in the recipient was delayed, possibly owing to the damage of the thymic microenvironment. These results suggest that a graft containing a mixture of thymic epithelia and BM cells would be a good choice for transplantation. This graft containing mixture would be beneficial to the functional reconstruction (including recovery of thymocytes and the thymic microenvironment) of the thymus in irradiated recipient.
In summary, our results demonstrated that the degree of radiosensitivity of thymocytes depends on their proliferative states and radiation impairs the maturation of donor-derived CD4+CD8+ thymocytes cells in recipient thymus.
Footnotes
This work was supported by an operating grant (No. BK2008440) to DSM from the Science and Technology Department of Jiangsu province, China.
The authors reported no conflict of interest.
References
- 1.Dzhagalov I, Phee H. How to find your way through the thymus: a practical guide for aspiring T cells. Cell Mol Life Sci. 2011;69:663–82. doi: 10.1007/s00018-011-0791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–79. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 3.McConkey DJ, Orrenius S, Jondal M. Cellular signalling in programmed cell death (apoptosis) Immunol Today. 1990;11:120–1. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- 4.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–9. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 5.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–7. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 6.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–24. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 7.Fukasawa K. P53, cyclin-dependent kinase and abnormal amplification of centrosomes. Biochim Biophys Acta. 2008;1786:15–23. doi: 10.1016/j.bbcan.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson G, Jenkinson WE, Jones T, Parnell SM, Kinsella FA, White AJ, et al. Establishment and functioning of intrathymic microenvironments. Immunol Rev. 2006;209:10–27. doi: 10.1111/j.0105-2896.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Barcellos-Hoff MH, Nguyen DH. Radiation-induced Microenvironments and their role in carcinogenesis. In: Jakowlew S, editor. Tumor-Associated Fibroblasts and Their Matrix. Berlin: Springer; 2011. pp. 267–82. [Google Scholar]
- 10.Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–39. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 11.Suetsugu-Maki R, Maki N, Fox TP, Nakamura K, Cowper Solari R, Tomlinson CR, et al. A complement receptor C5a antagonist regulates epithelial to mesenchymal transition and crystallin expression after lens cataract surgery in mice. Mol Vis. 2011;17:949–64. [PMC free article] [PubMed] [Google Scholar]
- 12.Shu L, Ji J, Zhu Q, Cao G, Karaplis A, Pollak MR, et al. The calcium-sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res. 2011;26:1057–71. doi: 10.1002/jbmr.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity. 2000;13:73–84. doi: 10.1016/s1074-7613(00)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izon DJ, Punt JA, Xu L, Karnell FG, Allman D, Myung PS, et al. Notch1 regulates maturation of CD4+ and CD8+ thymocytes by modulating TCR signal strength. Immunity. 2001;14:253–64. doi: 10.1016/s1074-7613(01)00107-8. [DOI] [PubMed] [Google Scholar]
- 16.Jehn BM, Bielke W, Pear WS, Osborne BA. Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J Immunol. 1999;162:635–8. [PubMed] [Google Scholar]
- 17.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–7. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohi H, Mishima Y, Kamimura K, Maruyama M, Sasai K, Kominami R. Multi-step lymphomagenesis deduced from DNA changes in thymic lymphomas and atrophic thymuses at various times after gamma-irradiation. Oncogene. 2007;26:5280–9. doi: 10.1038/sj.onc.1210325. [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Kim S, Lee Y, Choi MS, Choi MU. Alteration of lipid composition of rat thymus during thymic atrophy by whole-body X-irradiation. Int J Radiat Biol. 2006;82:129–37. doi: 10.1080/09553000600617189. [DOI] [PubMed] [Google Scholar]
- 20.Paulino AC, Constine LS, Rubin P, Williams JP. Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin Radiat Oncol. 2010;20:12–20. doi: 10.1016/j.semradonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 22.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859–66. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 23.Gray DH, Ueno T, Chidgey AP, Malin M, Goldberg GL, Takahama Y, et al. Controlling the thymic microenvironment. Curr Opin Immunol. 2005;17:137–43. doi: 10.1016/j.coi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Ochs L, Shu XO, Miller J, Enright H, Wagner J, Filipovich A, et al. Late infections after allogeneic bone marrow transplantations: comparison of incidence in related and unrelated donor transplant recipients. Blood. 1995;86:3979–86. [PubMed] [Google Scholar]
- 25.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 26.Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. Cytotherapy. 2001;3:41–54. doi: 10.1080/146532401753156403. [DOI] [PubMed] [Google Scholar]