Abstract
A number of recent studies utilizing both functional neuroimaging and lesion analysis techniques in neurologic patients have produced conflicting results with respect to the neural correlates of picture naming. Picture naming involves a number of cognitive processes, from visual perception/recognition to lexical-semantic retrieval to articulation. This middle process, the ability to retrieve a name associated with an object, has been attributed in some cases to posterior portions of the left lateral temporal lobe and in other cases, to anterior temporal cortex. In the current study, we used voxel-based lesion symptom mapping (VLSM) to identify neural correlates of picture naming in a large sample of well-characterized left hemisphere (LH) patients suffering from a range of naming deficits. We tested patients on the Boston Naming Test (BNT), a clinical, standardized measure of picture naming that is widely used in both clinical and research settings. We found that overall performance on the BNT was associated with a network of LH regions that included significant portions of the left anterior to posterior middle temporal gyrus (MTG) and superior temporal gyrus (STG) and underlying white matter, and extended into left inferior parietal cortex. However, when we added covariates to this analysis that controlled for deficits in visual recognition and motor speech in order to isolate brain regions specific to lexical-semantic retrieval, the significant regions that remained were confined almost exclusively to the left mid-posterior MTG and underlying white matter. These findings support the notion that a large network in left peri-Sylvian cortex supports picture naming, but that the left mid-posterior MTG and underlying white matter play a critical role in the core ability to retrieve a name associated with an object or picture.
Keywords: Picture naming, Word production, Temporal cortex, Wernicke’s aphasia, Aphasia, Middle temporal gyrus, Lexical-semantics
The ability to name a picture requires a number of cognitive processes, from visual perception/recognition to conceptual/ semantic processing to lexical selection and retrieval, and finally the planning and execution of an articulatory motor plan (Benson, 1979; Foundas et al., 1998; Kohn and Goodglass, 1985; Levelt, 1999). A long literature has been devoted to parsing these different cognitive aspects of naming, with more recent work focused on identifying the brain regions underlying these processes. Lesion studies, as well as functional neuroimaging and cortical stimulation studies, have been utilized to this end (see Indefrey and Levelt, 2004 and Whatmough and Chertkow, 2002 for reviews).
A number of previous studies of picture naming in neurologic patients have provided key insights into both the cognitive processes involved in naming as well as their neural basis. Such studies typically report that left temporal and frontal cortex are critical for naming, but the specific subregions vary, depending on task demands and content material. Damasio et al. (1996, 2004) assessed picture naming in neurologic patients and found that deficits were associated primarily with left anterior temporal and posterior lateral temporal cortex, although the precise location differed among subcategories of objects: Lesions in left temporopolar cortex disrupted naming famous faces; lesions in anterior inferotemporal (IT) cortex disrupted animal naming; and damage in posterolateral IT/temporo-occipital-parietal cortex disrupted patients’ ability to name tools. Hillis et al. (2006) identified brain regions critical to naming in acute stroke patients by identifying regions of reperfusion on perfusion-weighted magnetic resonance imaging (MRI) scans that correlated with improved naming on follow-up scans done 3–5 days after an initial scan. They found that the left posterior middle temporal gyrus (MTG) and inferior temporal/fusiform gyri, BA 37, were most strongly associated with improved naming; the posterior superior temporal gyrus (STG) and Broca’s area were secondarily implicated. DeLeon et al. (2007) also used perfusion imaging in a group of acute ischemic stroke patients and found that several left hemisphere (LH) regions were critical for naming. The left STG was most strongly associated with impaired lexical-semantics (i.e., the ability to associate a word with a picture), but the left anterior temporal pole and MTG were also implicated. Left posterior inferior temporal cortex was most strongly associated with anomia, that is, when patients knew the correct response but could not produce it (either in spoken or written form). Frontal regions such as Broca’s area were most closely linked to motor speech (i.e., output) aspects of naming. Trebuchon-Da Fonseca et al. (2009) assessed naming in patients with temporal lobe epilepsy and correlated naming performance with resting state positron emission tomography (PET). They found that impaired naming due to impaired lexical retrieval (with intact conceptual/ semantic understanding) correlated with reduced metabolism primarily in left posterior temporal cortex, specifically in the inferior temporal and superior temporal gyri. In a single case study, Raymer et al. (1997) described a patient with a lesion in the left posterior MTG (BA37) who exhibited impaired naming across modalities, along with relatively intact semantic/conceptual processing. They concluded that BA 37 is critical for associating stored concepts with lexical information (see also Foundas et al., 1998).
Other studies have analyzed picture naming decrements in neurologic patients with degenerative disorders (Brambati et al., 2006; Grossman et al., 2004; McMillan et al., 2004; Mesulam et al., 2009). Brambati et al. (2006) used voxel-based morphometry (VBM) to relate regions of atrophy in patients with degenerative disease (progressive aphasia and semantic dementia) to deficits in category-specific naming. This analysis showed that overall naming was associated with bilateral atrophy in inferior and superior temporal gyri, anterior fusiform gyri, and hippocampi, and left-sided atrophy in the parahippocampal gyrus, MTG, and the temporal pole. Grossman et al. (2004) (see also McMillan et al., 2004) examined naming deficits in three distinct groups of patients with dementia—Alzheimer’s disease, fronto-temporal dementia, and cortico-basal degeneration. VBM analyses showed that changes in the left lateral temporal lobe were common across patient groups and were related specifically to lexical retrieval impairments in all groups. Visual-perceptual processing aspects of naming were associated with the right inferolateral temporal and right dorsolateral frontal cortices.
A number of recent studies have focused on the left anterior temporal lobe as a critical region involved in naming (Drane et al., 2008; Lu et al., 2002; Price et al., 2005; Schwartz et al., 2009). Schwartz et al. (2009) found that semantic errors in naming on the Philadelphia Naming Test were associated primarily with the left anterior temporal lobe, extending back to the middle portion of the left MTG. Drane et al. (2008) reported that epilepsy patients with left anterior temporal lobe foci had significantly reduced naming, while right anterior temporal patients had more general recognition/conceptual deficits. Price et al. (2005) performed a meta-analysis of functional neuroimaging studies of picture naming in normal participants. They found that when baseline conditions were used that controlled for speech and visual processing, rather than simple fixation, anterior temporal cortex was typically activated. Such findings are also consistent with a number of recent studies in patients with semantic dementia, who have significant anterior temporal lobe atrophy and a striking impairment in naming (Mesulam et al., 2009). Patterson et al. (2007) has argued for a ‘distributed-plus-hub’ network model, in which the anterior temporal lobe is a hub that processes information about associations between different aspects of an object (e.g., between its name and shape, between its shape and color, etc.), regardless of the type of task or modality (i.e., naming, sorting, drawing, etc.).
In short, a number of previous studies of naming have implicated various regions within left mid-posterior lateral temporal cortex, while other studies point to more anterior temporal regions as critical. In the current study, we were interested in identifying brain regions involved in naming using a statistical, voxel-based lesion analysis tool that allowed us to visualize regions critical for picture naming as measured by a standard clinical test, the Boston Naming Test (BNT; Kaplan et al., 2001). The BNT is a standardized naming test that has been normed on several populations, published as a shortened version, and translated into several languages (e.g., Pedraza et al., 2009; Kohnert et al., 1998; Mack et al., 1992; Tombaugh and Hubley, 1997). The BNT has been used extensively as an outcome measure in clinical studies (e.g., Kendall et al., 2006; Naeser et al., 2010) and thus, understanding its neural underpinnings would add significant clinical value.
Our two main goals in the current study were: 1) to confirm/disconfirm previously implicated LH regions most critical to lexical-semantic retrieval as measured by picture naming (e.g., left MTG, STG, anterior pole); and 2) to characterize the neural underpinnings of a commonly used clinical test of naming, the BNT. To do this, we used voxel-based lesion symptom mapping (VLSM), a statistical, voxel-by-voxel analysis of brain regions implicated in a given behavioral measure. This approach is complementary to functional imaging techniques, in that VLSM identifies regions critical to task performance, rather than all potential regions recruited by a task. Also, unlike functional imaging techniques such as functional MRI (fMRI), VLSM can identify both grey and white matter regions that are critical for performance on a given task. In the current study, we first used VLSM to identify LH regions involved in overall picture-naming performance on the BNT. Next, in order to identify brain regions most critical to core aspects of lexical-semantic retrieval, we used patients’ speech fluency scores as a covariate in the VLSM analysis to partial out regions related to basic speech production aspects of naming. We also used patients’ performance on a 3-choice BNT recognition task to control for poor performance due to visual recognition deficits. In this way, we aimed to isolate brain regions most critical to lexical-semantic processing, specifically, the ability to retrieve a name associated with a picture. Based on previous studies, we predicted that a large network of left lateral temporal regions would be involved in overall naming and that the MTG and/or anterior temporal cortex would be most critical for lexical-semantic retrieval.
Methods
Participants
The study sample included 96 participants (21 female) who had suffered a single LH stroke. Study criteria included being a native English speaker, right-handed, and in the chronic phase of stroke (at least 10 months post-injury) so that behaviors were stabilized. All patients regardless of arterial distribution of their stroke were included; however, patients with clinical neglect or visual agnosia were excluded. Patients’ mean age was 60.0 years (SD = 11.2, range = 31–84), mean education was 14.6 years (SD= 3.2, range= 5–20), and mean time post-stroke was 58.8 months (SD = 54.6, range= 10–272). Patients were screened for prior history of neurologic/ psychiatric disorders and substance abuse, and all patients had normal or corrected-to-normal hearing and vision. A subset of the participants in the current study was included in an earlier study of category-specific deficits in naming (Baldo et al., 2009). All participants in the current study signed consent forms, and research was carried out in a manner consistent with the Helsinki Declaration.
Materials and procedures
Behavioral measures
Participants were administered the Western Aphasia Battery (WAB; Kertesz, 1982) and the BNT (Kaplan et al., 2001) as part of a larger neuropsychological battery. The WAB is a speech/ language battery that includes separate subtests that measure speech fluency, naming, repetition, and comprehension. Based on scores from these four main subtests, the WAB classifies examinees as to aphasia subtype. The current sample included 20 patients with an anomic aphasia, 18 patients with Broca’s aphasia, 9 patients with Wernicke’s aphasia, 6 patients with conduction aphasia, 1 patient with transcortical sensory aphasia, 2 patients with global aphasia, 7 patients with unclassifiable aphasia, and 33 patients who scored within-normal limits (WNL). WNL status is based on a cut-off score of 93.7% correct. These WNL patients had a prior history of clinical aphasia, but at the time of testing, their symptoms were too mild to be detected by the WAB. The mean score on the WAB was 74.2 out of 100 (SD = 27.1, range= 11.8–100).
On the BNT, spontaneous naming was tested by asking patients to name a series of 60 black and white line drawings of animate and inanimate items, ranging from high frequency items (e.g., bed ) to low frequency items (e.g., abacus). The percentage of drawings named correctly was the primary dependent variable used in the VLSM analyses described below. If the examinee was unable to name the item spontaneously, the examiner provided a cue. If the problem appeared to be one of a visual-perceptual nature (i.e., the examinee did not recognize the item), a semantic cue was provided (e.g., used in the mouth for toothbrush). The decision to provide a semantic cue on the standard BNT administration is subjective, based on the examiner’s impression that the patient may not be seeing the item properly (e.g., if they call the canoe a peapod ), but this cueing was rarely necessary with the current group of patients. Otherwise, only a phonemic cue was provided (e.g., /ko/ for comb), and this cue was provided for every item that the patient was unable to name spontaneously. Unlike the standard BNT administration, patients were tested on all 60 items, rather than discontinuing after six consecutive errors, so that all participants were tested on the entire stimulus set. Also, after patients had attempted to name all the BNT items, they were given an in-house, auditory, 3-choice recognition task on the items they had not been able to name. The examiner showed the BNT picture and presented the three choices aloud. The patient was allowed to respond non-verbally if necessary (e.g., by holding up 1, 2, or 3 fingers to indicate their choice). The two distracters in the recognition task began with the same two phonemes as the target and had the same number of syllables, but were semantically distinct (e.g., cabin and candle for the target cactus).
Brain imaging
Patients’ lesions were imaged with 3D CT or MRI scans at least 3 months post-onset so that lesion sites had stabilized. In 43 patients, high-resolution T1-weighted structural 3DMRI scans were obtained on a 1.5 T Phillips Eclipse scanner. T1-weighted images were acquired with a Spoiled Gradient Recall (SPGR) sequence (TR/TE = 15/4.47 msec, FOV = 240 mm, 256 × 256 imaging matrix, flip angle = 35°, .94 × 1.3 × .94mm3 voxels, 212 coronal slices). The lesions were traced directly on the patient’s T1 digital MRI image using MRIcro (Rorden and Brett, 2000) and then registered with the MNI template using the standard nonlinear spatial normalization procedure from SPM2 (Statistical Parametric Mapping, Wellcome Trust Center for Neuroimaging), with a cost function masking procedure to avoid distortions due to the presence of the lesion (Brett et al., 2001). T2 and FLAIR images were also available and were yoked to the T1 images in MRIcro so that the extent of the lesion could be verified on these image sequences. When 3D digital MRI images were not available, lesions were drawn from hard-copy CT (n = 31) or MRI films (n = 22). The CT scanner was a Siemens Somatom Emotion 16 CT scanner with 3 × 3 × 3 mm imaging, and MRI images were collected on the 1.5 T scanner described above. Based on the films, lesions were drawn onto an 11-slice, standardized template (based on the atlas by DeArmond et al., 1989) by a board-certified neurologist who was blind to the patient’s behavioral presentation and study predictions. Reliability has been demonstrated previously using this technique (Friedrich et al., 1998; Knight et al., 1988). These templates were then digitized and non-linearly transformed into MNI space (Collins et al., 1994) using SPM5. For this transformation, slices from the two templates were aligned using 50 control point pairs to match anatomical features on the two templates, and the slices were then aligned using a local weighted mean transformation implemented by the cpselect, cp2tform and imtransform functions in Matlab 6.5. These algorithms were then used to warp all the lesion reconstructions from the 11-slice template into MNI space. It should be noted that lesion reconstruction methods have limitations and that in some cases, there is likely lesioned/dysfunctional tissue that is not registered with these techniques, especially in the case of patients with very large lesions (Rorden and Karnath, 2004).
An overlay of patients’ lesions is shown in Fig. 1. The vast majority (95%) of our patients suffered middle cerebral artery strokes, with the other 5% sustaining posterior cerebral artery or anterior communicating artery strokes. As can be seen, the lesions covered the entire distribution of the middle cerebral artery. The average lesion volume was 105.2 cc (SD = 90.2).
Fig. 1.
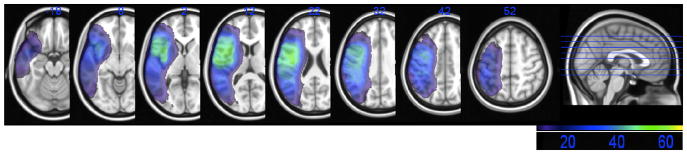
Lesion overlap map with a minimum of five patients per voxel, thus showing those voxels eligible for inclusion in the VLSM analyses. The color bar shows regions of minimal overlap of five patients (in dark purple) to maximal overlap of 52 patients (in green).
In order to determine whether we had sufficient power to detect differences in the current dataset, we generated a map showing the distribution of statistical power across the LH, based on a large effect size (.8) and an alpha of .05 (see Fig. 2; Cohen, 1988, 1992; Kimberg et al., 2007). Predictions for this study were confined to those regions with power ≥.6. As shown in Fig. 2, there was sufficient power in the left peri-Sylvian region and throughout much of the LH, encompassing those regions included in our predictions.
Fig. 2.
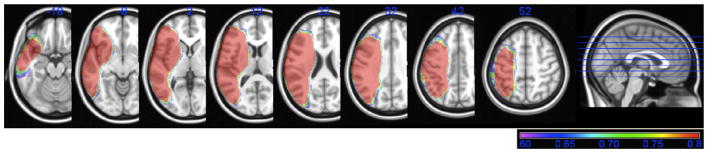
Power map, showing the degree of statistical power across brain regions in the VLSM analyses. The color bar shows power values ranging from .6 (in purple) to .8 and above (in red).
Lesion analysiss
To determine the anatomic correlates of picture naming on the BNT, we applied VLSM analyses to the lesion and behavioral data (http://neuroling.arizona.edu/resources.html; see Baldo et al., 2012; Bates et al., 2003). In VLSM, t-tests are run at every voxel, comparing performance on every measure in patients with a lesion versus without a lesion involving that voxel. Thus, for any particular voxel, a t-test is run with lesion status as the independent variable (lesioned or not) and behavioral performance (here, spontaneous naming on the BNT) as the dependent variable. In the current study, t-tests were confined to those voxels in which there were at least five patients with a lesion and five patients without a lesion, in order to avoid running analyses in voxels in which very few patients had lesions. To determine what constituted a “significant” voxel, a statistical threshold cut-off (t-value) was determined based on permutation testing (n = 1,000) with alpha set at .05 (see Kimberg et al., 2007). Permutation testing is a conservative method used to correct for multiple comparisons. With permutation testing, the patients’ behavioral scores are randomly reassigned across the voxels 1,000 times. For each permutated dataset, the statistics are re-run and the top 5% of t-values is calculated. These critical t-values for each VLSM analysis in the current study are listed in the Results. Only voxels exceeding these stringent cut-offs are shown in the VLSM figures below. In addition, only clusters of ≥50 voxels are discussed in the Results below, although the figures show all voxels surpassing the critical t-value, regardless of cluster size. Brain regions corresponding to the significant voxel locations were determined using the Brodmann and AAL atlases in MRIcroN (ver. 7).
In addition to the primary VLSM analysis of spontaneous naming on the BNT, we ran additional VLSM analyses using patients’ speech fluency and BNT recognition scores as covariates. These covariate analyses were run in order to control for deficits in general speech production and visual recognition, respectively, which could impact naming performance. Like a behavioral covariate analysis, these additional variables were entered into the VLSM analysis along with the primary variable (spontaneous naming performance on the BNT), with the resultant VLSM map indicating regions that remained significant once these covariates were factored out. The speech fluency score covariate was taken from the WAB. It is based on a spontaneous speech sample that is scored by the examiner: from 0 (“no words or short, meaningless utterances”) to 10 (“sentences of normal length and complexity, without definite slowing, halting, or articulatory difficulty”). The visual recognition covariate was the percent correct on the BNT 3-choice recognition procedure, described above. Last, because the patient sample was somewhat heterogeneous with respect to age and education, we included these variables as additional covariates in all of the VLSM analyses.
Results
Behavioral performance
Patients’ performance on the BNT, as well as overall language profile on the WAB language battery, is shown in Table 1. Spontaneous naming on the BNT (percent of items spontaneously named without a cue) averaged 57.0% correct (SD = 36.1) across the sample. The average percentage of items that were subsequently named correctly when patients were provided a semantic cue was 2.9% (SD = 7.7). Of those items that were neither spontaneously retrieved nor named with a semantic cue, the average percentage of items remaining that were named correctly with a phonemic cue was 44.6% (SD = 33.2). Last, of the items remaining that patients failed to name either spontaneously or with a cue, the average percentage of items correctly recognized with the 3-choice recognition task was 88.8% (SD= 18.3).
Table 1.
Patient Performance on BNT and WAB Language Subtests.
| Measure | Percent correct | SD | Range |
|---|---|---|---|
| BNT spontaneous naming | 57% | 36.1 | 0–100 |
| BNT with semantic cue | 3% | 7.7 | 0–50 |
| BNT with phonemic cue | 45% | 33.2 | 0–100 |
| BNT with forced recognition | 89% | 18.3 | 0–100 |
| WAB total score | 74% | 27.1 | 12–100 |
| WAB fluency | 72% | 3.0 | 0–100 |
| WAB spontaneous speech | 75% | 5.5 | 0–100 |
| WAB comprehension | 84% | 2.0 | 25–100 |
| WAB repetition | 69% | 3.6 | 0–100 |
| WAB naming | 68% | 3.3 | 0–100 |
Note: SD = standard deviation.
Lesion analysis findings
The VLSM analysis of spontaneous naming on the BNT (i.e., without cueing) is shown in Fig. 3. The map revealed significant foci primarily in the anterior, middle, and posterior portions of the left MTG and STG, as well as the underlying white matter. The maximum t-value was 9.21 (cluster volume = 9865) and was located in the white matter just medial to the MTG (MNI coordinates, −48,−24,−4). This cluster of significant voxels also extended anteriorly to the left superior temporal pole and superiorly into left inferior parietal cortex (angular and supramarginal gyri). The minimum cut-off t-value in this analysis was 4.41.
Fig. 3.
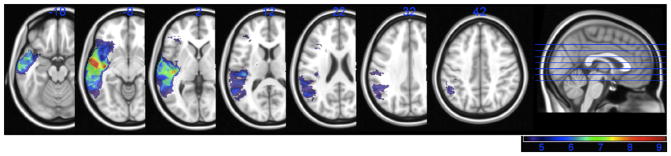
VLSM map showing significant voxels associated with impaired spontaneous naming performance on the BNT. All voxels shown in color exceeded the critical threshold for significance, and the colors reflect increasing t-values from 4.41 to 9.21 (shown in purple to red).
Since naming depends heavily on speech production, and many of the regions found in the above analysis are often implicated in speech production, we re-ran the VLSM analysis of naming performance, controlling for overall fluency in speech production. We used patients’ speech fluency score on the WAB as a covariate in the VLSM analysis. In this way, we controlled for the fluency of patients’ spontaneous speech performance so that the effects of speech production deficits on naming would be partialed out. When we re-ran the VLSM analysis with overall speech fluency as a covariate, the significant voxels were restricted primarily to the mid-to posterior portion of the MTG (BA 21 and 37), again with some extension into the white matter just medial to the MTG (see Fig. 4). The maximum t-value was 6.06 (cluster volume = 1092) and was located more posteriorly than the original analysis, in the white matter just medial to the posterior MTG (−46,−48, 0). For this analysis, the minimum t-value cut-off was 4.43.
Fig. 4.
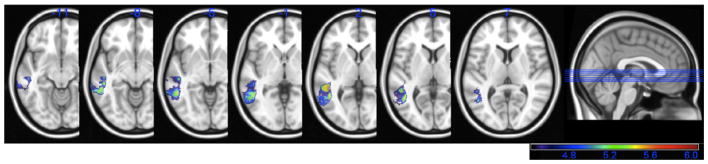
VLSM map showing significant voxels associated with impaired naming performance on the BNT, with the effect of speech production deficits partialed out. All voxels shown in color exceeded the critical threshold for significance, and the colors reflect increasing t-values from 4.43 to 6.06 (shown in purple to red).
Last, we added an additional covariate to this analysis, namely, performance on the 3-choice recognition task. We wanted to rule out the possibility that our findings might stem from brain regions critical for simple visual perception/ recognition of the items. When we included the recognition task performance as a covariate (in addition to the speech production covariate), the significant brain regions were almost identical to the prior analysis (thus, no additional figure is shown). Again, regions included predominantly the left mid-posterior MTG, including the white matter immediately adjacent to the left MTG (cluster volume = 1047). The minimum t-value was 4.46, and the maximum t-value was 6.02, centered in the same location as the previous analysis, in the white matter just medial to the left MTG (−46, −48, 0).
Discussion
In the current study, we found that spontaneous naming on the BNT was associated with lesions in a large network of left anterior, lateral, and posterior temporal regions and underlying white matter, as well as portions of left inferior parietal cortex. However, when we covaried for speech production and visual recognition deficits, the significant regions were confined primarily to the left mid-to posterior MTG and the white matter just medial to this region. These findings suggest that the ability to retrieve the correct name for a visually presented object is critically dependent on the mid-posterior left MTG and the adjacent white matter.
The fact that we observed a network of temporal and parietal regions in the primary VSLM map of picture naming is consistent with the fact that naming deficits are the most ubiquitous aphasic symptom following LH stroke and can arise from insult to a large number of different brain regions (Benson, 1979; Foundas et al., 1998; Ojemann and Whitaker, 1978; Ojemann et al., 1989). This is likely due to the fact that naming is multi-determined, involving a number of stages that include at a minimum: 1) visual-perceptual recognition/ understanding of the item; 2) associating a lexical representation with that representation (i.e., lexical-semantic retrieval); and 3) articulation of the item’s name (see Levelt, 1999 for discussion of more complex models of word production with sub-stages). In the current study, we were primarily interested in identifying brain regions critical to stage 2 in this model, the core ability of retrieving the correct name for an object (i.e., lexical-semantic retrieval). To isolate this stage, we used speech fluency as a covariate in the VLSM analysis of naming to control for speech production/articulation factors (stage 3 in the naming model). This speech fluency variable was based on examiner ratings of the fluency of patients’ spontaneous speech on the WAB that ranged from 0 (halting, short utterances) to 10 (normal, fluent speech without slowing or halting). Although fluency itself can be broken down into many sub-stages, we used this speech fluency score to control for deficits in motor output aspects of speech that can disrupt naming. To control for deficits in visual-perceptual processing (stage 1 in the naming model), we included the BNT visual recognition task as an additional covariate. With these covariates added to the analysis, a number of anterior and posterior brain regions dropped out, but the significant focus that remained was the left mid-posterior MTG and underlying white matter, suggesting that this region is critical for the middle stage described above, lexical-semantic retrieval.
The current findings are in keeping with previous studies of neurologic patients that have implicated the left middle-posterior portion of the MTG in picture naming (Damasio et al., 1996; DeLeon et al., 2007; Hillis et al., 2006; Raymer et al., 1997), although some of these studies also implicated other left temporal regions as well. Cortical stimulation studies have also reported disrupted naming following stimulation of the left posterior MTG in patients undergoing awake brain mapping (Corina et al., 2005; Hamberger et al., 2001; Malow et al., 1996; Ojemann et al., 1989; Schwartz et al., 1999). Schwartz et al. (1999) compared picture naming to speech arrest and found that naming disruption was most strongly associated with stimulation in the left posterior MTG, whereas speech arrest was most strongly associated with the left posterior STG. Malow et al. (1996) compared disruption of picture versus definitional naming (e.g., “What’s a barking pet?”) using cortical stimulation and found that both were disrupted by stimulation of posterior lateral temporal cortex, as well as inferior temporal and frontal cortex in the dominant hemisphere. Similarly, Hamberger et al. (2001) did a comparison in 20 patients and found that both picture naming and definitional naming were disrupted by left posterior MTG stimulation. Finally, Corina et al. (2005) found that cortical stimulation of the posterior MTG as well as the STG and supramarginal gyrus in the dominant hemisphere disrupted picture naming, which included naming pictures of both objects and actions.
The current findings are also consistent with findings from previous functional imaging studies of naming (Edwards et al., 2010; Indefrey and Levelt, 2004; Martin, 2007). In an fMRI study with healthy participants, Tomaszewki Farias et al. (2005) compared picture naming to definitional naming and found that picture naming was most strongly associated with the left posterior MTG, while the definition-naming task was associated with a larger portion of the temporal lobe, including anterior temporal cortex and inferior and superior temporal gyri. Indefrey and Levelt (2004) conducted a meta-analysis of data from 82 functional imaging and cortical stimulation studies of word production. They found that the mid-portion of the MTG was most critical for lexical selection aspects of naming and that the posterior MTG and STG were critical for lexical word form retrieval. The left posterior inferior frontal gyrus (IFG) was critical for “syllabification” (pre-articulation), and a number of bilateral motor regions were critical for end-stage articulation. It should be noted that there are additional brain areas, such as ventral temporal regions, that were not sampled in the current study that have been previously implicated in picture naming as well (e.g., Kherif et al., 2011; Martin, 2007).
Our findings are only partially consistent with previous studies suggesting a primary role for left anterior temporal cortex in picture naming (Damasio et al., 1996, 2004; Schwartz et al., 2009). Although we found that the left superior anterior temporal pole region was involved in overall naming, this region dropped out when we covaried for speech production deficits. It should be noted that lesion coverage in our patient sample did not include the entire left temporal pole but rather only superior portions of the left superior temporal pole, which may explain the discrepant findings. Another difference is that at least one study of picture naming, Schwartz et al. (2009); (see also Walker et al., 2011), found that left anterior temporal cortex was related to the production of semantic errors, as opposed to overall naming performance as in the current study. For comparison, we ran a VLSM analysis of semantic errors on the BNT in a subset of the current patient sample (n = 85), but no significant voxels emerged with our strict, permutation-based correction method. Another important consideration is that conceptual knowledge deficits and concomitant naming deficits such as those seen in semantic dementia may require bilateral anterior temporal lobe lesions, whereas naming deficits alone are likely to follow unilateral left temporal lobe lesions, as in our patients in the current study (Damasio et al., 2004; Lambon Ralph et al., 2001).
The current study focused on picture naming with a commonly used clinical tool that included a number of subcategories of items (e.g., animals, tools, etc.). A large literature is devoted to identifying distinct brain networks associated with naming objects belonging to particular categories (Gainotti, 2000; Hillis and Caramazza, 1991; Martin et al., 1996; Okada et al., 2000; Perani et al., 1995, 1999; Phillips et al., 2002; Smith et al., 2001; Spitzer et al., 1995; Tranel et al., 1997; Tyler and Moss, 2001). These studies are not always consistent (see Devlin et al., 2002 and Gerlach, 2007 for reviews), but many report that naming living items (e.g., animals) is more associated with left anterior temporal regions, while naming artifacts such as tools and manipulable items is associated with more posterior left lateral and inferior temporal cortex (see Damasio et al., 1996). It has been pointed out that the BNT contains a large number of man-made objects and thus might be biased toward detecting more posterior temporal lobe changes (Drane et al., 2008). However, in a previous paper (Baldo et al., 2009), we assessed category-specific naming on the BNT using VLSM and did not find this to be the case. Specifically, we compared the anatomic correlates of naming across different categories of items on the BNT, matched for frequency, difficulty, naming agreement, visual complexity and number of syllables: 1) man-made objects versus natural kinds, 2) animals versus tools, and 3) manipulable versus non-manipulable items. In that paper, we found largely overlapping distributions for these three comparisons that involved a similar naming network as in the current paper, including the left MTG, STG, and inferior parietal cortex. We also examined several patients in that study who showed strong behavioral dissociations across these various semantic categories; again, there was no consistency in the anatomy of these individuals’ lesions with respect to the pattern of dissociations across semantic categories.
Although the bulk of the significant voxels associated with naming in the current study were located in left temporal cortex, there was some extension into the underlying white matter, with the peak t-values in the VLSM maps located just medial to the left MTG. The role of white matter pathways in cognition are just now beginning to be analyzed more systematically, with newer techniques such as diffusion tensor and diffusion spectrum imaging (DTI, DSI; Breier et al., 2008; Catani and Mesulam, 2008; Papagno et al., 2011; Powell et al., 2008; Turken and Dronkers, 2011). Recently, Glasser and Rilling (2008) used DTI to identify two different segments of the arcuate fasciculus, one terminating in the left MTG. They reported that this termination overlapped with regions previously implicated in functional imaging studies of lexical-semantics, consistent with the current study.
In summary, the current study showed that, in a large group of unilateral LH patients with predominantly middle cerebral artery lesions, picture naming on the BNT was dependent on a large network of regions, including portions of the left anterior to posterior MTG and STG and underlying white matter, as well as inferior parietal cortex. However, when factors related to basic speech production and visual perception were factored out, performance was most critically dependent on the mid-to posterior portion of the left MTG and underlying white matter. This brain region, then, seems to play a critical role in lexical-semantic retrieval, specifically, the ability to retrieve the correct lexical label for a visually presented object, in right-handed patients with LH strokes who are impaired on picture naming. As has been noted (Foundas et al., 1998; Raymer et al., 1997), this brain region is well-situated in the brain to serve as a “supramodal” processing center that integrates elements of object recognition (inferior temporal cortex), visual perception (visual association cortex), and core language processing (left MTG/STG), all of which are critical to associating a name with a visual object. The MTG is highly connected with other peri-Sylvian regions via the arcuate fasciculus and associated pathways that pass beneath this cortical region, and it appears that the left MTG plays a critical role in this larger network (Turken and Dronkers, 2011). Further work is necessary to determine the precise aspects of lexical-semantic retrieval mediated by the mid-posterior MTG, as well as the degree to which it may play a more general role in associating meaning with symbolic representations, both linguistic and non-linguistic (Xu et al., 2009).
Acknowledgments
This material is based on work supported by the U.S. Department of Veterans Affairs, Office of Research and Development, Rehabilitation R&D Program, NIH/NINDS 5 P01 NS040813, and NIH/NIDCD 5 R01 DC00216. The contents reported/presented within do not represent the views of the Department of Veterans Affairs or the United States Government. We would like to thank And Turken, Stephanie Ries, StephenWilson, and David Wilkins for their helpful comments on an earlier version of this manuscript. We would also like to thank the research volunteers who took part in this study.
References
- Baldo JV, Wilson SM, Dronkers NF. Uncovering the neural substrates of language: A voxel-based lesion symptom mapping approach. In: Faust M, editor. Advances in the Neural Substrates of Language: Toward a Synthesis of Basic Science and Clinical Research. Oxford: Wiley-Blackwell; 2012. [Google Scholar]
- Baldo JV, Arevalo A, Wilkins DP, Dronkers NF. Voxel-based lesion analysis of category-specific naming on the Boston naming test. 2. Vol. 21. Center for Research in Language Technical Report, University of California; San Diego: 2009. pp. 1–2. [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno M, Knight RT, et al. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Benson F. Neurologic correlates of anomia. In: Whitaker H, Whitaker HA, editors. Studies in Neurolinguistics. Academic Press; 1979. [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin K, Allison SC, Rosen HJ, et al. The anatomy of category-specific object naming in neurodegenerative diseases. Journal of Cognitive Neuroscience. 2006;18(10):1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. American Journal of Neuroradiology. 2008;29(3):483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Earlbaum; 1988. [Google Scholar]
- Cohen JA. power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Corina DP, Gibson EK, Martin R, Poliakov A, Brinkley J, Ojemann GA. Dissociation of action and object naming: Evidence from cortical stimulation mapping. Human Brain Mapping. 2005;24(1):1–10. doi: 10.1002/hbm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Fusco MM, Dewey MM. Structure of the Human Brain. New York: Oxford University Press; 1989. [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, et al. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40(1):54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemenn GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Nagarajan S, Dalal S, Canolty RT, Kirsch HE, Barbaro NM, et al. Spatiotemporal imaging of cortical activation during verb generation and picture naming. NeuroImage. 2010;50(1):291–301. doi: 10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Daniels SK, Vasterling JJ. Anomia: Case studies with lesion localization. Neurocase. 1998;4(1):35–43. [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: A comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12:193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Gainotti G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: A review. Cortex. 2000;36(4):539–559. doi: 10.1016/s0010-9452(08)70537-9. [DOI] [PubMed] [Google Scholar]
- Gerlach C. A review of functional imaging studies on category specificity. Journal of Cognitive Neuroscience. 2007;19(2):296–314. doi: 10.1162/jocn.2007.19.2.296. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathways. Cerebral Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, Gee UJ. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Hamberger M, Goodman R, Perrine K, Tamny T. Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56(1):56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, et al. Restoring cerebral blood flow reveals neural regions critical for naming. Journal of Neuroscience. 2006;26(31):8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Category-specific naming and comprehension impairment: A double dissociation. Brain. 1991;114(5):2081–2094. doi: 10.1093/brain/114.5.2081. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Philadelphia: Lippincott, Williams, & Wilkins; 2001. [Google Scholar]
- Kendall DL, Nadeau SE, Conway T, Fuller RH, Riestra A, Gonzalez Rothi LJ. Treatability of different components of aphasia – Insights from a case study. Journal of Rehabilitation Research and Development. 2006;43(3):323–336. doi: 10.1682/jrrd.2005.01.0014. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kherif F, Josse G, Price C. Automatic top-down processing explains common left occipito-temporal responses to visual words and objects. Cerebral Cortex. 2011;21(1):103–114. doi: 10.1093/cercor/bhq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth C. The effects of lesions of superior temporal gyrus and inferior parietal lobe on temporal and vertex components of the human AEP. Electroencephalography and Clinical Neurophysiology. 1988;70:499–509. doi: 10.1016/0013-4694(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Kohn SE, Goodglass H. Picture-naming in aphasia. Brain and Language. 1985;24(2):266–283. doi: 10.1016/0093-934x(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Kohnert KJ, Hernandez AE, Bates E. Bilingual performance on the Boston Naming Test: Preliminary norms in Spanish and English. Brain and Language. 1998;65(3):422–440. doi: 10.1006/brln.1998.2001. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Levelt WJ. Models of word production. Trends in Cognitive Sciences. 1999;3(6):223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, et al. Category-specific naming deficits for objects and actions: Semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40(9):1608–1621. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- McMillan C, Gee J, Moore P, Dennis K, DeVita C, Grossman M. Confrontation naming and morphometric analyses of structural MRI in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2004;17(4):320–323. doi: 10.1159/000077163. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: Shortened versions for use in Alzheimer’s disease. The Journals of Gerontology. 1992;47(3):154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Malow B, Blaxton T, Sato S, Bookheimer SY, Kufta CV, Figlozzi CM, Theodore WH. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37(3):245–252. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132(9):2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, et al. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cognitive and Behavioral Neurology. 2010;23(1):29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Whitaker HA. Language localization and variability. Brain and Language. 1978;6(2):239–260. doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation maping investigation in 117 patients. Journal of Neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Okada T, Tanaka S, Nakai T, Nishizawa S, Inui T, Sadato N. Naming of animals and tools. A functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neuroscience Letters. 2000;296(1):33–36. doi: 10.1016/s0304-3940(00)01612-8. [DOI] [PubMed] [Google Scholar]
- Papagno C, Gallucci M, Casarotti A, Castellano A, Falini A, Fava E, et al. Connectivity constraints on cortical reorganization of neural circuits involved in object naming. NeuroImage. 2011;55(3):1306–1313. doi: 10.1016/j.neuroimage.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Neuroscience Reviews. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Graff-Radford NR, Smith GE, Ivnik RJ, Willis FB, Petersen RC, et al. Differential item functioning of the Boston Naming Test in cognitively normal African American and caucasian older adults. Journal of the International Neuropsychological Society. 2009;15(5):758–768. doi: 10.1017/S1355617709990361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Bettinardi V, Bressi S, Gorno-Tempini M, Matarrese M, et al. Different neural systems for the recognition of animals and man-made tools. NeuroReport. 1995;6(12):1637–1641. doi: 10.1097/00001756-199508000-00012. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti M, Gorno-Tempini M, Cappa SF, Fazio F. Word and picture matching: A PET study of semantic category effects. Neuropsychologia. 1999;37(3):293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Noppeney U, Humphreys GW, Price CJ. Can segregation within the semantic system account for category-specific deficits? Brain. 2002;125(Pt 9):2067–2080. doi: 10.1093/brain/awf215. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Barker GJ, et al. Imaging language pathways predicts postoperative naming deficits. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(3):327–330. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: Effect of baseline. Human Brain Mapping. 2005;25(1):70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymer AM, Foundas AL, Maher LM, Greenwald ML, Morris M, Rothi LJG, et al. Cognitive neuropsychological analysis and neuroanatomic correlates in a case of acute anomia. Brain and Language. 1997;58(1):137–156. doi: 10.1006/brln.1997.1786. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Devinsky O, Doyle W, Perrine K. Function-specific high-probability “nodes” identified in posterior language cortex. Epilepsia. 1999;40(5):575–583. doi: 10.1111/j.1528-1157.1999.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Differences in functional magnetic resonance imaging activation by category in a visual confrontation naming task. Journal of Neuroimaging. 2001;11(2):165–170. doi: 10.1111/j.1552-6569.2001.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Kwong KK, Kennedy W, Rosen BR, Belliveau JW. Category-specific brain activation in fMRI during picture naming. NeuroReport. 1995;6(16):2109–2112. doi: 10.1097/00001756-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Tomaszewki Farias S, Harrington G, Broomand C, Seyal M. Differences in functional MR imaging activation patterns associated with confrontation naming and responsive naming. American Journal of Neuroradiology. 2005;26(10):2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley AM. The 60-item Boston Naming Test: Norms for cognitively intact adults aged 25–88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19(6):922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR. Explaining category-related effects in the retrieval of conceptual and lexical knowledge for concrete entities: Operationalization and analysis of factors. Neuropsychologia. 1997;35(10):1329–1339. doi: 10.1016/s0028-3932(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Trebuchon-Da Fonseca A, Guedj E, Alario FX, Laguitton V, Mundler O, Chauvel P, et al. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;132(10):2772–2784. doi: 10.1093/brain/awp083. [DOI] [PubMed] [Google Scholar]
- Turken A, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5(1) doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Moss HE. Towards a distributed account of conceptual knowledge. Trends in Cognitive Sciences. 2001;5(6):244–252. doi: 10.1016/s1364-6613(00)01651-x. [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz M, Kimberg DY, Faseyitan O, Brecher A, Dell GS, et al. Support for anterior temporal lobe involvement in semantic error production in aphasia: New evidence from VLSM. Brain and Language. 2011;117(3):110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H. Neuroanatomical aspects of naming. In: Hillis AE, editor. Handbook of Adult Language Disorders. New York: Psychology Press; 2002. [Google Scholar]
- Xu J, Gannon PJ, Emmorey K, Smith JF, Braun AR. Symbolic gestures and spoken language are processed by a common neural system. Proceedings of the National Academy of Sciences. 2009;106(49):20664–20669. doi: 10.1073/pnas.0909197106. [DOI] [PMC free article] [PubMed] [Google Scholar]


