Abstract
Background
Chronic misuse of alcohol results in widespread damage to the brain. Prior morphometric studies have examined cortical atrophy in individuals with alcoholism; however, no previous studies have examined alcohol-associated atrophy using cortical thickness measurements to obtain regional mapping of tissue loss across the full cortical surface.
Methods
We compared cortical thickness measures from 31 abstinent individuals with a history of prior alcohol abuse to 34 healthy nonalcoholic control participants (total sample size = 65). Cortical surface models were created from high-resolution T1-weighted images, and cortical thickness was then estimated as the distance between the gray matter/white matter boundary and the outer cortical surface.
Results
Abstinent alcoholics showed reduced whole-brain thickness as compared to nonalcoholic participants. Decreases in thickness were found bilaterally in (i) superior frontal, (ii) precentral, (iii) postcentral, (iv) middle frontal, (v) middle/superior temporal, (vi) middle temporal, and (vii) lateral occipital cortical regions. Decreased cortical thickness in the alcoholic group was associated with severity of alcohol abuse.
Conclusions
These findings demonstrate widespread reduction in cortical thickness as a consequence of chronic alcoholism, with most severe reductions in frontal and temporal brain regions.
Keywords: Adult, Humans, Magnetic Resonance Imaging, Cortical Thickness, Alcoholism
Chronic misuse of alcohol results in morphological damages to the brain, as revealed with postmortem analyses and in vivo neuroimaging techniques. Abundant evidence from these different methodologies indicates that the effects of alcohol are widespread and include neuronal degeneration of the cerebral cortex, cerebellum, and brainstem, as well as changes in the white matter underlying the cerebral cortex (De la Monte, 1988). The vulnerability of the cerebellum and the white matter to the effects of alcohol is also well documented (e.g., Harper and Kril, 1989; Pfefferbaum et al., 2000; Pfefferbaum and Sullivan, 2002).
The impact of alcohol on the cerebral cortex, and in particular the frontal lobes, has received considerable attention for decades (Harper, 1982). Early neuropathological evidence provided by Harper (1982; Harper et al., 1987) showed a 22% reduction in the number of neurons in the superior frontal cortex of alcoholics. Later studies showed reduced brain weight of alcoholics relative to controls that was correlated with the rate and amount of lifetime alcohol consumption (Harding et al., 1996). Some pathology-based studies have further demonstrated that reductions in overall brain volume in alcoholics may in fact be largely accounted for by reduction in white matter (e.g., De la Monte, 1988; Harper and Kril, 1985). Although white matter pathology is an integral aspect of the neuropathological changes associated with alcoholism (Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2000, 2009), the evidence is conclusive that the volume of gray matter is also reduced (e.g., Jernigan et al., 1991; Mann et al., 2001; Pfefferbaum et al., 1995). In fact, Fein and colleagues (2002, 2010) demonstrated that cortical gray matter volumes in alcohol-dependent individuals were negatively associated with age and lifetime duration of alcohol use. Even individuals with less severe alcohol abuse histories showed reduced whole-brain, prefrontal, and parietal cortical gray matter compared with nonalcoholic control participants (Fein et al., 2002, 2010).
Volumetric studies examining the vulnerability of the brain to alcohol have additionally revealed greater alcohol-associated volume losses in the frontal lobes compared with other structures (Dao-Castellana et al., 1998;Miguel-Hidalgo et al., 2002). In particular, atrophy has been noted in the precentral gyrus, middle frontal gyrus, dorsolateral-prefrontal cortex, subcallosal, orbitofrontal, and cingulate cortices (Mechtcheriakov et al., 2007). Volumetric studies have also identified widespread alcohol-related changes to cerebral cortex extending beyond the frontal lobes most often encompassing temporal, insular, and parietal cortex (Chanraud et al., 2007; Gazdzinski et al., 2005; Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2001). Makris and colleagues (2008) examined whether alcoholics had reduced volume in a reward network encompassing many fronto-temporal cortical structures (dorsolateral-prefrontal cortex, insula, subcallosal, orbitofrontal, cingulate cortices, parahippocampal gyrus, and temporal pole). Morphometric analyses indicated decreased total reward network volume in alcoholic subjects, which was most pronounced in the right dorsolateral-prefrontal cortex, right anterior insula, right nucleus accumbens, and left amygdala. Neuroanatomic investigations in animals have typically paralleled the human findings demonstrating reduction in cortical thickness related to chronic alcohol exposure, with the most vulnerable brain regions consistently involving frontal, temporal, and parietal cortex (e.g., Santucci et al., 2004; Savage et al., 2000).
Although various methods of image analysis have been employed to estimate the extent of structural damage related to alcohol abuse, abnormalities in cortical thickness have not yet been examined in individuals with chronic alcoholism. Prior morphometry studies have largely examined brain volume in specified regions of interest (ROIs) in alcoholics compared with nonalcoholics. Cortical thickness analyses allow measurement of tissue integrity across the entire cortical mantle, not limited by arbitrary anatomical landmarks (Fischl and Dale, 2000). This approach has advantages in detecting regionally specific cortical atrophy associated with subtle brain changes without being limited by a priori regional hypotheses. In this study, we measured thickness of the cerebral cortex from MR images (Fischl and Dale, 2000; Fischl et al., 1999a) to determine the regional vulnerability of the brain to chronic alcohol abuse. Based on the available volumetric findings in humans (Chanraud et al., 2007; Dao-Castellana et al., 1998; Gazdzinski et al., 2005; Makris et al., 2008; Mechtcheriakov et al., 2007; Miguel-Hidalgo et al., 2002; Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2001) and demonstrated cortical thinning in animals (Santucci et al., 2004; Savage et al., 2000), we expected to find substantial alcohol-associated reductions in thickness, particularly in frontal cortex. We further hypothesized that the reduced cortical thickness might extend to parietal and temporal regions.
MATERIALS AND METHODS
Participants
High-resolution structuralMR scans were obtained from a total of 65 participants in 2 demographic groups: abstinent alcoholic participants (ALCs; n = 31) and nonalcoholic control participants (NCs; n = 34). Participants were recruited by the Geriatric Research Education and Clinical Center (GRECC) at the VA Boston Healthcare System, Boston, MA, by way of flyers and newspaper advertisements targeting adults aged 35 to 65 both with and without a history of alcohol abuse. As participants were recruited from veteran and nonveteran sources, to ensure that the groups did not differ with regard to veteran status, a nonparametric, independent sample Mann–Whitney U-group test was conducted and found not to be significant (p > 0.5). All participants were screened to be free of any neurologic disease or illness. Participants were also excluded for any central nervous system drugs, blackouts (NCs only), seizure disorder (NCs only), head injury, hospitalization in a psychiatric facility > 1 week, or any medications for/history of severe psychiatric disorders (e.g., schizophrenia, chronic intractable obsessive compulsive disorder, agoraphobia, current major depression). History of substance abuse/dependence other than alcohol, except nicotine (current or lifetime) and cannabis (lifetime), was cause for exclusion. Cannabis use in the year prior to testing was cause for exclusion.
ALC individuals included 20 men and 11 women, and NC participants included 20 men and 14 women. The mean age of the ALC group was 51 years (SD = 8.0). The mean education in years was 14 (SD = 2.5). Verbal intelligence as measured by the Wechsler Test of Adult Reading (WTAR) (Wechsler, 2001) was 108 (SD = 12.8). For 3 participants, WTAR estimated IQ was not available; in these cases, the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Verbal IQ (Wechsler, 1997) was used (WAIS-III mean Verbal IQ = 102, SD = 15.0). Additionally, for 1 ALC, neither measure was available.
The NC group was matched to the ALC participants with regard to sex distribution, age (M = 51, SD = 7.8), education (M = 15, SD = 4.2), and verbal intelligence as measured by WTAR (M = 110, SD = 9.3). For 2 participants, WTAR estimated IQ was not available; in these cases, the WAIS-III measure of Verbal IQ was used (WAIS-III mean Verbal IQ = 104, SD = 12.7). For 1 NC, neither measure was available. t-Tests indicated that the ALC participants and control participants were not significantly different on these measures (ps > 0.30).
We studied ALC participants to examine the effects of long-term, chronic alcohol abuse on cortical thickness in the absence of possible confounding influences of concurrent use of alcohol on the brain. To meet criteria for inclusion, the ALC participants must have self-reported to have abstained from drinking for at least 1 month prior to participating in the study, report a history of ≥21 drinks per week for 4 years or longer, and meet criteria for alcohol abuse or dependence (defined below). The mean duration of abstinence prior to enrollment in this study was 5.6 years (SD = 7.8). Duration of abstinence ranged from 1 month to 26 years.
On the Lifetime Drinking History (LDH) (Skinner and Sheu, 1982), ALCs reported a significant history of alcohol abuse that ranged in duration from 4 years to 46 years, with a mean length of abuse of 25 years (SD = 9.4). The LDH yields an estimate of total lifetime exposure to alcohol using number of standard drinks consumed and a standard drink conversion (grams absolute alcohol). ALCs reported an average lifetime total volume of alcohol exposure of 60,250 total drinks (SD = 116,420) or 819,400 g (SD = 1,583,312) absolute alcohol. Across all drinking phases of their lifetime, ALCs reported a mean of 10 (SD = 6.4) standard drinks per drinking day and a mean maximum of 15 (SD = 7.8) standard drinks per drinking day.
All alcoholic participants met criteria for abuse or dependence on either the Diagnostic Interview Schedule for DSM-IV (DIS-IV) or the Self-Administered Short Michigan Alcoholism Screening Test (SMAST). Twenty-one ALCs met DSM-IV diagnostic criteria for alcohol dependence on the DIS-IV (Robins et al., 1996), and 7 met criteria for alcohol abuse. The 3 participants who did not meet criteria for alcohol abuse/dependence on the DIS-IV scored positively for alcohol abuse on the SMAST (scores of 8, 9, and 7, respectively). The SMAST (Selzer et al., 1975) is a self-reported measure of the severity of alcoholic behavior. ALCs reported scores ranging from 3 to 13 with a mean score of 9.0 (SD = 2.7). Selzer and colleagues (1975) suggest that a score of 0 to 1 on the SMAST represents a nonalcoholic profile, a score of 2 indicates a possible alcoholic profile, and a score of 3 or higher represents an alcoholic profile. All NC participants denied any significant history of alcohol or other substance abuse. This was supported by results on the computerized DIS-IV and the SMAST, on which none of the NC participants met criteria for alcohol dependence or abuse.
MRI Image Acquisition
Two whole-brain high-resolution T1-weighted MPRAGE scans were collected and averaged for each participant (T1 = 1,000 ms, TR = 2.73 sec, TE = 3.31 ms, flip angle = 7°, slice thickness = 1.3 mm, 128 slices, field of view = 256 × 256 mm) to create a single image volume with high contrast-to-noise. These scans have been empirically optimized for high contrast between gray and white matter, as well as gray matter and cerebrospinal fluid (CSF) for optimal structural and surface segmentation. Total imaging time was approximately 20 minutes.
Image Analysis
T1-weighted MRI data were processed using the FreeSurfer morphometric analysis tools (http://surfer.nmr.mgh.harvard.edu). The technical details of these procedures are described in prior publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 1999a,b, 2001, 2002, 2004a,b; Han et al., 2006; Jovicich et al., 2006; Segonne et al., 2004). Cortical thickness measurements were obtained by reconstructing representations of the gray/white matter boundary (Dale et al., 1999) and the cortical surface and then calculating the distance between those surfaces at each point across the cortical mantle. This method uses both intensity and continuity information from the entire 3-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data thus are capable of detecting submillimeter differences between groups (Fischl and Dale, 2000). Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Salat et al., 2004). FreeSurfer morphometric procedures show good test–retest reliability across scanner manufacturers and across field strengths (Han et al., 2006).
Thickness measures were mapped on the “inflated” surface of each participant’s reconstructed brain (Fischl et al., 1999a). This procedure allows visualization of data across the entire cortical surface (i.e., both the gyri and sulci) without interference from cortical folding. Maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a standard deviation of 20 mm and averaged across participants using a nonrigid high-dimensional spherical averaging method to align cortical folding patterns (Fischl et al. 1999a). This procedure provides accurate matching of morphologically homologous cortical locations among participants on the basis of each individual’s anatomy while minimizing metric distortion, resulting in a mean measure of cortical thickness for each group at each point in the reconstructed surface.
Statistical comparisons of global data and surface maps were generated to examine group differences in thickness (ALC vs. NC) at each vertex. Thickness maps were then created, with areas of significance noted. Maps were created using statistical thresholds of p = 0.05 and p = 0.01 and were smoothed to a full-width half-maximum (FWHM) level of 20. Further analyses examining the group differences in cortical thickness were conducted using ROIs.
Clusterwise Correction for Multiple Comparisons
Multiple comparison correction was performed using a clusterwise procedure described previously (Hagler et al., 2006) for cortical surface analysis. This procedure, which is available as a part of the Free-Surfer processing stream, is a method that utilizes a simulation to obtain a measure of the distribution of the maximum cluster size under the null hypothesis. Briefly, a z map is synthesized and spatially smoothed by an FWHM equal to that of the residual error from the generalized linear model analysis of the real data. The smoothed map is rescaled and thresholded at a given voxelwise level. Clusters are defined as a contiguous set of voxels above this voxelwise threshold. The area of the maximum cluster size is recorded, and the procedure is repeated to build up a list of maximum cluster sizes from which the distribution of the maximum cluster size is computed. Clusters in the real data are then computed using the same voxelwise threshold as in the simulation. The p-value for each real cluster is computed as the proportion of clusters in the simulated data that have a cluster area the same or greater. For these analyses, a total of 5,000 iterations were performed for each comparison, using a threshold of p = 0.05 and an FWHM of 20. The simulation cluster analysis was run for group comparisons.
RESULTS
Global Measures
Mean global thickness measures for each group are presented in Table 1. Unpaired t-tests revealed alcohol-associated reduction in global cortical thickness in both left (p < 0.05) and right (p < 0.01) hemispheres. Effects of alcohol on global cortical thickness were independent of sex and age.
Table 1.
Abstinent Alcoholics Showed Decreased Whole-Brain Thickness as Compared to Nonalcoholic Participants. Mean Global Thickness Measures in the Left and Right Hemispheres (Standard Error) are Presented for Each Group
| Nonalcoholic | Abstinent | |
|---|---|---|
| controls | alcoholics | |
| Age | 50.8 (1.3) | 51.3 (1.4) |
| Gender | 20 Male | 20 Male |
| 14 Female | 11 Female | |
| Left hemisphere thickness | 2.071 (0.0222)* | 1.991 (0.0232)* |
| Right hemisphere thickness | 2.074 (0.0216)** | 1.990 (0.0226)** |
p < 0.05;
p < 0.01.
Regional Measures and Maps of Cortical Thinning
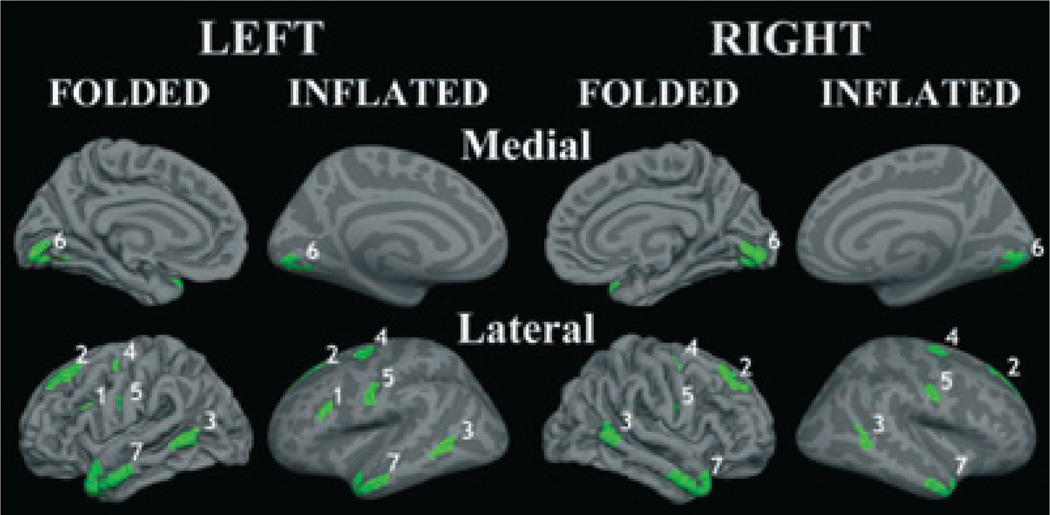
Significance maps of the group differences in cortical thickness are presented in Fig. 1. Alcohol-related reduction in cortical thickness was widespread and spanned a number of cortical regions. Further analyses examining the group differences in cortical thickness were conducted using ROIs created in regions showing substantially decreased thickness in the ALCs. These regions were also used in follow-up analyses examining the relationship between severity of alcohol abuse and cortical thickness. Bilateral ROIs were selected based on cortical thickness significance maps, displayed in Fig. 1; selected ROIs are shown in Fig. 2. The ROIs were derived by selecting areas of high significance between groups that were also validated in the simulation cluster analysis. ROIs were additionally constrained to be either gyral or sulcal, depending on the selected region. Seven ROIs were chosen in the following regions (all were bilateral): superior frontal, precentral, postcentral, middle frontal, middle/superior temporal, middle temporal, and lateral occipital. Thickness values were averaged across left and right hemispheres for bilateral ROIs, and this was performed for all 7 factors. Decreases in thickness in the ALCs as compared to NC participants were found in all 7 ROIs; group differences in thickness in each ROI can be seen in Fig. 2 and Table 2.
Fig. 1.
Significance maps of the group differences in cortical thickness in the abstinent alcoholics as compared to nonalcoholic control participants. Areas of significance were noted using light blue (p = 0.01) and dark blue (p = 0.05), indicating significantly decreased cortical thickness in the alcoholic individuals as compared to nonalcoholic control participants. The clusterwise analysis revealed areas of significant difference between groups remained after multiple comparison corrections procedures.
Fig. 2.
Decreases in thickness in the abstinent alcoholics as compared to nonalcoholic control participants were found in the following bilateral regions of interest: (1) middle frontal/pars opercularis, (2) superior frontal, (3) middle temporal, (4) precentral sulcus, (5) postcentral sulcus, (6) lateral occipital, and (7) middle/superior temporal.
Table 2.
Reduced Thickness in the Abstinent Alcoholics (ALCs) as Compared to Nonalcoholic Control (NC) Participants was Found in all 7 Regions of Interest (ROIs). Thickness Values were Averaged Across Left and Right Hemispheres for Bilateral ROIs. Means (Standard Error) and p-Values are Presented
| ROIs | ALC | NC | p-Value |
|---|---|---|---|
| Inferior frontal | 1.73 (0.033) | 1.90 (0.032) | 0.0005 |
| Superior frontal | 2.23 (0.034) | 2.41 (0.032) | 0.0487 |
| Frontal temporal | 2.97 (0.034) | 3.12 (0.032) | 0.0013 |
| Middle temporal | 2.54 (0.037) | 2.65 (0.035) | 0.0250 |
| Precentral sulcus | 1.76 (0.037) | 1.97 (0.035) | 0.0002 |
| Central sulcus | 1.54 (0.031) | 1.66 (0.030) | 0.0039 |
| Medial occipital | 1.68 (0.022) | 1.78 (0.021) | 0.0020 |
Results of Clusterwise Correction for Multiple Comparisons
The clusterwise analysis revealed areas of significant difference between groups that remained after multiple comparison corrections procedures. These areas were consistent with what was observed during the initial analyses (Fig. 1).
Effect of Alcohol Severity on Cortical Thickness
We used the total years of abuse, months of sobriety/abstinence, and the SMAST as our dependent measures of alcohol severity. Across all participants, total years of abuse were negatively correlated with global (whole brain) cortical thickness (r = −0.283; p = 0.022), indicating that longer durations of abuse were associated with decreased cortical thickness. Furthermore, total years of abuse were negatively correlated with cortical thickness in 6 of 7 ROIs (see Table 3; ps<0.05).
Table 3.
Bivariate Correlational Analyses Revealed Significant Correlations Between Self-Administered Short Michigan Alcoholism Screening Test (SMAST) Score and Cortical Thickness Regions of Interest (ROIs) for the Combined Group of Abstinent Alcoholics and Nonalcoholic Control Participants
| SMAST |
||||
|---|---|---|---|---|
| All participants |
Abstinent alcoholic only |
|||
| ROIs | r-Value | p-Value | r-Value | p-Value |
| Inferior frontal | −0.495** | 0.0001 | −0.441** | 0.013 |
| Superior frontal | −0.291* | 0.019 | −0.303 | 0.098 |
| Frontal temporal | −0.376** | 0.002 | −0.055 | 0.768 |
| Middle temporal | −0.249* | 0.045 | −0.008 | 0.965 |
| Precentral sulcus | −0.492** | 0.0001 | −0.367* | 0.042 |
| Central sulcus | −0.369** | 0.002 | −0.150 | 0.421 |
| Medial occipital | −0.399** | 0.001 | −0.149 | 0.425 |
p ≤ 0.01,
p ≤ 0.05.
SMAST score varied from 3 to 13 in the ALC participants and from 0 to 2 in the NCs. Correlational analyses were performed with the whole group then in the ALCs only. Across all participants (ALCs and NCs), SMAST score was negatively correlated with global (whole brain) cortical thickness (r = −0.353; p = 0.0040), indicating that more severe alcohol severity ratings (as depicted by higher SMAST score) were associated with decreased cortical thickness (see Fig. 3). Table 4 displays results from correlation analyses examining the relationship between SMAST and cortical thickness for each derived ROI. Across all participants, SMAST score was significantly negatively correlated with cortical thickness across all 7 ROIs indicating that more severe alcohol severity ratings were also associated with decreased cortical thickness in frontal and temporal brain regions. These effects remain when controlling for age (ps<0.05). Alcohol severity as assessed by the SMAST accounted for nearly 25% of the variance in the cortical thickness in the inferior frontal region as well as in the precentral sulcus region (see Table 4). Scatterplots of the relationship between alcohol severity and cortical thickness for all ROIs are presented in Fig. 3.
Fig. 3.
Scatterplots of the relationship between alcohol severity as measured by the Self-Administered Short Michigan Alcoholism Screening Test (SMAST) score and global (whole brain) cortical thickness and cortical thickness in regions of interest (*p < 0.05).
Table 4.
Bivariate Correlational Analyses Revealed Significant Correlations Between Years of Alcohol Abuse and Cortical Thickness Regions of Interest (ROIs) Across all Participants
| Years abuse |
||
|---|---|---|
| ROIs | r-Value | p-Value |
| Inferior frontal | −0.275* | 0.027 |
| Superior frontal | −0.110 | 0.383 |
| Frontal temporal | −0.289* | 0.020 |
| Middle temporal | −0.275* | 0.047 |
| Precentral sulcus | −0.355** | 0.004 |
| Central sulcus | −0.265* | 0.033 |
| Medial occipital | −0.255* | 0.041 |
p < 0.01,
p < 0.05.
A follow-up correlation analysis was performed to look at the relationship between alcohol abuse severity as assessed by the SMAST and cortical thickness only in the ALCs to determine whether the relationship between alcohol severity and cortical thickness would remain within the ALC group only (Table 4). Inferior frontal (p < 0.01) and precentral sulcus (p < 0.05) regions were still significantly correlated with alcohol severity; and a trend was observed in superior frontal cortex (p < 0.10).
Effect of Abstinence on Cortical Thickness
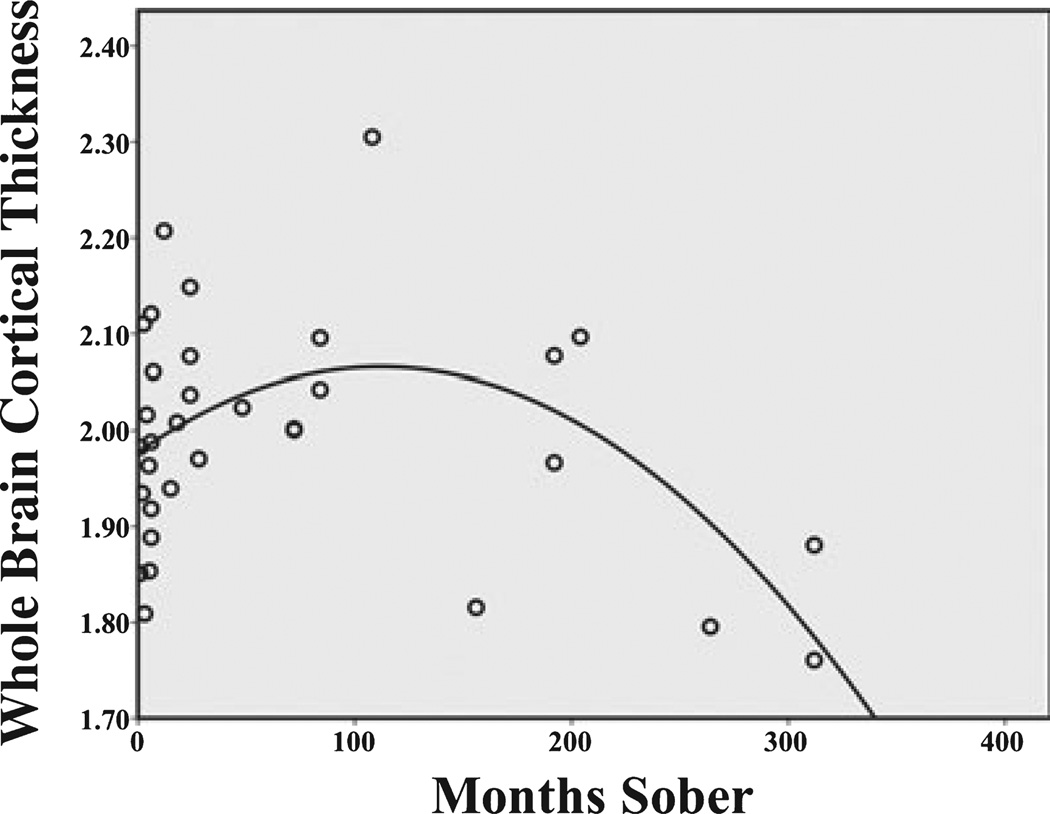
We next examined the association between thickness and length of sobriety/abstinence from alcohol. As seen in Table 3, the linear relationship between months of sobriety/abstinence and mean global cortical thickness failed to reach significance, but the distribution of the data suggested that the relationship might be better understood in terms of a quadratic function where by most recovery occurs early then levels off (see Fig. 4). The second-order quadratic relationship as determined by multiple regression was significant (F = 5.41, p = 0.01, R2 = 0.265). The equation demonstrated a positive association between cortical thickness and length of sobriety up to approximately 8 years (100 months sobriety), with the greatest increase occurring in the first 2 years of sobriety. There appears to be a stable relationship between cortical thickness and months of sobriety spanning 8 to 12 years, after which thickness decreases among individuals with 12 years or more of maintained abstinence.
Fig. 4.
Scatterplot of the relationship between length of sobriety and global (whole brain) cortical thickness demonstrating a positive association between cortical thickness and length of sobriety up to approximately 12 years (150 months sobriety), with the greatest increase occurring in the first 2 years of sobriety. The quadratic equation is shown.
DISCUSSION
The current study applied a novel technique to measure the thickness of the cortical mantle in ALCs and healthy NC participants, without a priori defined volumetric ROIs. This method provided an objective and sensitive assessment of alcohol’s effects across the whole brain by calculating group differences first as function of cortical thickness across the entire mantle followed by statistically guided, data-driven region-by-region comparison, as opposed to a straight a priori region-by-region comparison. This analysis showed that ALCs have decreased whole-brain cortical thickness as compared to NC participants. The data-driven approach of the current method suggests that an in vivo measurement of the human cortex reveals it to be thinner overall in ALCs as compared to NCs; a finding that is highly consistent with the neuropathological literature demonstrating conclusively that alcoholic brains are lighter overall than nonalcoholic brains (e.g., Harding et al., 1996; Harper et al., 1987). However, while some pathological studies have attributed low brain weight to a reduction in white matter volume (e.g., De la Monte, 1988; Harper andKril, 1985), the current data support evidence that cortical gray matter is also reduced in volume and thus may contribute to low brain weight (e.g., Jernigan et al., 1991; Mann et al., 2001; Pfefferbaum et al., 1995).
Mapping the thickness of the cortex across the brain revealed regionally specific areas that were thinner in ALCs compared with NC participants. Specifically, there were pronounced bilateral decreases in cortical thickness in inferior frontal, superior frontal, lateral frontal, middle temporal, precentral sulcus, central sulcus, and medial occipital cortical regions in ALCs. The regional distribution of differences suggests that the frontal lobes are especially vulnerable to thinning, followed by temporal and occipital regions. Thus, the current results support Harper and Kril’s (1989) early pathological work and subsequent suggestion that alcohol may preferentially target large pyramidal cells in frontal cortex.
These findings are consistent with other volumetric techniques in humans (e.g., Chanraud et al., 2007; Dao-Castellana et al., 1998; Gazdzinski et al., 2005; Makris et al., 2008; Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2001) showing selective neuronal loss occurring most consistently in frontal cortex. In particular, our results are highly consistent with a recent voxel-based morphometry study of gray matter (and white matter) in alcohol-dependent individuals by Mechtcheriakov and colleagues (2007). This study documented atrophy in the precentral gyrus, middle frontal gyrus, dorsolateral-prefrontal cortex, subcallosal, orbitofrontal, and cingulate cortices (as well as the subcortical structures of the dorsal hippocampus and anterior thalamus and the cerebellum). The overlap of regions most susceptible to alcoholrelated pathology in this volumetric study as compared to those identified using the cortical thickness whole-brain approach taken in this investigation is striking and provides strong support for this emerging consensus of vulnerable regions within the frontal lobes using different morphological techniques.
The regions identified as showing the greatest reduction in cortical thickness are consistent also with the well-documented neuropsychological effects of alcohol. Investigations of recently detoxified individuals with chronic alcoholism have consistently shown significant impairments in the cognitive domains of executive function, nonverbal memory, visuospatial function, and gait and balance (e.g., Oscar-Berman, 2000; Oscar-Berman and Marinkovic, 2007; Parsons, 1993). Deficits in these functions are suggestive of dysfunction of the cerebellar cortex and vermis, posterior parietal lobe, and most significantly, frontal lobes (specifically, orbitofrontal and prefrontal cortex) (Sullivan et al., 2000, 2002) and are consistent with the ROI identified in this investigation.
Last, this is the first study to date to relate severity of alcohol abuse to reduction in cortical thickness. Length of alcohol abuse was related to a greater reduction in cortical thickness in this sample. Self-report of alcoholic behaviors as assessed with the SMAST was used to quantify alcohol abuse severity.
The SMAST is aimed at identifying alcoholic dependency through the endorsement of alcoholic behaviors. It is assumed that higher scores on the SMAST are associated with higher levels of alcohol consumption, dependency, tolerance, and maladaptive behaviors that interfere with social/occupational functioning. Thus, high endorsement of such alcoholic behaviors was associated with greater reduction in cortical thickness among recovered alcoholics. Severity of alcohol abuse was associated with greater reduction in thickness across the entire cortical mantle, and analyses with global thickness and ROI demonstrated that these relationships were independent of age. Correlations were highly significant for those regions found to evidence the greatest group differences. For example, the measures of thickness in the inferior frontal region as well as in the precentral sulcus region were most strongly related to severity of alcoholism. This latter finding in particular might have far-reaching consequences, as it is possible that reduction in frontal cortical tissue leads to maladaptive alcoholic behaviors or that the behaviors themselves are associated with greater alcohol consumption, which then exerts a neurotoxic effect on frontal cortical tissue. The causality of the relationship should be explored in future investigations.
Follow-up regression analyses with global thickness revealed a quadratic relationship between length of abstinence from alcohol and cortical thickness. Global cortical thickness was higher in this cross-sectional sample of ALCs with maintained sobriety for up to 12 years, after which thickness across the cortical mantle began to decline. This analysis supports the idea that changes to brain structure may occur within the initial 2 years of abstinence, but a longitudinal study would need to address this possibility directly. While certainly in need of further examination in a larger sample, this analysis supports other investigations demonstrating the brain’s ability to recover tissue integrity and function with maintained abstinence, particularly during the initial 2 years of sobriety. Why cortical thickness was decreased in individuals with 12 years or more of maintained sobriety is unclear, but likely is related to other mediating factors rather than to alcoholism or abstinence per se. It is important to note that there are not many individuals in the sample who have achieved sobriety for more than 12 years.
Although our approach is a very sensitive and accurate way of measuring neuropathology in vivo, there are other brain structures that we did not investigate but are nonetheless impacted by alcohol, most importantly, the cerebellum. The cerebellum is in fact the primary neurologic target of alcohol’s deleterious effects. Given the large body of literature confirming cerebellar shrinkage among chronic and ALCs, if we had been able to visualize cerebellar cortex, we would predict that we would have seen alcohol-related thinning in the cerebellum as well. We continue to develop our ability to assess volume, cortex, and white matter in the cerebellum and hope to address the association between cerebellar and cortical atrophy in future investigations.
These findings demonstrate widespread reduction in cortical thickness as a consequence of alcoholism in a sample of community dwelling former alcoholics. It should be stressed that the current findings were derived using a whole-brain analysis that yielded regions of greatest significance, rather than an a priori region-by-region comparison that can be susceptible to variations in the statistical sensitivity of each isolated region/comparison (Fischl and Dale, 2000). This approach has advantages in detecting regionally specific cortical atrophy associated with subtle brain changes without being limited by a priori regional hypotheses. Future investigations should continue to quantify cortical thickness alterations related to alcohol and to relate cortical thickness to severity of alcoholism. Particularly, cortical thickness should be related to measures of alcohol consumption across a variety of drinkers to investigate whether quantity of alcohol consumed can predict brain changes. Further, relationships between cognitive dysfunction and cortical thickness measures in alcoholics should be explored.
ACKNOWLEDGMENTS
This research was supported by NIH NIAAA 14205, NIH AG08796, NIH MH 53673, NIH NINR R01NR010827, NIH NIA K01AG024898, and VA Merit Review Awards to CBF and REM.
REFERENCES
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot J-L. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- De la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Fein G, di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naive actively drinking alcohol-dependent sample. Alcohol Clin Exp Res. 2010;34:175–182. doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Lui A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE TransMed Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation. Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat D, van der Kouwe A, Makris N, Segonne F, Dale A. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale A. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale A. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux E, Halgren E, Segonne D, Salat D, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JL, Harper CG, Kril JJ. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and timedependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- Harper C. Neuropathology of brain damage caused by alcohol. Med J Aust. 1982;2:277–282. doi: 10.5694/j.1326-5377.1982.tb124389.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. J Neurol Neurosurg Psychiatry. 1985;48:211–217. doi: 10.1136/jnnp.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurons away? Br Med J. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, Drobes DJ, George MS, Bares R, Machulla H-J, Mundle G, Reimold M, Heinz A. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):104S–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Nororha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism Research Monograph No. 34; 2000. pp. 437–471. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol Induced Brain Damage NIAAA Research Monograph No. 22. Bethesda, MD: 1993. pp. 173–194. NIH Pub. No. 93-3549. [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moselesy M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan EV. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Robins LN, Marcus L, Reich W, Cunningham R, Gallagher T. Diagnostic Interview Schedule, Version IV. St. Louis, MO: Department of Psychiatry, Washington University School of Medicine Louis; 1996. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Mercado M, Bettica A, Cortes C, York D, Moody E. Residual behavioral and neuroanatomical effects of short-term chronic ethanol consumption in rats. Brain Res Cogn Brain Res. 2004;20:449–461. doi: 10.1016/j.cogbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Savage LM, Candon PM, Hohmann HL. Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcohol Clin Exp Res. 2000;24:465–475. [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered shortMichigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: the Lifetime Drinking History and theMAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Regional cerebellar volume decline in normal aging, uncomplicated alcoholism, and Korsakoff’s Syndrome. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]