Abstract
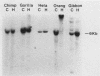
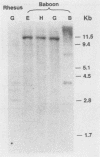
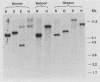
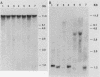
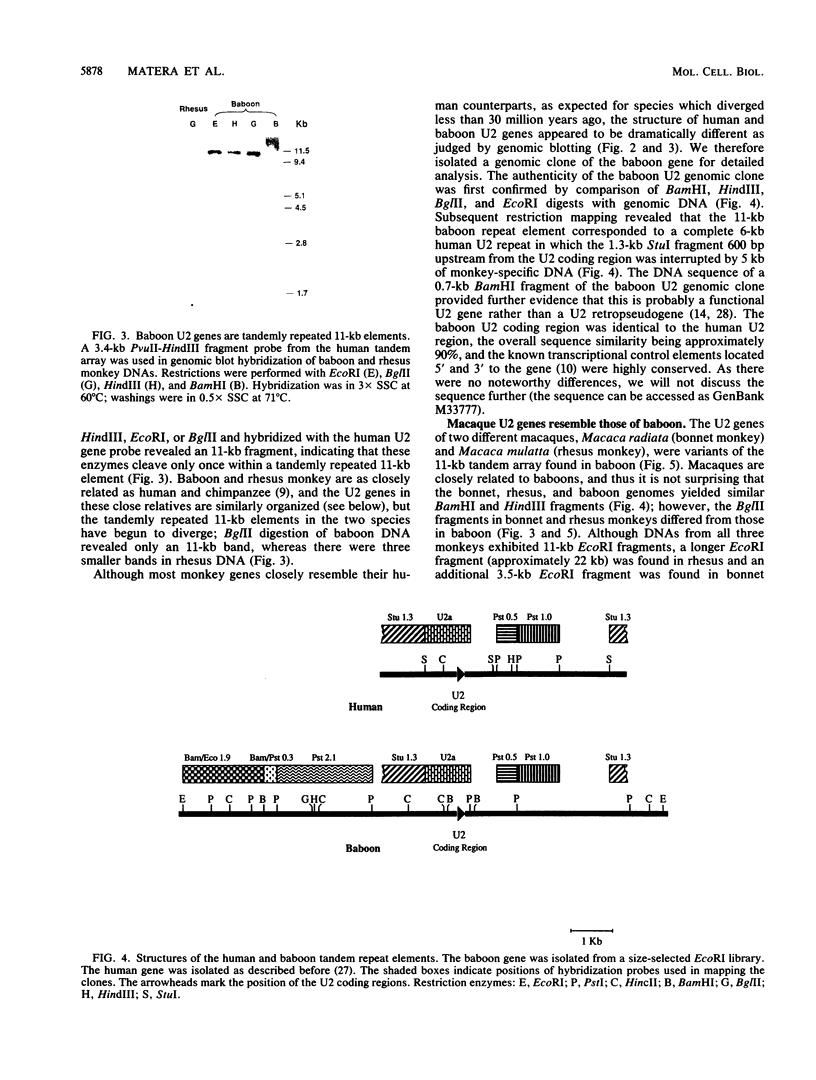
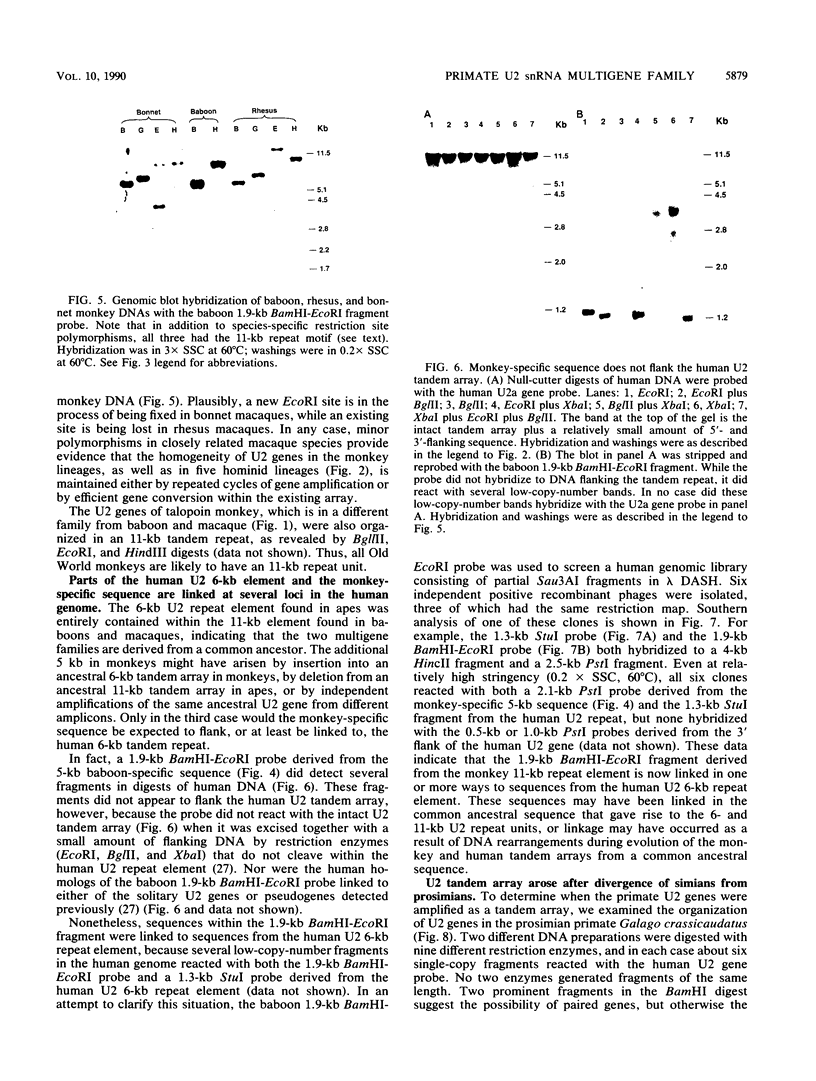
The organization of U2 genes was compared in apes, Old World monkeys, and the prosimian galago. In humans and all apes (gibbon, orangutan, gorilla, and chimpanzee), the U2 genes were organized as a tandem repeat of a 6-kb element; however, the restriction maps of the 6-kb elements in these divergent species differed slightly, demonstrating that mechanisms must exist for maintaining sequence homogeneity within this tandem array. In Old World monkeys, the U2 genes were organized as a tandem repeat of an 11-kb element; the restriction maps of the 11-kb elements in baboon and two closely related macaques, bonnet and rhesus monkeys, also differed slightly, confirming that efficient sequence homogenization is an intrinsic property of the U2 tandem array. Interestingly, the 11-kb monkey repeat unit differed from the 6-kb hominid repeat unit by a 5-kb block of monkey-specific sequence. Finally, we found that the U2 genes of the prosimian galago were dispersed rather than tandemly repeated, suggesting that the hominid and Old World monkey U2 tandem arrays resulted from independent amplifications of a common ancestral U2 gene. Alternatively, the 5-kb monkey-specific sequence could have been inserted into the 6-kb array or deleted from the 11-kb array soon after divergence of the hominid and Old World monkey lineages.
Full text
PDF

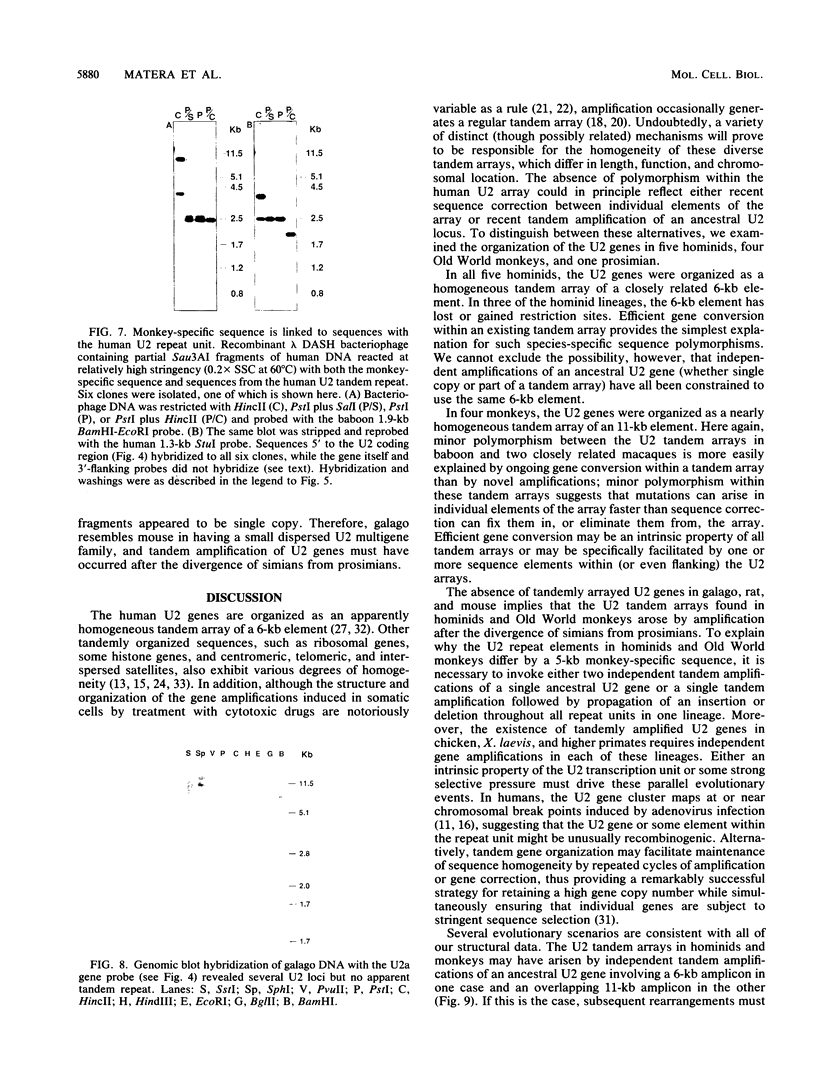
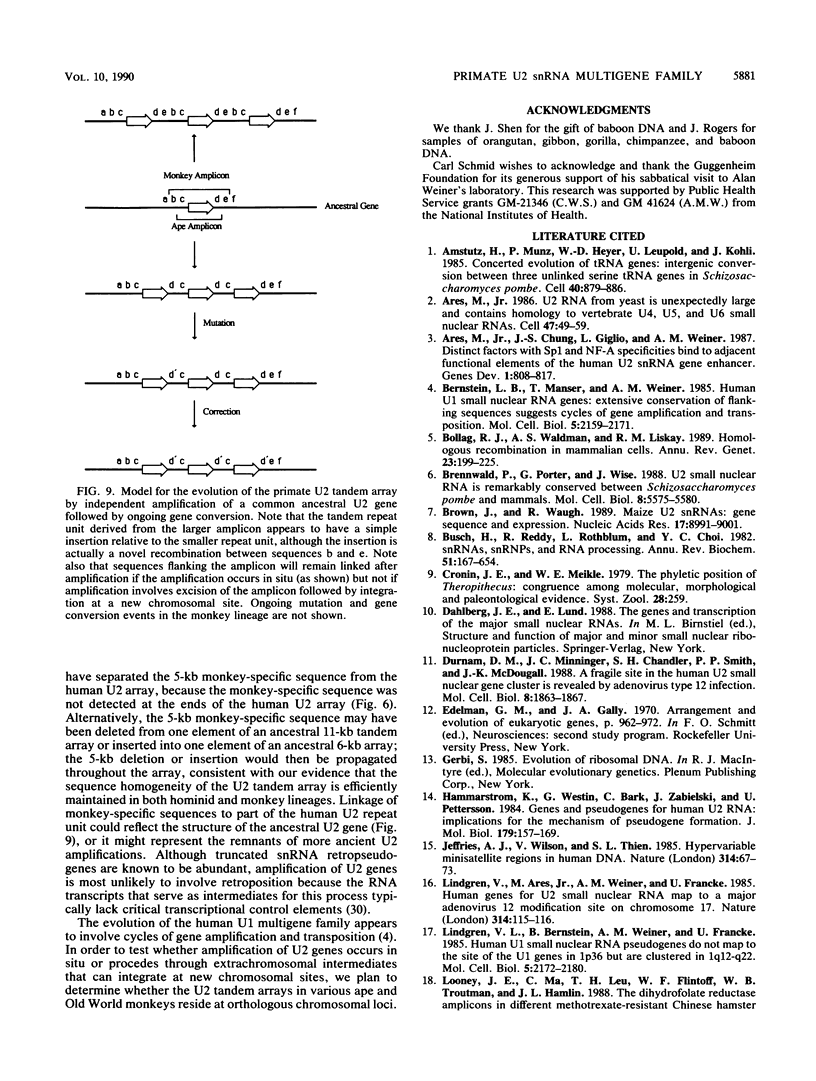

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstutz H., Munz P., Heyer W. D., Leupoid U., Kohli J. Concerted evolution of tRNA genes: intergenic conversion among three unlinked serine tRNA genes in S. pombe. Cell. 1985 Apr;40(4):879–886. doi: 10.1016/0092-8674(85)90347-2. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Chung J. S., Giglio L., Weiner A. M. Distinct factors with Sp1 and NF-A specificities bind to adjacent functional elements of the human U2 snRNA gene enhancer. Genes Dev. 1987 Oct;1(8):808–817. doi: 10.1101/gad.1.8.808. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr U2 RNA from yeast is unexpectedly large and contains homology to vertebrate U4, U5, and U6 small nuclear RNAs. Cell. 1986 Oct 10;47(1):49–59. doi: 10.1016/0092-8674(86)90365-x. [DOI] [PubMed] [Google Scholar]
- Bernstein L. B., Manser T., Weiner A. M. Human U1 small nuclear RNA genes: extensive conservation of flanking sequences suggests cycles of gene amplification and transposition. Mol Cell Biol. 1985 Sep;5(9):2159–2171. doi: 10.1128/mcb.5.9.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Brennwald P., Porter G., Wise J. A. U2 small nuclear RNA is remarkably conserved between Schizosaccharomyces pombe and mammals. Mol Cell Biol. 1988 Dec;8(12):5575–5580. doi: 10.1128/mcb.8.12.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Waugh R. Maize U2 snRNAs: gene sequence and expression. Nucleic Acids Res. 1989 Nov 25;17(22):8991–9001. doi: 10.1093/nar/17.22.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Menninger J. C., Chandler S. H., Smith P. P., McDougall J. K. A fragile site in the human U2 small nuclear RNA gene cluster is revealed by adenovirus type 12 infection. Mol Cell Biol. 1988 May;8(5):1863–1867. doi: 10.1128/mcb.8.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström K., Westin G., Bark C., Zabielski J., Petterson U. Genes and pseudogenes for human U2 RNA. Implications for the mechanism of pseudogene formation. J Mol Biol. 1984 Oct 25;179(2):157–169. doi: 10.1016/0022-2836(84)90463-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Ares M., Jr, Weiner A. M., Francke U. Human genes for U2 small nuclear RNA map to a major adenovirus 12 modification site on chromosome 17. Nature. 1985 Mar 7;314(6006):115–116. doi: 10.1038/314115a0. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Bernstein L. B., Weiner A. M., Francke U. Human U1 small nuclear RNA pseudogenes do not map to the site of the U1 genes in 1p36 but are clustered in 1q12-q22. Mol Cell Biol. 1985 Sep;5(9):2172–2180. doi: 10.1128/mcb.5.9.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passananti C., Davies B., Ford M., Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification. EMBO J. 1987 Jun;6(6):1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26(1-2):99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Base sequence and evolution of guinea-pig alpha-satellite DNA. Nature. 1970 Aug 22;227(5260):794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Weiner A. M. Human genes for U2 small nuclear RNA are tandemly repeated. Mol Cell Biol. 1984 Mar;4(3):492–499. doi: 10.1128/mcb.4.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Weiner A. M. Pseudogenes for human U2 small nuclear RNA do not have a fixed site of 3' truncation. Nucleic Acids Res. 1984 Feb 10;12(3):1463–1471. doi: 10.1093/nar/12.3.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. Structure of U2 snRNA genes of Arabidopsis thaliana and their expression in electroporated plant protoplasts. EMBO J. 1988 Mar;7(3):791–799. doi: 10.1002/j.1460-2075.1988.tb02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Denison R. A. Either gene amplification or gene conversion may maintain the homogeneity of the multigene family encoding human U1 small nuclear RNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1141–1149. doi: 10.1101/sqb.1983.047.01.129. [DOI] [PubMed] [Google Scholar]
- Westin G., Zabielski J., Hammarström K., Monstein H. J., Bark C., Pettersson U. Clustered genes for human U2 RNA. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3811–3815. doi: 10.1073/pnas.81.12.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]