Abstract
Background
The electrocardiographic PR interval increases with aging, differs by race, and is associated with atrial fibrillation (AF), pacemaker implantation and all-cause mortality. We sought to determine the associations between PR interval and heart failure, AF, and mortality in a biracial cohort of older adults.
Methods and results
The Health, Aging, and Body Composition (Health ABC) Study is a prospective, biracial cohort. We employed multivariable Cox proportional hazards models to examine PR interval (hazard ratios expressed per standard deviation (SD) increase) and 10-year risks of heart failure, AF, and all-cause mortality. Multivariable models included demographic, anthropometric, and clinical variables in addition to established cardiovascular risk factors. We examined 2722 Health ABC participants (age 74±3 years, 51.9% women, and 41% black). We did not identify significant effect modification by race for the outcomes studied. Following multivariable adjustment, every SD increase (29 ms) in PR interval was associated with a 13% greater 10-year risk of heart failure (95% confidence interval [CI], 1.02 to 1.25) and a 13% increased risk of incident AF (95% CI, 1.04 to 1.23). PR interval >200 ms was associated with a 46% increased risk of incident heart failure (95% CI, 1.11 to 1.93). PR interval was not associated with increased all-cause mortality.
Conclusions
We identified significant relations of PR interval to heart failure and AF in older adults. Our findings extend prior investigations by examining PR interval and associations with adverse outcomes in a biracial cohort of older men and women.
Keywords: PR interval, epidemiology, heart failure, atrial fibrillation, mortality, aging
The PR interval, accessible from the electrocardiogram (ECG), measures the duration of atrial and atrioventricular (AV) nodal conduction. Automated software routinely quantifies the median PR interval in clinical settings. As established by large-sized cohorts, PR interval increases progressively with age.1,2
Previously PR interval ≥200 ms, was considered benign.3 More recently, PR interval prolongation has been associated with adverse outcomes including a 1.44 to 2-fold5 increased risk of atrial fibrillation (AF). The association with mortality has been varied: in follow-up over several decades, increased PR interval duration in a community-based cohort was associated with a 1.4-fold increased risk of all-cause mortality.5 In contrast, a National Health and Nutrition Examination Survey analysis found no association with all-cause or cardiovascular mortality.6 Studies examining racial differences have had varied results. Whereas a cross-sectional multi-ethnic study identified no significant ethnic differences in PR interval duration,7 a large community-based study reported significantly prolonged PR interval duration in blacks compared to whites.4
Heart failure, AF, and mortality are common in older age groups. We examined PR interval in the Health, Aging, and Body Composition Study (Health ABC), a prospective, biracial cohort of older adults. We hypothesized that PR interval would be significantly associated with heart failure, AF, and all-cause mortality in a cohort of older adults after accounting for established clinical risk factors. We sought to identify racial differences in PR interval as an exposure, especially because blacks have been reported to have less incident AF than whites8 despite observed differences in PR duration.9
Methods
Study cohort
Health ABC is a longitudinal cohort study examining the association of body composition with health outcomes. Participants were recruited from a random sampling of white Medicare beneficiaries in areas surrounding Pittsburgh, Pennsylvania, and Memphis, Tennessee, and all age-eligible blacks in the same zip codes. Enrollment criteria included age 70–79 years, white or black race, capacity to perform mobility-related activities of daily living, and absence of functional disability.10 The initial examination included a standardized physical, anthropometric assessment, laboratory, motor and cognitive assessments, body imaging, and medications. Health ABC’s baseline examination (1997–1998) consisted of 3,075 participants (52% women and 42% black) who have been followed with telephone contacts at six-month intervals and clinic examinations in years 1–6, 8, 10 and 11. The study completed its 14th year 2010–2011.
The present analysis excluded participants with missing ECG data or PR intervals <80 ms (n=81); atrial or other supraventricular arrhythmias including AF, ventricular pre-excitation (Wolff-Parkinson-White), second degree or higher heart block, or paced rhythm (n=136); absence of the examined covariates (n=133); and lacking outcome data (n=3). Participants with prevalent heart failure (n=64) were excluded from the analysis examining heart failure as an outcome. Participants with prevalent AF (n=126) or lacking Center for Medicare and Medicaid (CMS) data (n=23) were excluded from analysis examining incident AF. Health ABC’s study protocols were approved by the Institutional Review Boards at the University of Pittsburgh and the University of Tennessee. All participants provided written informed consent.
Electrocardiography and PR interval measurement
Twelve-lead ECGs for the present investigation were collected at the baseline examination (Marquette Electronics MAC PC) by standardized protocol. ECG tracings were submitted to the St. Louis University Core ECG Laboratory (St. Louis, Missouri) for analysis, where ECG analysis was blinded to participant demographics. Two independent coders analyzed each ECG for standard intervals (heart rate, PR, QRS and QT intervals) and amplitudes (R, S, and T wave and J and ST-segment) as described elsewhere.11 The PR interval was determined as beginning at the T-P junction at the start of the P wave to the initiation of the QRS segment. Measurements of the PR interval were made in lead II using a 7-power magnifying loupe on a grid with 0.1 mm calibration. The PR interval was determined as the average measure from 3 consecutive beats or 2 at slower heart rates (<50 beats per minute). Interreader discrepancies were resolved by direct comparison and adjudication by a supervisor. The St. Louis University Core ECG Laboratory has reported excellent reliability assessments.11 Interreader reproducibility assessments for PR interval measurement found a coefficient of reliability of 0.997 and Pearson’s correlation coefficient 0.997. A paired t-test comparing interreader PR measurements did not achieve statistical significance (P=0.22).
Study measurements and clinical assessments
The Year 1 visit comprised the baseline examination for the present analysis. Subsequent examinations included interim health history updates, hospitalizations, and current medications. Race (black or white), smoking (current/former or never), and alcohol use were determined by self-report. Moderate-to-heavy alcohol consumption was determined by self-report of ≥14 drinks for men and ≥7 drinks weekly for women. Systolic and diastolic blood pressure (BP) consisted of two measurements averaged with participants in a seated position. Body mass index (BMI) was derived using weight divided by height squared (kg/m2). Diabetes was determined from self-reported history, use of oral hypoglycemics or insulins, or fasting glucose ≥126 mg/dL. Blood samples were obtained after an 8-hour fast. Serum total and HDL cholesterol measures were assayed according to standardized methods (Ortho-Clinical Diagnostics, Rochester, NY). Medications taken within two weeks were brought to each examination and classified according to the Iowa Drug Information System, thereby identifying antihypertensive, oral hypoglycemic or insulin medications, and medications with atrioventricular nodal blocking properties (amiodarone, oral beta blockers, cardiac glycosides and calcium channel blockers). Prevalent heart failure was determined by self-reported history or use of vasodilator, cardiac glycoside or diuretic therapies. Prevalent coronary heart disease (CHD) was determined by self-reported history of coronary artery bypass graft surgery, coronary angioplasty, history of myocardial infarction, angina or ECG evidence of myocardial infarction by identification of an ECG major Q-wave abnormality. ECG left ventricular hypertrophy (LVH) was categorized using an automated measurement of the R wave amplitude >26 mm in precordial leads V5 or V6; R wave amplitude >20 mm in limb leads I, II or III, or aVF; or R wave amplitude >12 mm in limb lead aVL.
Study events and outcomes
Outcomes occurred during 10-year follow-up after the baseline examination and consisted of heart failure, AF, and all-cause mortality. Medical history for interim events and incident disease were reviewed at annual examinations and 6-month telephone contacts. Incident heart failure was determined by a physician diagnosis of congestive heart failure and medical treatment for heart failure (i.e. combination of a diuretic and cardiac glycoside or vasodilator) requiring overnight hospitalization. Further criteria included presence of cardiomegaly or pulmonary edema by chest radiograph or evidence of ventricular dilatation by cardiac imaging when available. Records from hospitalizations were obtained and reviewed. Incident heart failure cases underwent adjudication by physicians at the local research site as described previously.12 Incident atrial fibrillation (AF) was obtained by linking unique Health ABC identifiers to the CMS database for International Classification of Diseases, Ninth Revision (ICD-9), codes 427.31 or 427.32 obtained from the ambulatory or in-patient setting through 2008. Health ABC investigators did not have access to hospital ECGs, and AF was not an adjudicated study end-point. Use of ICD-9 coding for ascertainment of incident AF has been demonstrated to have 84% sensitivity and 98% specificity.8 Date of death was ascertained from participant proxy or other participant representative, hospital records, obituary, or search of the National Death Index. The Health ABC central Diagnosis and Disease Ascertainment Committee integrates data to review all identified deaths. Follow-up duration was determined from the baseline visit until the first event or the day of death or 10 years. Outcomes were considered independently, and participants were censored from the analysis at the date of the event or last known study contact.
Statistical analyses
Continuous variables were examined for their mean and standard deviations and categorical variables for their distributions. We examined the graphical and numeric distribution of the PR interval and determined it did not depart from normality. The relations of covariates to the PR interval were estimated using general linear models. Models were initially adjusted for demographic variables (age, sex, race and site). A multivariable model then adjusted for the demographic variables and the following clinical variables: smoking history, BMI, systolic and diastolic BP, heart rate, medications (amiodarone, cardiac glycosides, calcium channel blocker and oral beta blocker), ratio of total to HDL cholesterol, ECG LVH, hypertension treatment, prevalent heart failure (for AF and mortality analyses), coronary heart disease, and diabetes. The 10-year incident rates for the outcomes of heart failure, AF, and mortality were determined. Multivariable Cox proportional hazards regression models for each outcome examined PR interval per standard deviation increase and dichotomized as ≤ or >200 ms.
We assessed for effect modification between PR interval and age, sex, and race in examining the association between PR and the outcomes of heart failure, AF and mortality. We did not identify significant effect modification in the initial (age-, sex-, race- and site-adjusted) or the complete multivariable adjusted Cox proportional hazards regression models. Survival curves based on stratified Cox regression models were constructed to examine differences between categorical PR and the associations with the outcomes of heart failure, AF and mortality. We verified that the proportionality of hazards assumption was not violated.
Secondary analyses were conducted adjusting for interim heart failure prior to AF and for interim AF prior to heart failure, and using medications as time-varying covariates during follow-up. The relations of PR interval to the outcomes of AF and heart failure were examined further by constructing restricted cubic splines using the cohort mean PR as the reference and incorporating knots at 5, 27.5, 50, 72.5 and 95 quantiles as recommended by Harrell.13 All statistical analyses employed SAS version 9.2 (SAS Institute, Cary, NC). A 2-sided P<0.05 was considered statistically significant.
Results
Following exclusions, 2722 Health ABC participants comprised the present analysis. The cohort’s median PR interval was 168 (IQR 152, 188) ms and mean age was 74±3 years at baseline; 52.4% were women and 41.0% were black (Table 1). Race was not significantly correlated with PR interval in age-, sex- and site-adjusted analyses (P=0.28). The chief significant clinical correlates of PR interval were age, sex, heart rate, body mass index, and hypertension treatment.
Table 1.
Baseline characteristics and the clinical correlates of PR interval in the Health, Aging and Body Composition cohort (N=2722)
| Clinical Characteristics | Distribution | Age, sex, site, race adjusted | Multivariable adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PR (continuous) | PR > 200 | PR (continuous) | PR > 200 | ||||||
| Change in PR ±SD | P | OR (95% CI) | P | Change in PR ±SD | P | OR (95% CI) | P | ||
| Age | 74 ± 3 | 1.7 ± 0.6 | 0.003 | 1.19 (1.06, 1.33) | 0.003 | 1.7 ± 0.5 | 0.002 | 1.19 (1.06, 1.34) | 0.004 |
| Female sex | 1427 (52.4) | −12.5 ± 1.1 | <.0001 | 0.36 (0.28, 0.46) | <.0001 | −12.4 ± 1.2 | <.0001 | 0.35 (0.26, 0.46) | <.0001 |
| Memphis site | 1372 (50.4) | −1.8 ± 1.1 | 0.010 | 0.92 (0.73, 1.15) | 0.48 | −0.4 ± 1.1 | 0.75 | 1.03 (0.80, 1.32) | 0.82 |
| Black race | 1115 (41.0%) | 1.4 ± 1.1 | 0.22 | 1.14 (0.90, 1.44) | 0.28 | −0.2 ± 1.2 | 0.84 | 1.03 (0.80, 1.34) | 0.82 |
| Smoking (current and former) | 1515 (55.7) | −3.1 ± 1.2 | 0.007 | 0.88 (0.69, 1.13) | 0.33 | −2.7 ± 1.1 | 0.016 | 0.91 (0.71, 1.16) | 0.44 |
| Body mass index | 27.4 ± 4.8 | 3.6 ± 0.6 | <.0001 | 1.26 (1.12, 1.42) | 0.0002 | 3.1 ± 0.6 | <.0001 | 1.22 (1.07, 1.39) | 0.003 |
| Systolic BP, mm Hg | 136 ± 21 | 0.9 ± 0.6 | 0.11 | 1.13 (1.01, 1.27) | 0.031 | −0.2 ± 0.7 | 0.82 | 1.05 (0.91, 1.21) | 0.52 |
| Diastolic BP, mm Hg | 71 ± 12 | −0.3 ± 0.6 | 0.61 | 1.02 (0.91, 1.16) | 0.71 | 0.2 ± 0.7 | 0.78 | 1.02 (0.88, 1.18) | 0.82 |
| Heart failure | 64 (2.4) | 6.8 ± 3.6 | 0.06 | 1.57 (0.83, 2.96) | 0.16 | 3.6 ± 3.6 | 0.32 | 1.31 (0.67, 2.55) | 0.43 |
| Coronary heart disease | 565 (20.8) | 5.2 ± 1.4 | 0.0002 | 1.28 (0.98, 1.66) | 0.07 | 1.7 ± 1.5 | 0.24 | 0.96 (0.71, 1.29) | 0.77 |
| Stroke | 214 (7.9) | 0.8 ± 2.1 | 0.70 | 1.09 (0.72, 1.66) | 0.68 | −0.9 ± 2.0 | 0.67 | 0.97 (0.63, 1.50) | 0.90 |
| Diabetes | 400 (14.7) | 2.4 ± 1.6 | 0.13 | 1.22 (0.90, 1.67) | 0.20 | 1.7 ± 1.6 | 0.27 | 1.17 (0.85, 1.63) | 0.34 |
| Total/HDL Cholesterol ratio | 4.1 ± 1.3 | 1.7 ± 0. 6 | 0.003 | 1.15 (1.03, 1.28) | 0.016 | 0.7 ± 0.6 | 0.19 | 1.09 (0.97, 1.22) | 0.17 |
| Hypertension treatment | 1459 (53.6) | 7. 3 ± 1.1 | <.0001 | 1.70 (1.34, 2.16) | <.0001 | 2.1 ± 1.4 | 0.14 | 1.12 (0.80, 1.56) | 0.52 |
| Selected medications | 1126 (41.3%) | 8.6 ± 1.1 | <.0001 | 1.91 (1.51, 2.42) | <.0001 | 4.8 ± 1.5 | 0.001 | 1.56 (1.12, 2.18) | 0.009 |
| Heart rate, beats per minute | 65 ± 11 | −4.9 ± 0.6 | <.0001 | 0.73 (0.64, 0.83) | <.0001 | −4.9 ± 0.6 | <.0001 | 0.73 (0.64, 0.83) | <.0001 |
| ECG left ventricular hypertrophy | 237 (8.7) | 5.2 ± 2.0 | 0.009 | 1.58 (1.09, 2.28) | 0.015 | 4.6 ± 1.9 | 0.016 | 1.47 (1.01, 2.16) | 0.047 |
| PR interval, ms | 168 (152, 188) | ||||||||
Distributions are means (SD) or n (%) for anthropometric, clinical, and laboratory variables. Age, sex, site and race adjusted models: each of age, sex, site and race was adjusted for the other three variables. Comprehensive multivariable adjustment included adjustment for age, sex, site and race and the remainder of the variables listed here. Selected medications are amiodarone, cardiac glycosides, calcium channel blockers, beta blockers. Continuous variables expressed per standard deviation and categorical for the presence of variable. Estimates show the change in PR interval per standard deviation change for continuous variables or for the presence of that variable for categorical variables. PR expressed as median and interquartile range. BP, blood pressure.
Over the course of the 10-year follow-up, 369 participants were diagnosed with heart failure, 537 with incident AF and 832 died. Table 2 presents the incidence rate per 1000 person-years for the outcomes. There was no significant interaction between race and PR interval with the outcome of heart failure (P=0.26), AF (P=0.89), or all-cause mortality (P=0.66). Incidence rates by race are in Supplementary Table 1.
Table 2.
Ten year incidence rate per 1000 Person-Years follow up
| Outcome | Events | Total sample (N=2722) | PR≤200 ms (N=2383) | PR>200 ms (N=339) |
|---|---|---|---|---|
| Event Rate per 1000 Person-Years (95% CI) | Event Rate per 1000 Person-Years (95% CI) | Event Rate per 1000 Person-Years (95% CI) | ||
| Heart Failure | 369 | 16.7 (15.0, 18.4) | 15.4 (13.7, 17.1) | 26.9 (20.6, 33.3) |
| Atrial fibrillation | 537 | 26.3 (24.1, 28.5) | 25.0 (22.7, 27.3) | 36.6 (28.8, 44.4) |
| Mortality | 832 | 35.6 (33.2, 38.0) | 34.5 (32.0, 37.0) | 43.5 (35.8, 51.2) |
The events column indicates the number of participants with the designated outcome out of the total eligible participants. CI, confidence interval. Heart failure (white, n=201; black, n=168); atrial fibrillation (white, n=359; black, n=178); mortality, (white, n=435; black, n=397).
In multivariable-adjusted analyses (Table 3), every SD increase (29 ms) of the baseline PR interval was associated with a 13% increased 10-year risk of heart failure (hazard ratio [HR], 1.13; 95% CI, 1.02 to 1.25), and a 13% increased 10-year risk of incident AF (HR, 1.13; 95% CI, 1.04 to 1.23). PR interval was not associated with all-cause mortality. Results for AF by race are particularly of interest given prior findings that risk of AF varies by race. The HR per SD of PR interval adjusted for age, sex, and site was 1.14 (95% CI, 1.03 to 1.26, P=0.01) for whites and 1.17 (95% CI, 1.01 to 1.34, P=0.04) for blacks. Racially stratified results are summarized in Supplementary Table 2.
Table 3.
Hazard ratio per 1-SD* increase in PR for selected outcomes in Health ABC.
| Adjusted for age, sex, site, race | Multivariable Adjusted* | |||
|---|---|---|---|---|
| End Point | HR (95% CI) | P | HR (95% CI) | P |
| HR per 1-SD increase in PR | ||||
| Incident heart | 1.16 (1.05, 1.28) | 0.003 | 1.13 (1.02, 1.25) | 0.019 |
| failure | ||||
| Incident AF | 1.15 (1.06, 1.25) | 0.0010 | 1.13 (1.04, 1.23) | 0.005 |
| All-cause mortality | 0.99 (0.93, 1.07) | 0.95 | 1.03 (0.96, 1.11) | 0.37 |
| HR comparing PR >200ms versus ≤ 200ms (reference) | ||||
| Incident heart | 1.60 (1.22, 2.09) | 0.0006 | 1.46 (1.11, 1.93) | 0.006 |
| failure | ||||
| Incident AF | 1.33 (1.05, 1.68) | 0.019 | 1.26 (0.99, 1.61) | 0.059 |
| All-cause mortality | 1.08 (0.89, 1.31) | 0.44 | 1.14 (0.94, 1.39) | 0.19 |
Health ABC indicates Dynamics of Health, Aging and Body Composition Study; AF, atrial fibrillation.
1-SD PR increase corresponds to 29 ms.
Multivariable models adjusted for age, sex, site, body mass index, heart rate, systolic and diastolic blood pressures, past/current smoking, ratio of total to high density lipoprotein, electrocardiographic left ventricular hypertrophy, hypertension treatment, selected medications (amiodarone, cardiac glycosides, calcium channel blockers, beta blockers) and prevalent cardiovascular disease.
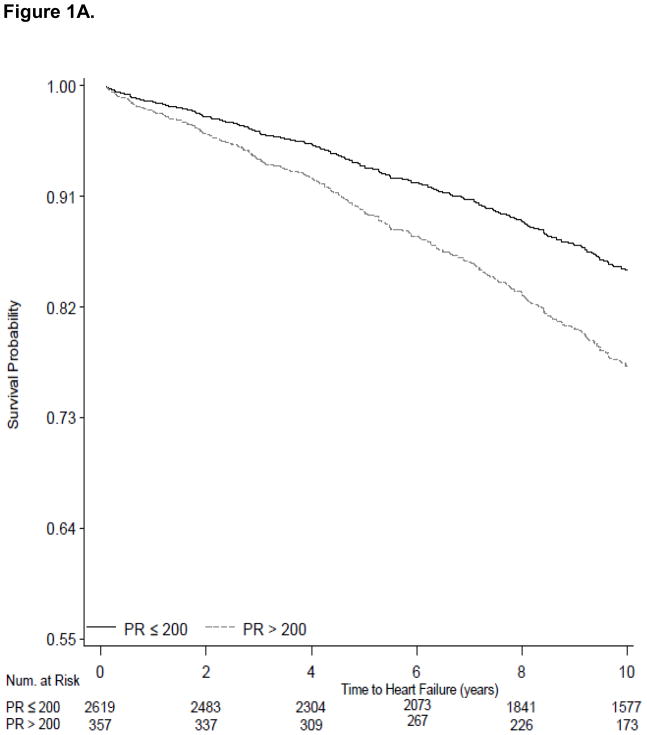
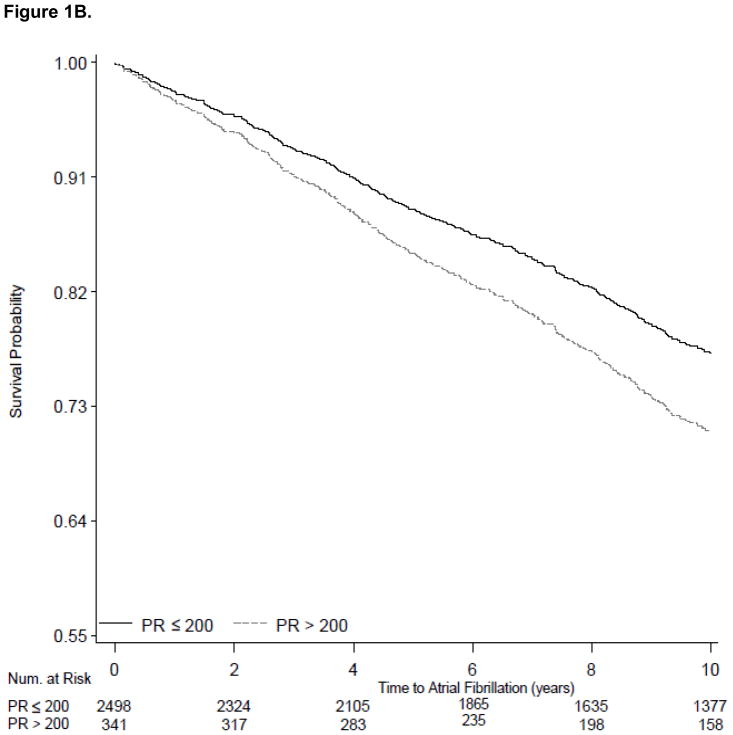
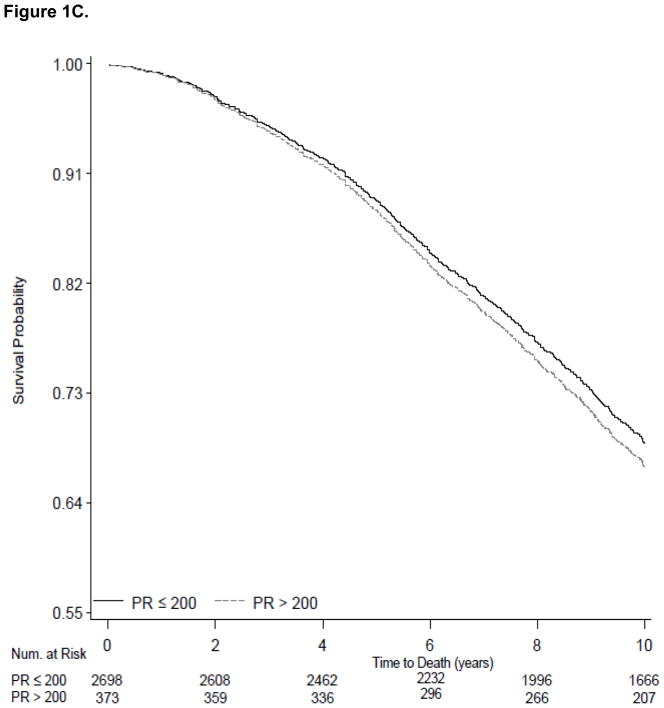
The 339 Health ABC participants in our sample with PR>200 ms had 46% increased risk of incident heart failure (95% CI, 1.11 to 1.93; Table 3). In multivariable models PR>200 ms was not significantly associated with 10-year risk of AF (HR 1.26; 95% CI, 0.99 to 1.61) and was not associated with increased all-cause risk of death (HR 1.16; 95% CI, 0.96 to 1.39. We present survival curves for the outcomes of heart failure, AF and all-cause mortality adjusted for age, sex, race and site in Figure 1A, 1B, and 1C using PR stratified as ≤ 200 ms versus >200 ms.
Figure 1.
Survival curves showing the electrocardiographic PR interval stratified at ≤200 ms and >200 ms and incident outcomes examined during 10-year follow-up. Figure 1A shows variation in heart failure incidence according to PR categories. Figure 1B shows variation in atrial fibrillation incidence according to PR categories. Figure 1C shows the absence of variation in all-cause mortality between the PR strata. All survival curves were adjusted for age, sex, race and site.
We developed restricted cubic splines adjusting for sex, age, race, site and clinical correlates and employed the mean PR interval, 170 ms, as the reference value because of its proximity to the median value in our cohort. Supplementary Figure 1 shows the splines for heart failure, and Supplementary Figure 2 shows the splines for AF. The restricted cubic splines employing the median PR value as the referent to determine hazard ratios. The splines (hazard ratio shown in red solid line and dashed line indicating the 95% confidence interval) demonstrate a progressive increase in hazard ratios with the rise in PR interval beyond the reference value of 170 ms (Wald Chi-square overall association 5.81, P=0.01 for heart failure and 5.81, P=0.005 for AF). We further note the limited rise in the hazard ratio for both heart failure and AF identified when considering PR values less than the referent. The wide confidence intervals, however, indicate the association is not significant. We report the hazard ratio estimates derived from the splines for heart failure and AF using a PR of 170 ms as the referent value in Supplementary Table 3.
Secondary analyses examined PR as a continuous measure employing multivariable models and adjusting for interim events. After adjusting for interim AF, an incremental 1-SD increase in PR interval remained associated with a 13% increased risk of heart failure during 10-year follow-up (95% CI, 1.02 to 1.25, P=0.006). Similarly, a 1-SD increase in PR interval remained associated with AF after adjustment for interim heart failure (HR 1.13; 95% CI, 1.04 to 1.23, P=0.02).
Discussion
We examined the associations of the ECG PR interval with the 10-year risks of incident heart failure, AF and mortality in a biracial cohort of older adults. We identified a significant association between baseline PR interval and incident heart failure and AF. Chiefly, the analysis identified a 46% increased heart failure risk during the 10-year follow-up.
Our findings extend prior investigations of PR interval beyond younger or more racially homogenous cohorts. In the Framingham Heart Study, investigators identified an association between PR interval and AF highly consistent with our findings.5,14 In the ARIC Study, Soliman et al. found a 41% increase in AF risk with each SD increase in PR interval.4 However, only 10 black ARIC Study participants developed AF during the approximately 7-year mean follow-up period, suggesting a lower risk of AF in blacks. The ARIC participants’ mean age (54 years) was younger, limiting comparison of the findings presented here. Our results validated these prior studies concerning the importance of PR prolongation as a marker of cardiovascular risk, and extended their findings by identifying that PR was associated with heart failure and AF in a markedly older, biracial cohort.
We showed that baseline PR interval in this older cohort had no association with 10-year risk of all-cause mortality in contrast to prior studies. Our study participants were older, and hence likely had a higher burden of comorbidities with greater impact on prognosis than PR.
Several mechanisms may contribute towards the association of PR interval prolongation and incident heart failure. PR interval shares clinical correlates with established heart failure risk factors. Prolongation of the PR has been associated with obesity, increased waist circumference, and components of the metabolic syndrome,15 exposures that are associated with incident heart failure.16–19 Metabolic syndrome has been previously associated with heart failure in Health ABC.20 Hypertension is similarly a chief etiology for heart failure with preserved and compromised systolic function21–23 and may promote elevated intracardiac pressures that alter atrial electrical function.
PR interval progression is a marker of atrial electrical and structural remodeling. Invasive electrophysiologic studies have demonstrated severely altered atrial electrical properties in the setting of left ventricular systolic dysfunction.24 Electrophysiologic studies have quantified increased atrial refractoriness and conduction times accompanying aging.25,26 PR interval prolongation may consequently be consistent with generalized atrial aging or subclinical disease. Unaccounted for residual confounding may contribute towards the findings described here as well. Further study is needed to examine how modulation of risk factors may impact atrial and AV nodal conduction.
Potential mechanisms for the pathophysiologic association between PR prolongation and AF mirror those proposed for heart failure. PR interval has been associated with AF in multiple longitudinal cohort studies, as has its constituent component, P wave duration.14,27,28 There has been discussion regarding the decreased prevalence of AF in blacks, despite both clinical risk factors and prolonged measures of atrial electrical function, compared to whites.9,29 In this study of older white and black adults, we found no interaction by race between PR and the outcomes of heart failure and AF. Our findings suggest that racial differences in atrial electrical function may be less relevant in older adults. PR interval reflects intra- and interatrial conduction (the P wave) through the atrioventricular node, and age-related changes in atrial conduction may not be related to race. Further studies are essential to evaluate racial differences in atrial electrical function and their clinical relevance in relation to AF.
Our analysis has multiple strengths. Health ABC’s standardized protocol and examinations provide for comprehensive assessment of the covariates employed here. Second, our cohort has a unique composition of community-dwelling whites and black older adults with preserved high-level baseline functional status. Our findings are relevant to the increasing number of independent older adults who are aging successfully with limited impairment. Third, electrocardiographic analyses were conducted by standardized reading and coding, thereby enhancing reliability and limiting misclassification. Fourth, heart failure and mortality are adjudicated outcomes in Health ABC.
Our study has multiple limitations. Health ABC enrolled highly functional, older adults of white and black race in the Memphis, TN, and Pittsburgh, PA regions. Generalization to other races, ethnicities, and regions is uncertain and generalization to less healthy older adults may also be limited. Second, the study was not designed to examine AF risk factors in older adults. Hence, AF was not an adjudicated outcome in Health ABC. The reliance on CMS coding for AF determination is another limitation as it may result in misclassification of AF. Third, heart failure events were determined by review of hospitalizations. As recognized in an analysis of heart failure in Health ABC, this may underestimate heart failure cases and bias towards identification of more severe heart failure.12 We were not able to distinguish between heart failure with preserved or compromised ejection fractions, which may vary in their association with PR interval. Furthermore, Health ABC did not include baseline echocardiography. Inclusion of additional data not available here, such as echocardiographic diastolic indices, systolic function, chamber sizes and left ventricular mass, may potentially eliminate the predictive value of the PR interval. Another limitation consisted in our using a single determination of PR interval at the initial Health ABC examination. Residual confounding from PR interval variability is not accounted for in our analysis. However, we would expect misclassification of PR interval would be non-differential and bias our results towards the null. Finally, we cannot exclude residual confounding from other clinical variables that were not included in our multivariable adjustment.
In conclusion, we examined the association of PR interval with adverse outcomes. We found an association between PR interval and heart failure risk. We extended prior analyses of the relation of PR interval to AF by examining AF risk in an older, biracial cohort. Contrary to our expectations based on prior literature, we found no interaction between race and PR interval in examining the association of PR interval with the outcomes we studied. The ECG is widely used in clinical practice, is low-cost, and includes automated determination of PR interval. Our analyses adjusted for anthropometric and clinical risk factors relevant to cardiovascular disease and related to incident heart failure and AF.12,30,31 Inclusion of such diverse covariates is meaningful: clinicians evaluate PR interval in the context of widely established risk factors and clinical history without considering PR interval in isolation. As the general population continues to age, further strategies for heart failure and AF risk stratification are essential for evaluating and treating those at increased risk. Our investigation suggests that PR interval may contribute towards identifying older adults at increased risk for the adverse outcomes examined here. Further investigation is necessary to incorporate PR interval into risk classification schemes for prevention strategies and to determine if such treatment strategies will reduce risks of heart failure or AF.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Funding: This research was funded by American Heart Association Award 09FTF2190028 (JWM); by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, NINR grant R01-NR012459, 1RC1HL101056; 1R01HL102214; 1R01AG028321; and by NIH grants 1RO1HL092577(EJB and PTE), 5R21DA027021, 1RO1HL104156, 1K24HL105780 (PTE) and K23DK089118 (RD).
Footnotes
Disclosures: None.
Reference List
- 1.Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Kors JA, Rijnbeek PR, van Herpen G, Lu Z, Xu C. Normal limits of the electrocardiogram in Chinese subjects. Int J Cardiol. 2003;87:37–51. doi: 10.1016/s0167-5273(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 3.Bexton RS, Camm AJ. First degree atrioventricular block. Eur Heart J. 1984;5 (Suppl A):107–109. doi: 10.1093/eurheartj/5.suppl_a.107. [DOI] [PubMed] [Google Scholar]
- 4.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnani JW, Gorodeski EZ, Johnson VM, Sullivan LM, Hamburg NM, Benjamin EJ, Ellinor PT. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011;8:93–100. doi: 10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansi IA, Nash IS. Ethnic differences in electrocardiographic intervals and axes. J Electrocardiol. 2001;34:303–307. doi: 10.1054/jelc.2001.27453. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman EZ, Goff DC., Jr Paradox of racial distribution of atrial fibrillation. J Natl Med Assoc. 2008;100:447–448. doi: 10.1016/s0027-9684(15)31282-7. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity--the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58:715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 11.Chaitman BR, Zhou SH, Tamesis B, Rosen A, Terry AB, Zumbehl KM, Stocke K, Takase B, Gussak I, Rautaharju PM. Methodology of serial ECG classification using an adaptation of the NOVACODE for Q wave myocardial infarction in the Bypass Angioplasty Revascularization Investigation (BARI) J Electrocardiol. 1996;29:265–277. doi: 10.1016/s0022-0736(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell FE., Jr . Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 14.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P Wave Indices, Obesity, and the Metabolic Syndrome: The Atherosclerosis Risk in Communities Study. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 17.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 19.Ingelsson E, Arnlov J, Lind L, Sundstrom J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92:1409–1413. doi: 10.1136/hrt.2006.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de RN, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 21.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 23.Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789–1796. [PubMed] [Google Scholar]
- 24.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 25.Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol. 2006;17:120–127. doi: 10.1111/j.1540-8167.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ. P Wave Duration and Risk of Longitudinal Atrial Fibrillation Risk in Persons >/=60 Years Old (from the Framingham Heart Study) Am J Cardiol. 2011;107:917–921. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–556. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 30.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 31.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.