Abstract
The oxidative stress theory has been associated with atherosclerosis and has prompted a multitude of studies to evaluate the effects of antioxidants on cardiovascular disease prevention. Resveratrol, a relatively new antioxidant has gained considerable curiosity. This polyphenol stilbene identified in grape skin, is believed to be the main component contributing to the anti-atherosclerotic benefits linked to red wine consumption. It has demonstrated the ability to protect endothelial cells from lipid damage, promote vasodilation via modulation of nitric oxide synthesis, and inhibit platelet aggregation and smooth muscle proliferation. Although the complete mechanism of Resveratrol has yet to be fully elucidated, the Sirtuin system, consisting of 7 highly conserved families of regulator genes, are thought to be instrumental in establishing the various health benefits.
In this article we assess the current applications, mechanism, pharmacokinetics, bioavailability, and safety profile of the novel antioxidant Resveratrol and provide an in-depth review of the influence of the Sirtuin system on the Resveratrol mechanism of action. We resolve that while early data on Resveratrol are promising, the anti-oxidative and ultimately, anti-atherosclerotic potential depends on further clarification of the intricate and complex relationship between Resveratrol and the Sirtruin system.
Keywords: Resveratrol, Reactive oxygen species, Atherosclerosis, Cardiovascular disease
Introduction
Maintaining a healthy diet and lifestyle remains the mainstay of cardiovascular disease risk reduction. The “French Paradox” reflects the reduced incidence of Cardiovascular Disease (CVD) found in some French populations with moderate wine consumption despite a high-fat intake, low exercise, and increased tobacco use [1]. These and other such studies were monumental in developing the current concepts of the widely accepted “Mediterranean Diet”. A staple of the diet, red wine contains various cardio protective antioxidants and moderate intake of wine has been linked to reduced cardiovascular eve [2,3].
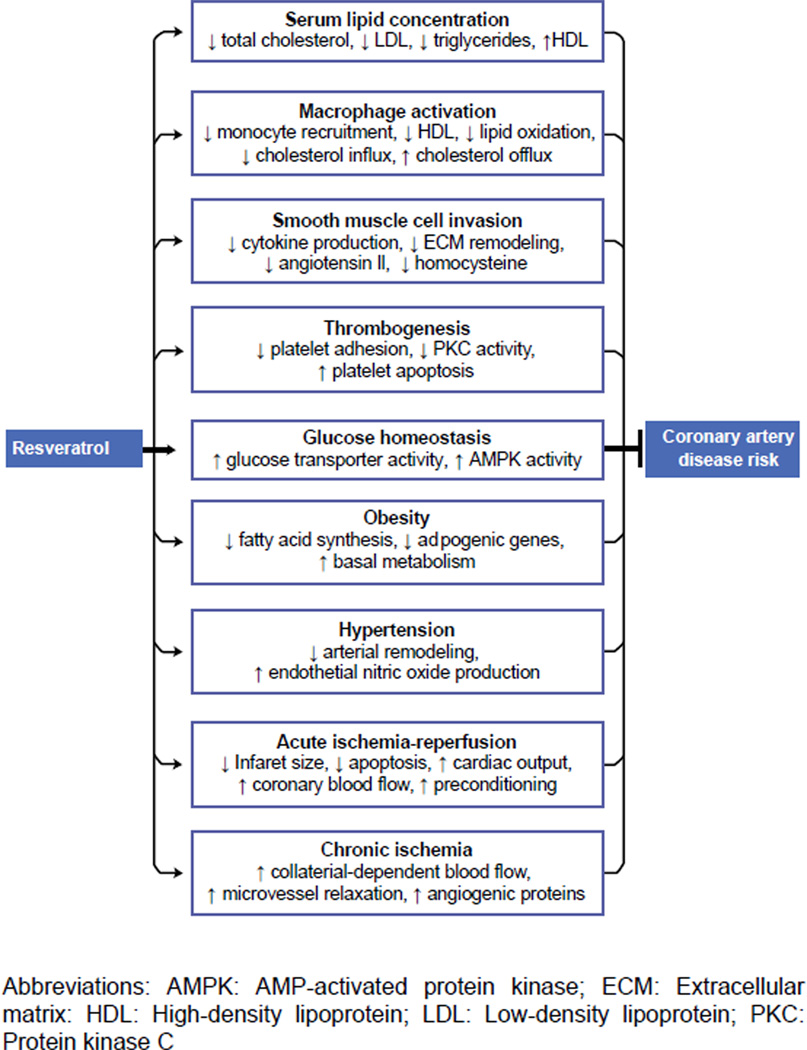
Progressive research on organic antioxidants found in natural products, such as red wine, have held a central focus in current antioxidant research models. The main ingredient of red wine, the grape, is a well known source of protective phenols and polyphenols that have been associated with CVD risk reduction and prevention against lipoprotein peroxidation. Moreover, Resveratrol is a polyphenol compound, has been attributed to various cardiovascular benefits secondary to its antioxidant properties [4–6] (Figure 1). While there appears to be an observational consensus about the cardiovascular benefit of Resveratrol, human clinical trials are still ongoing and premature. Furthermore, pre-clinical human studies have found it difficult to replicate the bioavailability and efficacy of Resveratrol found in early animal models (Table 1).
Figure 1.
The many effects of resveratrol on coronary artery disease risk. Resveratrol has been shown to modify a large number of factors that contribute to coronary artery disease. This diagram summarizes the proposed mechanisms by which resveratrol influences these factors and reduces the risk of CAD [51].
Table 1.
Pre-Clinical Studies of Resveratrol in Heart Disease.
| Study | Investigation Type/Model | Animal Class | Dose | Result |
|---|---|---|---|---|
|
Ischemic Heart Disease +/− Reperfusion Studies | ||||
| Sato M et al. [38] | Ischemia/reperfusion | Rat | 10 µM | Decreased infarct size |
| Hung LM et al. [39] | Ischemia/reperfusion by temporary occlusion of Left Main coronary artery | Rat | 0.23,2.3,23 µg/kg | Decreased incidence of ventricular fibrillation and tachycardia, decreased LDH, and increased vascular NO |
| Imamura G et al. [40] | Ischemia/reperfusion | Mouse | 10 µM | Decreased infarct size and apoptosis |
| Bradamante S et al. [41] | Ischemia/reperfusion 24 hrs. after treatment | Rat | 1 mg/kg | Functional recovery improvement and increased coronary flow |
| Das S et al. [42] | Ischemia/reperfusion | Rat | 2.5 mg/kg | Functional recovery improvement, decrease in infarct size and apoptosis, induction of VEGF, eNOS, iNOS |
| Dernek S et al. [43] | Ischemia/reperfusion | Rat | 20 µM | Post ischemic recovery improved with chronic treatment, only partial protection with acute dosing |
| Kaga S et al. [44] | LAD occlusion | Rat | 1 mg/kg | Improved ventricular function, decreased infarct size, increased VEGF expression |
|
Coronary Risk Factor Studies | ||||
| Wang Z et al. [45] | Hyperlipidemia | Rabbit | 3 mg/kg | Decreased thoracic intima layer thickness and plaques, block of dilation by flow mediation |
| Auger C et al. [46] | Hyperlipidemia | Hamster | 0.1428 mg/kg | Decreased Aortic fatty streak accumulation |
| Fukaw H et al. [47] | Endothelial injury | Mouse | 9.6, 96 mg/kg | Decrease in thrombus size and atheroma |
| Zou JG et al. [48] | Endothelial function | Rabbit | 3 mg/kg | Increase in endothelin, decrease of NO blocked |
| Wang Z et al. [49] | Platelet aggregation | Rabbit | 4 mg/kg | Reduced platelet aggregation |
| Arichi H et al. [50] | Triglyceride synthesis | Mouse | 25, 50 mg/kg | Reduced hepatic and adipose lipogenesis |
Resveratrol
The polyphenol stilbene molecule Resveratrol has recently gained much publicity and interest as a novel antioxidant molecule, potentially holding the key towards unlocking the cardio protective factors of red wine. Also known as (3,4’,5-trihydroxystilbene), this ancient stilbene has also long been utilized as a component of (Veratrum formosanum) in traditional East Asian medicine [7] and in pre-Colombian medicine in South America (Cassia quinquangulata) [8]. Analysis into recent studies have revealed Resveratrol as a known activator of the protein deacetylase sirtuin 1 (SIRT1) gene, which is thought to mediate anti-proliferative and anti-inflammatory activity due to alteration of gene expression and modulation of numerous metabolic pathways [9–11].
Applications
Resveratrol has demonstrated a wealth of promising benefits, such as anti-aging [12], anti-inflammatory [13], and anti-diabetic [14] effects; and further as a potent anti-viral [15] and anti-neoplastic [16] agent. Additionally, Resveratrol offers great potential as a cardiovascular disease modulating agent, as demonstrated by the abundance of preclinical trials related to heart disease (Table 1).
As a novel agent isolated in the skin of grapes, Resveratrol has demonstrated pronounced cardioprotective potential as it has been shown to enhance coronary relaxation, reduce ventricular arrhythmias, inhibit platelet aggregation and smooth muscle proliferation, improve glucose metabolism, reduce Low-density lipoprotein (LDL) while elevating High-density lipoprotein (HDL) [17]. As such, these cardiovascular effects promote vascular wall integrity and inhibit endothelial dysfunction, thus disrupting the atherosclerotic process (Figure 1).
Mechanism
Despite possessing such promising potential in treating an assortment of human diseases, the exact mechanism by which Resveratrol demonstrates these effects has yet to be fully elucidated as human clinical trials are lacking. Further, despite what seems to be broad and multifactorial applications, Resveratrol is quite a poor direct antioxidant. It was suggested that Resveratrol is less potent then other antioxidants, such as vitamin-C and cysteine [18].
This inadequacy to delineate one encompassing mechanism is likely due to the fact Resveratrol acts as a secondary regulator of a larger more complex biological response system. Recent studies have focused on the Sirtuin system, a complicated regulatory biological response process known to be activated by Resveratrol. This highly conserved family of genes termed silent information regulator genes (SIR) are comprised of seven Sirtuin genes in mammals, SIRT 1 through SIRT 7, and are widely expressed in a variety of tissues. These genes encode for specific (SIRT) NAD+ dependent de-acetylating enzymes and are considered regulatory proteins. Specifically, the subcellular locations of these Sirtuins include the nucleus (SIRT 1,-2,-6,-7), cytoplasm (SIRT-1,-2) and mitochondria (SIRT 3,-4,-5) [19].
SIRT 1 is one of the more extensively studied Sirtuins within the cardiovascular system. In addition to being modulated by Resveratrol, SIRT 1 is highly expressed in the vasculature. It appears to modulate angiogenic activity in periods of vascular remodeling and vascular growth [20,21]. In fact, SIRT-1 has been shown to target endothelial nitric oxide synthase (eNOS) for de-acetylation, thus activating and enhancing endothelial nitric oxide production [22].
The relationship between Resveratrol and SIRT 1 is further apparent via studies which have shown anti-hypertensive effects via modulation of the angiotensin II type I receptor. In vascular smooth muscle, in vivo studies reveal Resveratrol’s ability to propagate SIRT1 expression resulted in decreased angiotensin II-induced hypertension [23]. Additionally, SIRT1 has been reported be a regulator of tissue metalloproteinase 3 (TIMP3), an endogenous enzyme which defenses vascular inflammation and is involved in the prevention of atherosclerosis [24].
Furthermore, nuclear SIRT-1 enhanced by Resveratrol was found to induce superoxide dismutase, an antioxidant enzyme which suppressed cell death in cardiomyocytes [25]. These and other studies support the protective effects of Sirtuins on cardiomyocytes via activation of antioxidant encoding genes, thus helping to mitigate the cellular burden of reactive oxygen species [26].
Beyond SIRT-1, SIRT-3 and SIRT-7 also appear to be vital for optimal cardiovascular function. In SIRT-3 deficient mouse models, signs of severe cardiac hypertrophy and interstitial fibrosis were present, when a hypertrophic stimulus was given [27]. Conversely, transgenic mouse models engineered for over-expression of SIRT-3 were significantly less amenable to stimuli-induced hypertrophy [26]. In similar fashion, SIRT-7 deficient mice had myocardium defined by extensive fibrosis and high basal rates of apoptosis, resulting in high levels of cardiomyopathy [28].
Bioavailability
The alcoholic red wine matrix seems to be vital for activity, as primary supplementation with isolated Resveratrol results in low bioavailability [29]. Only when Resveratrol is ingested in the red wine matrix does it posses the bioavailability to achieve the efficacious levels to provide improvement of endothelial dysfunction [30]. Furthermore, studies of Resveratrol in other matrices such as white wine, grape juice, and vegetable juice/homogenate have not yielded promising results [31]. These studies have further underlined the importance of the ripe chemical environment specifically provided by red wine [32].
Metabolism and pharmacokinetics
Studies have postulated the role of detoxifying hepatic enzymes such as, cytochrome p450, as possessing particular importance in the catabolism of Resveratrol [29]. Furthermore, it is quickly metabolized as the active primary Resveratrol molecule possesses a relatively short half life of ~8–14min [33]. Additionally, Quercetine and Ethanol are compounds identified in red wine, which are perhaps necessary to amend the activity of catabolic enzymes, thus augmenting Resveratrol activity [34].
Safety/Adverse effects
Resveratrol has not been identified as toxic or causing adverse effects. However, human clinical trials are lacking at this point. In animal models, up to 300 mg/kg were administered over an extended period without note of adverse effects [35]. Additionally, through in vitro studies Resveratrol has been known to inhibit platelet aggregation [36]. As such, potential for bleeding may occur with concurrent administration of anticoagulants Heparin or Warfarin, and anti-platelet agents Aspirin or Plavix.
Resveratrol is metabolized by cytochrome P450, and has also been shown to inhibit the activity of the cytochrome [37]. Although not identified in humans, drugs taken concurrently with Resveratrol, which are also metabolized via the hepatic cP450 enzyme, may have increased bioavailability and the potential for subsequent toxicity exists. These drugs include but are not limited to: HMG-CoA reductase inhibitors, calcium channel blockers, and certain anti-arrhythmics, such as Amiodarone.
Conclusions
Resveratrol, a novel molecule found in red wine, and hypothesized to be the key ingredient and possible answer to the “French Paradox”, offers great potential benefit as an agent which may amend a multitude of human disorders, chiefly among these, Cardiovascular Disease.
Resveratrol modulates these favorable effects via interaction with Sirtuin molecules, a highly complex regulatory response system which appears sensitive to various metabolic influences. Human clinical research elucidating the relationship between Resveratrol and the Sirtuin system remains vital and necessary. Further investigation is currently ongoing on what appears to be a complex, yet broadly applicable regulatory process.
Acknowledgement
The National Institutes of Health supported this work under grant number KHL097158A, issued under responsible party and listed co-author Dr. ShaistaMalik.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 2.German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- 3.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, et al. Alcohol dosing and total mortality in men and women: An updated Meta-Analysis of 34 Prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 4.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Maulik N, Das DK. Cardioprotection with alcohol: role of both alcohol and polyphenolic antioxidants. Ann N Y Acad Sci. 2002;957:122–135. doi: 10.1111/j.1749-6632.2002.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 6.Berrougui H, Grenier G, Loued S, Drouin G, Khalil A. A new insight into resveratrol as an atheroprotective compound: Inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis. 2009;207:420–427. doi: 10.1016/j.atherosclerosis.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 8.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 9.Vanamala J, Reddivari L, Tarver CC, Murano PS, Lupton JR, et al. Resveratrol phenocopies the suppressive effects of insulin-like growth factor receptor-1 siRNA on IGF-1 promoted colon cancer cell proliferation. FASEB J. 2008;22:158. [Google Scholar]
- 10.Putics A, Vegh EM, Csermely P, Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid Redox Signal. 2008;10:65–75. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- 11.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 13.Khanduja KL, Bhardwaj A, Kaushik G. Resveratrol inhibits N-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J Nutr Sci Vitaminol (Tokyo) 2004;50:61–65. [PubMed] [Google Scholar]
- 14.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docherty JJ, Fu MM, Hah JM, Sweet TJ, Faith SA, et al. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 2005;67:155–162. doi: 10.1016/j.antiviral.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Jan M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 17.Carluccio MA, Ancora MA, Massaro M, Carluccio M, Scoditti E, et al. Homocysteine induces VCAM-1gene expression through NF-kappaB and NAD(P)H oxidase activation: protective role of Mediterranean diet polyphenolic antioxidants. Am J Physiol Heart Circ Physiol. 2007;293:H2344–H2354. doi: 10.1152/ajpheart.00432.2007. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli AAE. Modulatory effect of resveratrol, a natural phytoalexin, in endothelial adhesion molecules and intracellular signal transduction. Pharm Biol. 1998;36:44–52. [Google Scholar]
- 19.Sauve AA. Sirtuin chemical mechanisms. Biochim Biophys Acta. 2010;1804:1591–1603. doi: 10.1016/j.bbapap.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balestrieri ML, Rienzo M, Felice F, Rossiello R, Grimaldi V, et al. High glucose downregulates endothelial progenitor cell number via SIRT1. Biochim Biophys Acta. 2008;1784:936–945. doi: 10.1016/j.bbapap.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 24.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn BH, Kim HS, Song S, Lee IH, Liu J, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 29.Walle T, Hsieh F, Delegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 30.Bertelli AAE, Giovannini L, Stradi R, Bertelli A, Tillement JP. Plasma, urine and tissue levels of trans- and cis-reveratrol (3,4�,5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. Int J Tissue React. 1996;18:67–71. [PubMed] [Google Scholar]
- 31.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 32.Bertelli AAE. Resveratrol in Health and Disease. Boca Raton, FL: CRC Press; 2006. Pharmacokinetics and metabolism of resveratrol; pp. 631–647. [Google Scholar]
- 33.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 34.de Santi C, Pietrabissa A, Mosca F, Pacifici GM. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica. 2000;30:1047–1054. doi: 10.1080/00498250010002487. [DOI] [PubMed] [Google Scholar]
- 35.Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 36.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 37.Regev-Shoshani G, Shoseyov O, Kerem Z. Influence of lipophilicity on the interactions of hydroxy stilbenes with cytochrome P450 3A4. Biochem Biophys Res Commun. 2004;323:668–673. doi: 10.1016/j.bbrc.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, et al. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000;35:263–268. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 40.Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, et al. Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2002;282:H1996–2003. doi: 10.1152/ajpheart.01013.2001. [DOI] [PubMed] [Google Scholar]
- 41.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, et al. resveratrol provides late-phase cardioprotection by means of nitric oxide-and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465:115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, et al. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: A potential mechanism for resveratrol preconditioning of heart. Vascul Pharmacol. 2005;42:281–289. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Dernek S, Ikizler M, Erkasap N, Ergun B, Koken T, et al. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand Cardiovasc J. 2004;38:245–254. doi: 10.1080/14017430410035476. [DOI] [PubMed] [Google Scholar]
- 44.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Zou J, Cao K, Hsieh TC, Huang Y, et al. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int J Mol Med. 2005;16:533–540. [PubMed] [Google Scholar]
- 46.Auger C, Teissedre PL, Gérain P, Lequeux N, Bornet A, et al. Dietary Wine Phenolics Catechin, Quercetin, and Resveratrol Efficiently Protect Hypercholesterolemic Hamsters against Aortic Fatty Streak Accumulation. J Agric Food Chem. 2005;53:2015–2021. doi: 10.1021/jf048177q. [DOI] [PubMed] [Google Scholar]
- 47.Fukao H, Ijiri Y, Miura M, Hashimoto M, Yamashita T, et al. Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein E-deficient and low-densty lipoprotein receptor-deficient mice. Blood Coagul Fibrinolysis. 2004;15:441–446. doi: 10.1097/00001721-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med. 2003;11:317–320. [PubMed] [Google Scholar]
- 49.Wang Z, Zou J, Huang Y, Cao K, Xu Y, et al. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin Med J (Engl) 2002;115:378–380. [PubMed] [Google Scholar]
- 50.Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, et al. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et zucc. on lipid metabolism. Chem pharm Bull (Tokyo) 1982;30:1766–1770. doi: 10.1248/cpb.30.1766. [DOI] [PubMed] [Google Scholar]
- 51.Chu LM, Lassaletta AD, Robich MP, Sellke FW. Resveratrol in the prevention and treatment of coronary artery disease. Curr Atheroscler Rep. 2011;13:439–446. doi: 10.1007/s11883-011-0202-3. [DOI] [PubMed] [Google Scholar]