Abstract
The purpose of this article is to illustrate the imaging findings of typical and atypical metastatic sites of recurrent endometrial carcinoma. Typical sites include local pelvic recurrence, pelvic and para-aortic nodes, peritoneum, and lungs. Atypical sites include extra-abdominal lymph nodes, liver, adrenals, brain, bones and soft tissue. It is important for radiologists to recognize the typical and atypical sites of metastases in patients with recurrent endometrial carcinoma to facilitate earlier diagnosis and treatment.
Keywords: Recurrent endometrial carcinoma, oncologic imaging, computed tomography, magnetic resonance imaging, positron emission tomography
Introduction
Endometrial cancer is the most common gynecologic malignancy and accounts for 6% of all cancers in women. An estimated 43,470 cases are diagnosed with an estimated 7950 deaths in 2010[1]. Endometrial carcinoma is divided into several histologic categories based on cell type. Endometrioid is the most common cell type, accounting for 75–80% of cases, and subdivided into grade 1 (well differentiated) to grade 3 (poorly differentiated). Other aggressive pathologic variants with a high risk of metastatic disease include papillary serous carcinoma (<10%), clear cell carcinoma (4%), squamous cell carcinoma (<1%), mixed (10%) and undifferentiated types[2].
Local and distant recurrences continue to be a major problem in high-risk patients after surgical treatment of the primary endometrial carcinoma[3]. Adjuvant pelvic radiotherapy has been associated with decreased risk of local recurrence[4]. The median time of recurrence was 2–3 years and 75–80% of recurrences were extra-pelvic. Recurrence is defined as local tumor regrowth or the development of distant metastasis discovered 6 months or more after complete regression of the treated tumor[5]. Sixty-four percent of recurrences occur within 2 years and 87% of all recurrences occur by the third year after primary treatment[5].
Typical sites of recurrent endometrial carcinoma such as pelvic and para-aortic lymph nodes, vagina, peritoneum, and lungs are well recognized through various imaging modalities. Atypical sites such as intra-abdominal organs, bones, brain, abdominal wall, and muscle also occur. These less common patterns of recurrence are being recognized with greater frequency due to improvements in imaging techniques and prompt imaging of patients with suspected recurrence[6]. Some studies have shown that aggressive histopathologic types (such as papillary, clear cell, and adenosquamous) have mild higher incidence of relapse, with a higher incidence of extrauterine disease at presentation compared with the endometrioid type[2,3].
In this article, we discuss and illustrate the spectrum of typical and atypical sites of recurrent endometrial carcinoma on computed tomography (CT), magnetic resonance imaging (MRI), and [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT. All the patients included in this review were treated for primary endometrial carcinoma, and referred for recurrence or relapse with histopathologic proof of local and distant recurrences.
Typical sites of recurrent endometrial carcinoma
Local recurrence
Local or pelvic recurrence in endometrial carcinoma is commonly seen in patients treated with adjuvant chemotherapy alone, and involves the vagina, pelvic nodes and to a lesser extent the cervix, adnexa, and peritoneum[7]. Isolated vaginal recurrence is most commonly seen in patients with stage 1 and 2 disease, in patients without adjuvant radiation therapy[8]. The most common location of recurrence within the vagina is the apex and middle vaginal canal, followed by the distal vagina, and suburethral area[8] (Fig. 1).
Figure 1.
Axial (A) and coronal (B) contrast-enhanced CT images in an 80-year-old woman with recurrent endometrial carcinoma, with predominant endometrioid and papillary serous features, show a large centrally necrotic mass in the vaginal apex (arrows).
Vaginal recurrence is also highly correlated with cervical involvement, whereas extravaginal pelvic recurrence is correlated with deep myometrial and parametrial invasion and pelvic lymph nodes[5]. In general, patients with an isolated vaginal recurrence have a higher chance of cure than those with pelvic or abdominal recurrences, who in turn do better than those with distant metastases.
Pelvic and para-aortic nodal recurrence
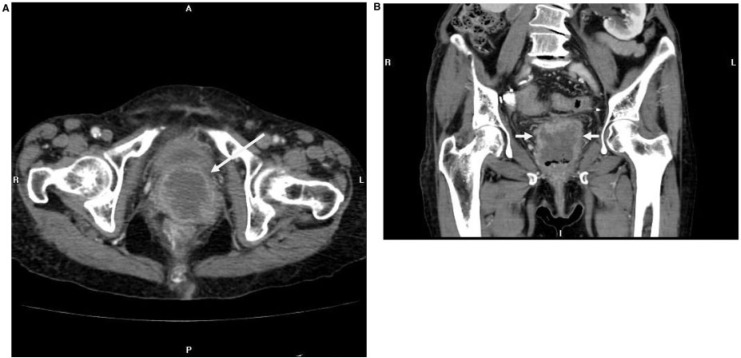
Pelvic and para-aortic stations (Figs. 3 and 4) are most commonly involved in nodal spread from endometrial adenocarcinoma. The external iliac lymph nodes are most commonly involved pelvic lymph nodes in endometrial carcinoma, followed by the obturator and common iliac nodes[6].
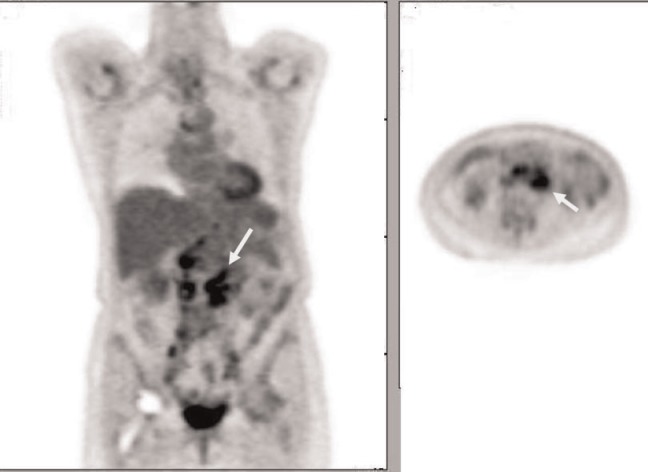
Figure 3.
Coronal and axial FDG-PET images of a 72-year-old patient with clear cell-type endometrial adenocarcinoma show intense FDG uptake in extensive para-aortic and pelvic adenopathy.
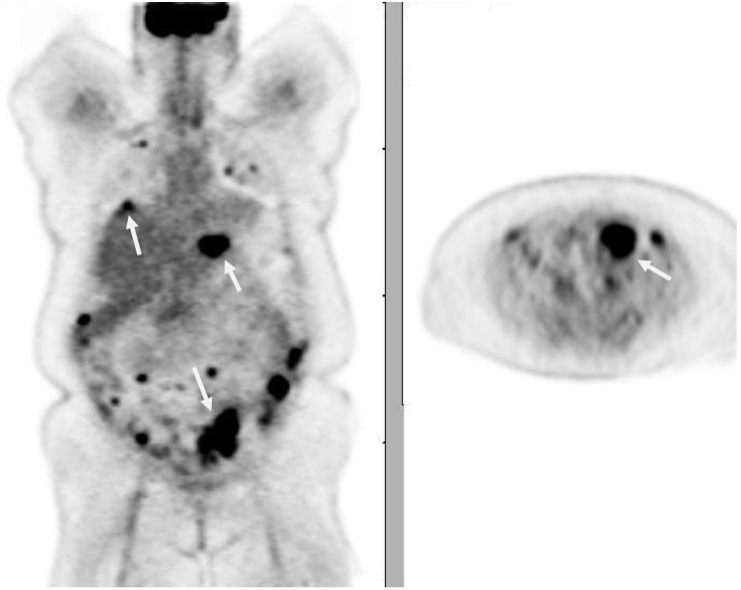
Figure 4.
PET image of a 51-year-old woman with endometrioid endometrial carcinoma showing intense FDG avidity of metastases in the lungs, liver, peritoneum, as well as para-aortic and mesenteric lymph nodes.
Peritoneal recurrence
Peritoneal carcinomatosis is one of the most typical sites of recurrent endometrial carcinoma, with 28% of relapses occurring in the peritoneum[6]. Peritoneal involvement may be seen as huge peritoneal masses (Fig. 2), peritoneal nodules (Fig. 4), and serosal implants that may cause extrinsic compression of the bowel.
Figure 2.
Coronal (A) and axial (B) contrast-enhanced CT images in a 66-year-old woman with endometrioid-type endometrial adenocarcinoma with squamous cell differentiation show multiple soft tissue nodules in the greater omentum.
Lungs
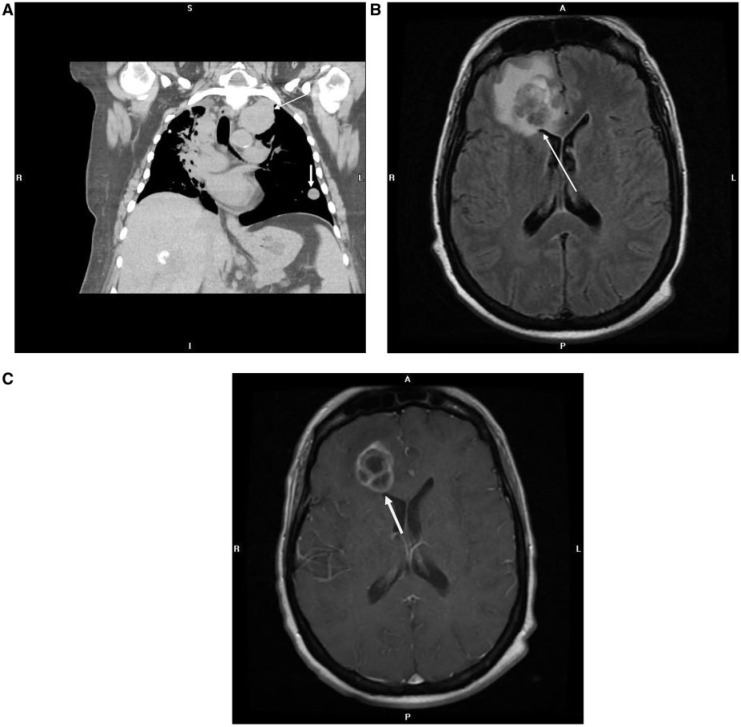
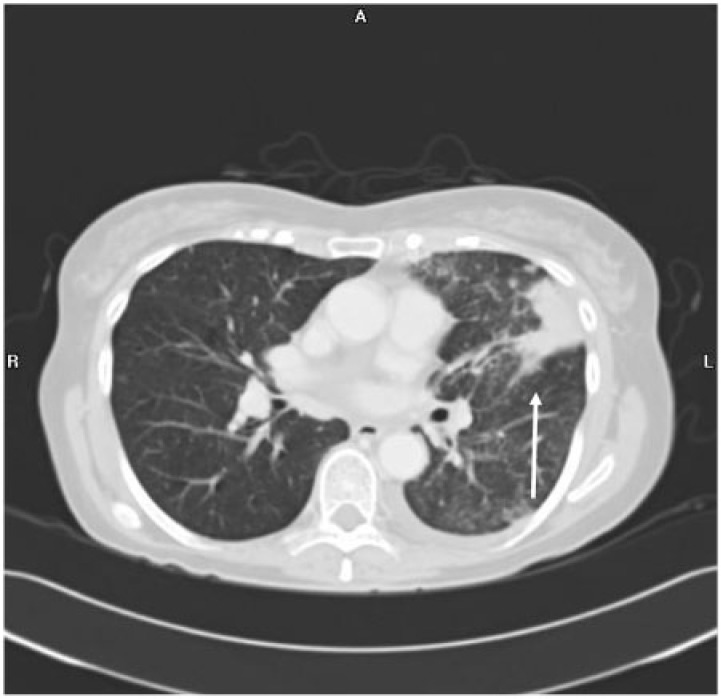
Endometrial carcinoma has the highest frequency of pulmonary metastases compared with other gynecologic malignancies, up to 20–25%[5,6]. Although frequently multiple, bilateral nodules are seen (Fig. 6A); a solitary nodule mimicking primary lung cancer may also be seen (Fig. 5A). Cavitation can be seen, especially with the squamous cell type. Lymphangitic carcinomatosis and endobronchial tumor spread are other pulmonary manifestations that are rarely seen (Fig. 7). Pleural involvement in the form of effusions, nodularity and thickening is seen infrequently (Fig. 6B).
Figure 6.
Coronal contrast-enhanced CT images in (A) lung and (B) mediastinal windows in a 62-year-old woman with clear cell endometrial adenocarcinoma reveal multiple right lower lobe pulmonary nodules (arrows in A) and left suprahilar and right infrahilar endobronchial masses (arrows in B). Axial gadolinium-enhanced T1-weighted MR image (C) shows a large heterogeneously enhancing solid right cerebellar metastasis (pathologic diagnosis of right cerebellar mass showed metastatic clear cell carcinoma, involving the cerebellum, consistent with spread from recurrent endometrial carcinoma).
Figure 5.
Coronal contrast-enhanced CT image of a 66-year-old woman with poorly differentiated endometrioid-type endometrial adenocarcinoma shows (A) mediastinal lymphadenopathy and a left lower lobe parenchymal lung nodule on a coronal CT. (B) Axial fluid-attenuated inversion recovery (FLAIR) and (C) gadolinium-enhanced axial T1-weighted MR images demonstrate a right frontal brain metastasis with surrounding vasogenic edema (arrow in B) and thin peripheral enhancement (arrow in C).
Figure 7.
Axial CT image in a lung algorithm in a 76-year-old woman with clear cell endometrial adenocarcinoma shows a consolidative left upper lobe mass with adjacent nodular septal thickening and peribronchial thickening representing lymphangitic carcinomatosis. Small nodules are seen elsewhere within the imaged lungs.
Atypical sites of recurrent endometrial carcinoma
Extra-abdominal nodal disease
The incidence of isolated extra-abdominal nodal recurrences is uncommon (0.4–1%[9,10]. Among these extra-abdominal nodal sites, supraclavicular nodal recurrence has a higher incidence compared with mediastinal and axillary node involvement (Fig. 8). Most supraclavicular and mediastinal recurrences were on the left side due to the location of the thoracic duct (Figs. 5A and 8).
Figure 8.
Axial contrast-enhanced CT image of a 65-year-old woman with papillary serous endometrial adenocarcinoma shows rounded, enlarged left posterior cervical lymph node metastases. (Fine-needle aspiration of the node interpreted positive malignant cells consistent with spread from serous adenocarcinoma).
Intra-abdominal organs
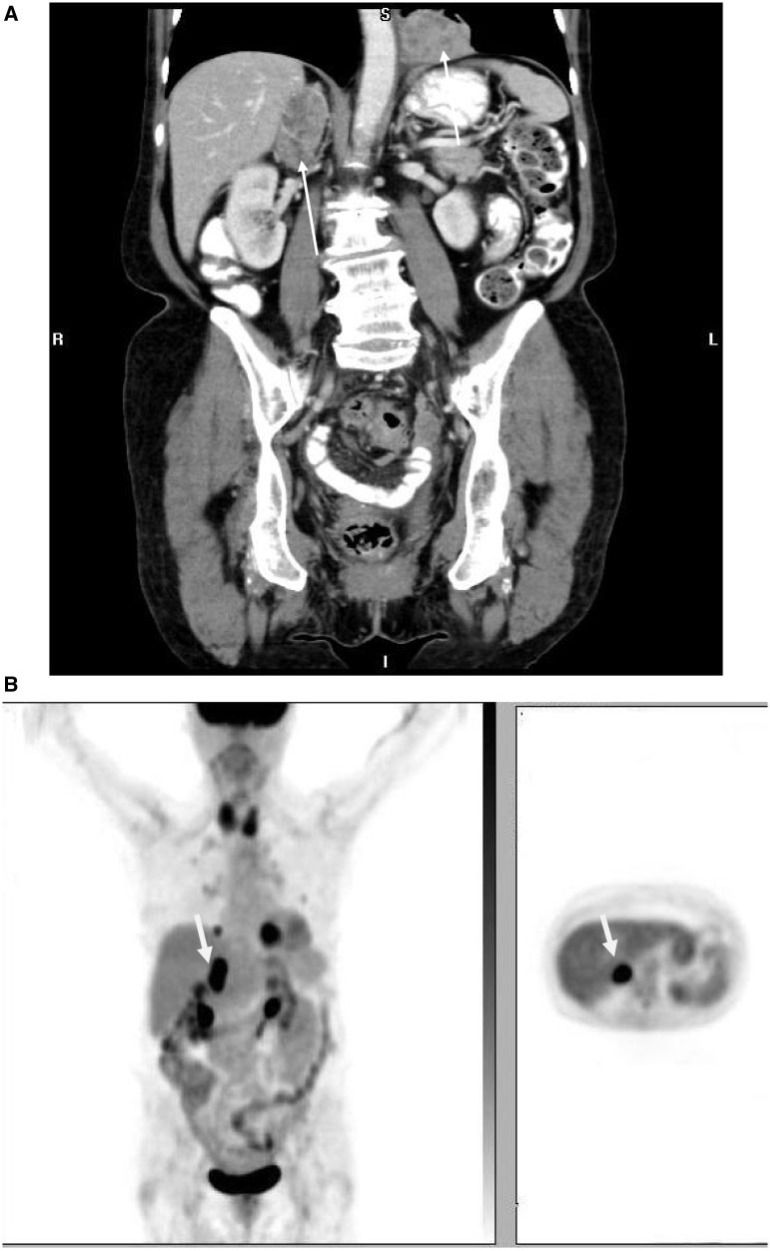
Compared with the typical metastatic sites such as the pelvic nodes, peritoneum, and lungs, the incidence of recurrent endometrial carcinoma is less frequent in intra-abdominal organs[5,6]. It has been observed that aggressive pathologic types such as clear cell and papillary serous types are more prone to relapse in the abdomen, including the omentum[11]. The most common intra-abdominal solid organ involved is the liver (7%). Liver metastases are seen as single or multiple liver lesions with peripheral enhancement, and as a large solitary liver mass or lesion (Fig. 9).The other atypical intra-abdominal organs are the adrenal glands (Fig. 10) and spleen (1%)[6].
Figure 9.
Contrast-enhanced coronal CT image in a 70-year-old woman with mixed serous/endometrioid endometrial cancer shows a typical hepatic metastasis in the right lobe, with peripheral soft tissue attenuation and central hypodensity.
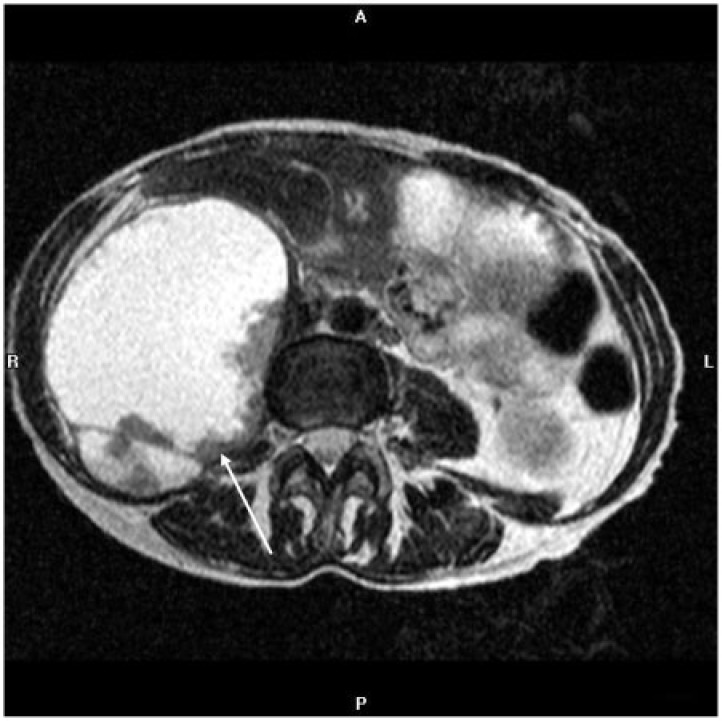
Figure 10.
Coronal contrast-enhanced CT (A) and PET images (B) in an 81-year-old woman with endometrioid endometrial adenocarcinoma show a heterogeneously enhancing right adrenal mass and left lower lung mass (arrows) with intense FDG uptake representing metastases.
Central nervous system metastases
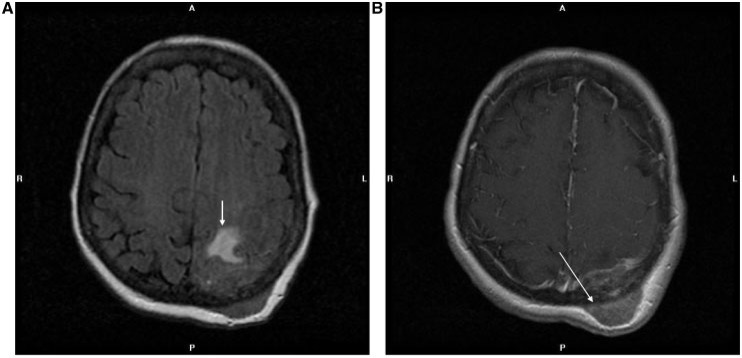
Central nervous system involvement is rare (<1%)[5,6,12]. Brain metastases may present as heterogeneously enhancing masses in cerebral and cerebellar regions. Lesions tend to have lobulated margins and may be hypointense on T1- and T2-weighted images (Figs. 5B,C and 6C). Dural-based lesions with or without osseous involvement may also be seen (Fig. 11).
Figure 11.
Axial FLAIR (A) and gadolinium-enhanced axial T1-weighted (B) MR images of a 61-year-old woman with endometrioid endometrial adenocarcinoma show a transcalvarial metastasis in the left occipital region, with underlying left parieto-occipital white matter edema (arrow in A) and epidural extension (arrow in B).
Musculoskeletal and soft tissue recurrence
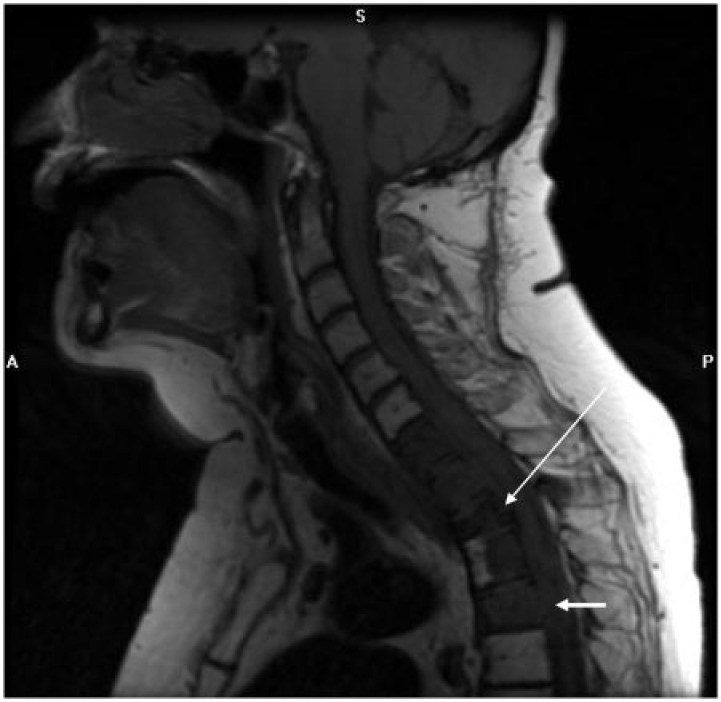
The prevalence of osseous metastases in endometrial carcinoma ranges from 4% to 7%[5,6,13]. Endometrial metastases to the bone are generally restricted to the axial skeleton, including the pelvis and thoracolumbar vertebrae[13] (Fig. 13). Skeletal metastases in the appendicular skeleton are very unusual. There are isolated case reports of unusual metastatic sites to extremities, such as the tibia, tarsus, calcaneus (Fig. 12).
Figure 13.
Sagittal T1-weighted image of the cervical spine in a 61-year-old woman with endometrioid endometrial adenocarcinoma reveals a nearly diffuse abnormal hypointense signal in C7–T4 vertebral bodies with pathologic fracture of T2 (long arrow) and epidural extension posterior to several of the affected vertebral bodies (short arrows).
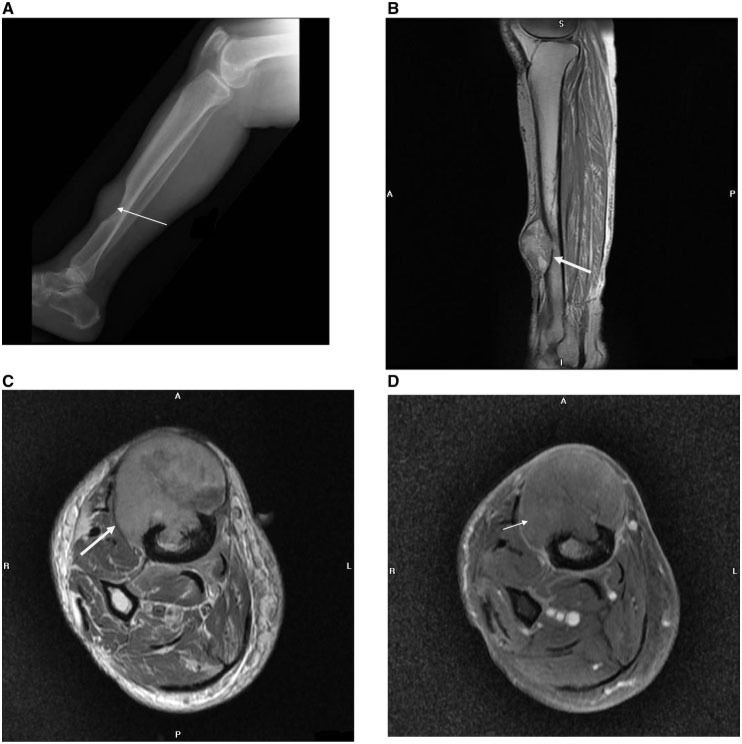
Figure 12.
(A) Lateral radiograph of the lower right leg shows a large elliptical lytic metastasis destroying the right anterior distal tibial diaphysis with overlying soft tissue mass (arrow) in a 72-year-old woman with endometrioid endometrial adenocarcinoma. Sagittal T1-weighted (B), axial pre-contrast T1-weighted (C), and axial post-contrast T1-weighted (D) MR images highlight the extent of the metastatic lesion destroying the distal tibia, with a large extra-osseous soft tissue mass (short arrow), cortical destruction (long arrow) and intramedullary extent. (On pathologic diagnosis, the soft tissue mass excision showed metastatic adenocarcinoma, morphologically consistent with recurrent endometrioid adenocarcinoma).
The prevalence of muscular and soft tissue metastases is 2–6%[6] (Fig. 14). These manifest as enhancing nodules or masses in the abdominal wall along the laparotomy scar or at remote locations (Fig. 15).
Figure 14.
Axial T2-weighted MR image of the abdomen of a 59-year-old woman with clear cell endometrial adenocarcinoma shows a large, predominately hyperintense mass that expands the right psoas muscle and displaces the bowel anteriorly, with peripheral intermediate intensity components representing tissue (arrow). Resection revealed a fluid-containing endometrial cancer metastasis.
Figure 15.
Contrast-enhanced axial CT image of the abdomen of a 58-year-old woman with endometrioid endometrial adenocarcinoma shows a lobulated, enhancing abdominal wall metastasis in the right rectus musculature (arrow).
Role of PET/CT in recurrent endometrial carcinoma
FDG-PET/CT is a highly sensitive, specific and useful modality for providing good anatomic and functional localization of sites of recurrence and distant metastasis in the preclinical stage before it becomes evident by conventional imaging modalities. PET/CT makes it possible to acquire both metabolic and anatomic imaging data and provides precise anatomic localization of suspicious areas of increased FDG uptake[9]. PET/CT may have the greatest usefulness in situations in which tumor marker levels are increasing and conventional imaging studies show negative results. Various studies have shown the percentage of cases in which clinical treatment was changed on the basis of PET/CT findings in patients with endometrial carcinoma with suspected recurrence and metastasis[14].
Conclusions
Although some of the manifestations of recurrent endometrial carcinoma such as pelvic (vaginal), nodal, peritoneal and lung metastases are well known, other manifestations involving sites such as intra-abdominal organs (liver, adrenals, spleen), brain, osseous and soft tissue metastases are less well known. Aggressive histopathologic types such as clear cell and papillary serous types are more prone to relapse in atypical sites compared with endometrioid type. Advances in treatment options such as radiation therapy and chemotherapy are being made and as patients with recurrent endometrial carcinoma are imaged with greater frequency, the unusual and atypical sites of recurrent disease may be encountered more often.
Conflict of interest
None declared
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage 1 carcinoma of endometrium. Gynecol Oncol. 2004;95:593–596. doi: 10.1016/j.ygyno.2004.08.019. . PMid:15581969. [DOI] [PubMed] [Google Scholar]
- 3.Grigsby PW, Perez CA, Galakatos AE, et al. Clinical stage 1 endometrial cancer: prognostic factors for local control and distant metastasis and implications of the new FIGO surgical staging system. Int J Radiat Oncol Biol Phys. 1992;22:905–911. doi: 10.1016/0360-3016(92)90786-H. . PMid:1555983. [DOI] [PubMed] [Google Scholar]
- 4.Elliott P, Green D, Coates A, et al. The efficacy of postoperative vaginal irradiation in preventing vaginal recurrence in endometrial cancer. Int J Gynecol Cancer. 1994;4:84–90. doi: 10.1046/j.1525-1438.1994.04020084.x. . PMid:11578390. [DOI] [PubMed] [Google Scholar]
- 5.Aalders JG, Abeler V, Kolstad P. Recurrent adenocarcinoma of the endometrium. A clinical and histopathological study of 379 patients. Gynecol Oncol. 1984;17:85–103. doi: 10.1016/0090-8258(84)90063-5. . PMid:6693055. [DOI] [PubMed] [Google Scholar]
- 6.Sohaib SA, Houghton SL, Rexnek RH, et al. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62:28–34. doi: 10.1016/j.crad.2006.06.015. . PMid:17145260. [DOI] [PubMed] [Google Scholar]
- 7.Mundt AJ, McBride R, Connell PP, et al. Significant pelvic recurrence in high-risk pathologic stage 1–4 endometrial carcinoma patients after adjuvant chemotherapy alone. Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145–1153. doi: 10.1016/S0360-3016(01)01566-8. . PMid:11483323. [DOI] [PubMed] [Google Scholar]
- 8.Sears JD, Greven KM, Randall ME, et al. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer. 1994;74:1303–1308. doi: 10.1002/1097-0142(19940815)74:4<1303::AID-CNCR2820740420>3.0.CO;2-G. . PMid:8055452. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima K, Murakami K, Sugumura K, et al. Performance of FDG-PET/CT in the diagnosis of recurrent endometrial cancer. Ann Nucl Med. 2008;22:103–109. doi: 10.1007/s12149-007-0087-y. . PMid:18311534. [DOI] [PubMed] [Google Scholar]
- 10.Foote RL, Schray MF, Malkasian GD, et al. Isolated peripheral lymph node recurrence of endometrial carcinoma. Cancer. 1988;61:2561–2565. doi: 10.1002/1097-0142(19880615)61:12<2561::AID-CNCR2820611229>3.0.CO;2-2. . PMid:3365675. [DOI] [PubMed] [Google Scholar]
- 11.Mariani A, Webb MJ, Podratz KC, et al. Routes of lymphatic spread. A study of 112 consecutive patients with endometrial cancer. Gynecol Oncol. 2001;81:100–104. doi: 10.1006/gyno.2000.6111. . PMid:11277658. [DOI] [PubMed] [Google Scholar]
- 12.Cormio G, Lissoni A, Mangioni C, et al. Brain metastases from endometrial carcinoma. Gynecol Oncol. 1996;61:40–43. doi: 10.1006/gyno.1996.0093. . PMid:8626115. [DOI] [PubMed] [Google Scholar]
- 13.Dursun P, Gultekin M, Ayhan A, et al. Bilateral bone metastasis in endometrial adenocarcinoma. Lancet Oncol. 2003;4:247. doi: 10.1016/s1470-2045(03)01193-8. [DOI] [PubMed] [Google Scholar]
- 14.Chung HH, Kang WJ, Kim JW, et al. The clinical impact of FDG PET/CT for the management of recurrent endometrial cancer: correlation with clinical and histological findings. Eur J Nucl Med Mol Imaging. 2008;35:1081–1088. doi: 10.1007/s00259-007-0687-8. . PMid:18180917. [DOI] [PubMed] [Google Scholar]