Abstract
CNTO 530 is an erythropoietin receptor agonist MIMETIBODYTM construct. CNTO 530 has been shown to be active in a number of rodent models of acquired anemia (e.g. renal insufficiency and chemotherapy induced anemia). We investigated the efficacy of CNTO 530 in murine models of β-thalassemia and sickle cell anemia (Berkeley mice). β- thalassemic mice are deficient in expression of α-globin chain and heterozygous mice are characterized by a clinical syndrome similar to the human β-thalassemia intermedia. Berkeley mice are knocked out for murine alpha and beta globin and are transgenic for human alpha, beta (sickle) and gamma globin genes. Berkeley mice thus express human sickle hemoglobin A (HbS) and can also express human fetal hemoglobin. These mice express a severe compensated hypochromic microcytic anemia and display the sickle cell phenotype. To test the effectiveness of CNTO 530, mice from both genotypes received a single subcutaneous (s.c.) dose of CNTO 530 or darbepoetin-α (as a comparator) at 10,000 U/kg, a dose shown to cause a similar increase in reticulocytes and hemoglobin in normal mice. Hematologic parameters were evaluated over time. CNTO 530, but not darbepoetin-α, increased reticulocytes, red blood cells and total hemoglobin in β- thalassemic mice. In Berkeley mice CNTO 530 showed an increase in reticulocytes, red blood cells, F-cells, total hemoglobin and fetal hemoglobin. In conclusion, CNTO 530 is effective in murine models of β-thalassemia and sickle cell anemia. These data suggest that CNTO 530 may have beneficial effects in patients with genetically mediated hemoglobinopathies.
Keywords: Erythropoiesis, Flow Cytometry, Fusion protein, Hematology, Ion Exchange Chromatography, Pharmacodynamics.
INTRODUCTION
Background
Erythropoiesis, the formation of red blood cells (RBC) from multipotent stem cells in the bone marrow, is an exquisitely regulated process in which the glycoprotein hormone erythropoietin (EPO) plays a central role [1]. Recently, a novel long-acting erythropoietin receptor agonist (EPO-R) has been described [2]. CNTO 530 is a 57 kD glycoprotein homodimer MIMETIBODYTM construct with two copies of EMP-1 (an erythropoietic peptide [3]) presented near the N-terminus. CNTO 530, like EPO, binds EPO-R and stimulates erythropoiesis. However, CNTO 530 bears no amino acid sequence homology to EPO.
β-thalassemia and sickle cell anemia (SCA) are two of the most common hemoglobinopathies [4, 5]. The adult hemoglobin molecule (HbA) is composed of two α-type and two β-type globin chains. In β-thalassemia, underproduction of to the β-globin chain results in precipitation of the unpaired α-chains, which in turn, triggers the destruction of RBC and red cell precursors in the bone marrow [6]. Numerous β-globin mutations can result in β-thalassemia. Depending on the mutation, the degree of underproduction of β-globin chain varies. In some patients, the imbalance of globin chain production is minimal and they remain symptom free (thalassemia minor). In others, there is a greater imbalance resulting in transfusion dependent anemia (thalassemia intermedia and thalassemia major). In these latter types, increased destruction of RBC and repeated transfusions leads to hemochromatosis.
In contrast, SCA is the result of a unique point mutation in the β globin gene that results in production of sickle hemoglobin (HbS) [5]. In sickle cell disease, individuals homozygous for the mutant β-globin gene produce no HbA and exclusively HbS. However, they retain some production of fetal hemoglobin (HbF). The solubility of the deoxygenated form of HbS is less than HbA. This lower solubility results in precipitation of HbS in the red cells, causing deformity of the cells (sickle cells) and leading to destruction of RBC, formation of micro-thrombi and sever pain. Exacerbation of this process is referred to as sickle cell crisis and can lead to infarctions in numerous tissues, especially the spleen, liver and brain [7].
Rationale
In baboons, rhEPO has been shown to increase the percent of reticulocytes containing HbF from 1-2% to as high as 50% [8] and rhEPO has been shown to have some, albeit limited, benefit in β-thalassemia and SCA (see below). This beneficial effect is believed, at least in part, to be derived from an up-regulation of gamma-globin chain utilization, leading to production of increased levels of fetal hemoglobin (HbF). The purpose of this study was to determine if CNTO 530 has beneficial effects in murine models of β-thalassemia and SCA.
MATERIALS AND METHODS
Test Articles
CNTO 530 and formulation buffer was supplied by Centocor, R&D (Radnor PA, Lot # TN101806). Darbepoetin-α (AranesepTM, Amgen, Thousand Oaks, CA) was purchased commercially. Phosphate buffered saline was purchased from Invitrogen (Carlsbad, CA).
Because CNTO 530 and darbepoetin-α have different molecular weights and different affinity for EPO-R, doses were expressed as UT-7 Units/kg. UT-7 units were determined from an in vitro assay described previously [2]. Briefly, using epoetin-α (mw = 34 kD) with an activity of 120 IU/μg as a standard, the EC50 for each test article for mediating proliferation of UT-7EPO cells and their respective molecular weight were used to calculate UT-7 units using the following equation:
UT-7 Units/µg = (Mol wt of ERA)*C/(EC50 for ERA),
where C = (120 Units/µg)*(EC50 for rHuEPO)/34 kD
This gave values of 199 U/µg for darbepoetin-α and 30 U/µg for CNTO 530.
Mice
Female C57BL/6 mice were obtained from Ace Animals, Boyertown, PA. Male and female Th3+/C57BL/6 (heterozygous) (β-thalassemic mice) [9] were obtained from a colony maintained in the pathogen-free vivarium at Centocor R&D, Inc., Radnor PA. Founder Th3+/C57BL/6 mice for the colony was obtained from M Weiss, University of Pennsylvania. The breeding stock was maintained as heterozygotes for a deletion of both the b1 and b2 globin genes. Th3+/C57BL/6 were selected for study based on a pale visual appearance and gross splenomegally in comparison with their Th3-/C57BL/6 littermates.
Hbatm1Paz Hbbtm1Tow Tg(HBA-HBBs)41Paz/J (Berkeley) mice [10] were bred at Ace Animals (Boyertown, PA). Founder mice for the transgenic colony were obtained from Jackson Laboratories (Stock number 003342, Bar Harbor, ME) under a nonexclusive bailment agreement with the Regents of the University of California through the EOL Berkeley National Laboratory. As recommended by Jackson Laboratories, dams heterozygous for murine β-globin (non-sickle) were bred to murine β-globin knockout (sickle) males. All mice were genotyped to ensure that they were murine β-globin knockout and transgene + (Tg/Tg or Tg/o). Only mice that were murine β-globin homozygous knockout and expressed the transgene were used in these experiments.
All mice were group housed in filter-topped plastic shoebox style cages. The animals were individually identified with ear tags, placed at least 1 week prior to the start of the study. All mice were maintained in the pathogen-free vivarium at Centocor R&D, Inc., Radnor PA. The Institutional Animal Care and Use Committee at Centocor approved all associated procedures.
Pharmacodynamics
Mice received a single subcutaneous (s.c.) dose of CNTO 530 or darbepoetin-α at 10,000 U/kg. Control mice received an equivalent volume of the saline vehicle or formulation buffer. Blood was collected from mice anesthetized with a CO2 mixture via open chest cardiac puncture into commercially prepared EDTA coated microtubes. Hematology analyses were performed on whole blood using an ADVIA® 120 hematology analyzer (Siemens Medical Solutions Diagnostics, Tarrytown NY, USA).
Ion exchange chromatorgraphic analysis of HbF was performed after the method of Morin and Barton [11]. Briefly, 50 μL fresh whole blood was lysed in 200 μL distilled H2O containing 0.1% Triton-X 100 and 200 mM KCN and 1 mL adsorption buffer was added. (The adsorption buffer contained 200 mM Bis-Tris acetate (pH 4.5), 200 mM KCN and a trace amount of trichlorobutanol as a preservative.) One cm disposable mini-columns were packed with 1 mL Sephadex CM-50 in adsorption buffer. The column was allowed to drain under minimal vacuum, the packing covered with a glass frit and washed with adsorption buffer. One ml of sample was layered on the packing and the column washed with 2 aliquots of adsorption buffer. The column was eluted with 2 mL aliquots of elution buffer and fractions were collected under gravity. (The elution buffer contained 100 mM Bis-Tris acetate (pH 6), 4.8 g/L magnesium acetate, 200 mM KCN, and a trace amount of trichlorobutanol as a preservative.) Aliquots of the collected fractions were transferred to a 96 well plate and the OD 415 (Soret peak for hemoglobin (Hgb)) read on a Molecular Devices SpectraMax 340PC (Sunnyvale, CA). Pre- and post-dose HbF ratio was calculated using the value for the peak fraction. To calculate HbF concentration, 250 μL of the remaining whole blood lysate was diluted to 2 mL and serial 2 fold dilutions prepared in adsorption buffer. Aliquots of these dilutions (0.33 mL) were transferred to a 96 well plate and the OD 415 read. These values were plotted as a function of dilution of total Hgb (measured by the automated hematology analyzer) and linear regression analysis was used to calculate an extinction coefficient for each mouse. This value was used with the OD of the HbF peak to calculate the concentration of HbF.
Flow cytometric analysis of RBC and reticulocytes expressing HbF was performed after the method of B Davis and K Davis (Current Protocols in Cytometry, 2004). Briefly, ~25x106 RBC were fixed with 1 mL cold 0.05% glutaraldehyde in phosphate buffered saline (PBS) for 10 minutes. The cells were washed with 2 mL 0.1% bovine serum albumin (BSA) 0.1% sodium azide in PBS and permeabilized in 500 μL 0.1% Triton-X 100 for 3-5 minutes, washed and resuspended in 500 μL BSA-PBS. Ten μL aliquots were stained with anti-HbF antibodies (5 μL in 80 μL BSA-PBS) in a 96 well round bottom plate for 15 minutes (Murine monoclonal anti-human HbF, clone HbF-1 (12) Cy5 (TRI-COLOR®, TC), Invitrogen). The cells were washed and resuspended in 200 μL thiazole orange (Retic-Count™ Reticulocyte Reagent System, Becton Dickinson Biosciences, San Jose, CA) for 15-30 minutes. Staining was controlled with a Fetal Hemoglobin Control Kit (Fetaltrol), Invitrogen, and BD Retic-Count™ Control Kit (Tri-Level Control). Data were acquired on a Becton Dickinson FACSCalibur. Monodisperse cells were gated on the basis of forward and side scatter. Cells stained with HbF-1 were counted as HbF+ and cells stained with thiazole orange were counted as reticulocytes. Data are expressed as %Total RBC HbF+ and %Reticulocytes HbF+.
Data Analysis and Statistics
Data are expressed as mean ± standard deviation. Statistical significance was determined by t-test or analysis of variance with Bonferroni’s correction for multiple comparisons. When the data were not normally distributed or if there was unequal variance, significance was determined by Mann-Whitney Rank Sum or analysis of variance on ranks with Dunn’s correction for multiple comparisons. P values <0.05 were accepted as statistically significant.
RESULTS
Characterization of β-thalassemic Mice
The results of the comparison of Th3+/C57BL/6 (β-thalassemic) with C57BL/6 (normal) are shown in (Table 1 and Table 2). Hematologic analysis confirmed dysregulated erythropoiesis in these mice with dramatically decreased RBC, Hgb, and hematocrit (Hct), by up to 40% compared to normal C57Bl/6 mice. Reticulocyte counts were elevated approximately 6 fold concurrent with decreased mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC). Moreover, red cell distribution width (RDW), a measure of size heterogeneity, was increased by approximately 3 fold. In addition, splenomegaly was observed consistent with increased hematopoiesis. Thus, Th3+/C57BL/6 mice can be described as exhibiting a marked microcytic, hypochromic, regenerative anemia and ineffective erythropoiesis.
Table 1.
Differences in the Erythron Between C57Bl/6, β-Thalassemic, and Berkeley Mice. Asterisk (*) Indicates Statistically Different from Control, t-test, (P<0.05).
| Mouse Strain | Statistics | Reticulocytes | Mature Erythrocytes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retic (x109cells/L) | CHr (pg) | Retic_ RDW (%) | Retic_ HDW (g/dL) | RBC (x106/uL) | HGB (g/dL) | HCT (%) | RDW (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | HDW (g/dL) | ||
| C57BL/6 n=30 | Mean Std. Dev. | 304.59 ± 47.98 | 16.11 ± 0.30 | 11.61 ± 0.66 | 1.91 ± 0.09 | 9.74 ± 0.31 | 15.22 ± 0.43 | 53.42 ± 1.74 | 12.39 ± 1.18 | 54.86 ± 1.04 | 15.63 ± 0.29 | 28.49 ± 0.40 | 1.36 ± 0.04 |
| TH3+/C57BL/6 n=34 | Mean Std. Dev. | 1751.45* ± 388.28 | 13.97* ± 0.55 | 17.39* ± 1.11 | 3.64* ± 0.20 | 9.09* ± 0.65 | 8.94* ± 0.58 | 36.64* ± 1.95 | 34.51* ± 2.79 | 40.13* ± 1.72 | 9.81* ± 0.61 | 24.45* ± 1.18 | 4.27* ± 0.34 |
| Berkeley n=18 | Mean Std. Dev. | 1880.09* ± 453.38 | 13.93* ± 1.38 | 17.23* ± 2.34 | 3.35* ± 0.49 | 5.27* ± 1.12 | 5.59* ± 1.03 | 27.01* ± 3.87 | 25.16* ± 3.52 | 52.42 ± 7.87 | 10.91* ± 2.18 | 20.68* ± 1.57 | 3.57* ± 0.27 |
Table 2.
Differences in Leukocytes and Platelets Between C57Bl/6, β-Thalassemic, and Berkeley Mice. Asterisk (*) Indicates Statistically Different from Control, t-test, (P<0.05).
| Mouse Strain | Statistics | Leukocytes | Platelets | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WBC (x103/µL) | Neuts (x103cells/µL) | Lymphs (x103cells/µL) | Mono (x103cells/µL) | Plt (x103/µL) | MPV (fL) | PDW (%) | MPC (g/dL) | ||
| C57BL/6 n=30 | Mean Std. Dev. | 10.64 ± 3.08 | 0.98 ± 0.35 | 9.10 ± 2.65 | 0.12 ± 0.06 | 1195.27 ± 227.57 | 5.73 ± 0.46 | 48.72 ± 2.16 | 23.13 ± 1.68 |
| TH3+/C57BL/ 6n=34 | Mean Std. Dev. | 17.52* ± 5.23 | 1.19 ± 0.50 | 15.63* ± 4.90 | 0.25 ± 0.13 | 1370.21 ± 188.77 | 6.54 ± 0.78 | 59.33* ± 14.09 | 23.22 ± 2.09 |
| Berkeley n=18 | Mean Std. Dev. | 25.48* ± 19.06 | 1.92* ± 0.92 | 22.42* ± 17.86 | 0.13 ± 0.15 | 898.56 ± 384.24 | 9.10* ± 1.19 | 83.02* ± 14.97 | 19.27* ± 1.61 |
Characterization of Berkeley Mice
The results of the comparison of Berkeley (sickle cell) with C57BL/6 (normal) mice are shown in (Table 1 and Table 2). Compared to normal C57Bl/6 mice, Berkeley mice showed a number of changes in RBC parameters such as a decrease in RBC, Hgb, Hct, MCH and MCHC with an increase in retic and RDW. Although the mean MCV were not different, there was a difference in variance, probably reflecting fragmentation of RBC. These data indicate that the Berkeley mice show a poorly compensated, normocytic, hypochromic anemia.
Pharmacodynamics of CNTO 530 and Darbepoetin-α
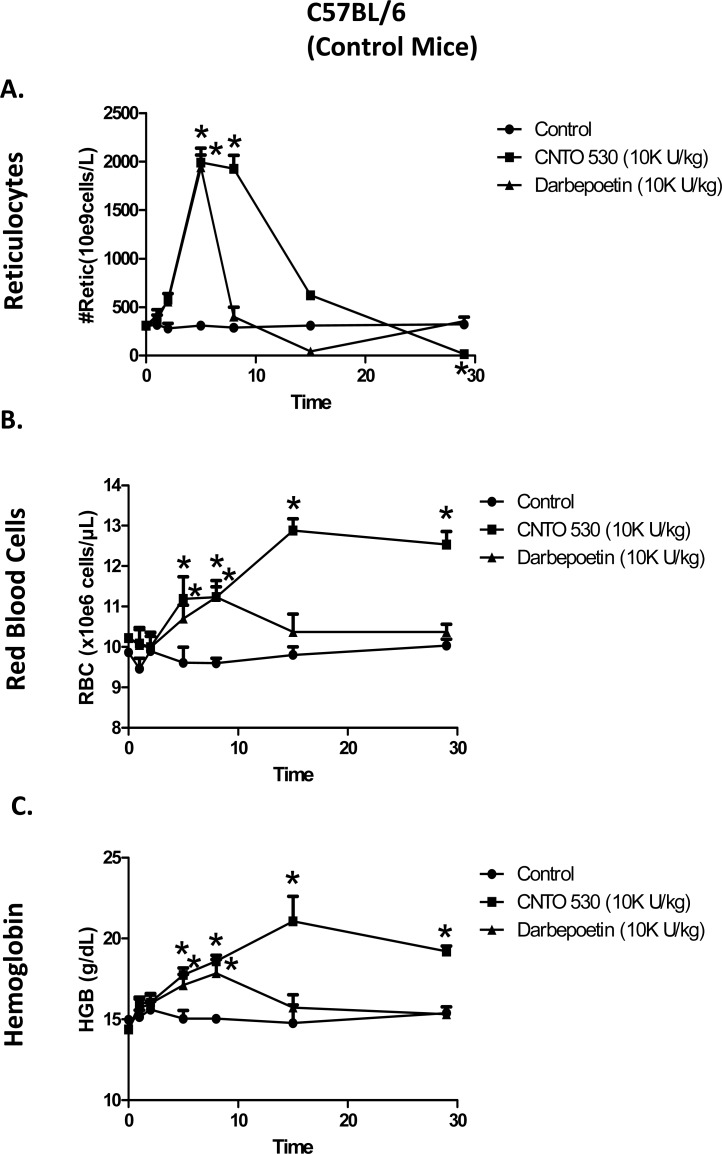
To determine the effects of CNTO 530 or darbepoetin-α in normal, β-thalassemic and sickle cell mice, all were given a single s.c. dose of 10,000 U/kg of CNTO 530 or darbepoetin-α. As shown in (Fig. 1a), this dose of CNTO 530 and darbepoetin-α caused a similar peak reticulocytosis in normal C57BL/6 mice. However, the duration of the elevation of reticulocytes was longer with CNTO 530. The longer duration of reticulocytosis was reflected in a greater and longer-lived increase in RBC (Fig. 1b) and Hgb (Fig. 1c).
Fig. (1).
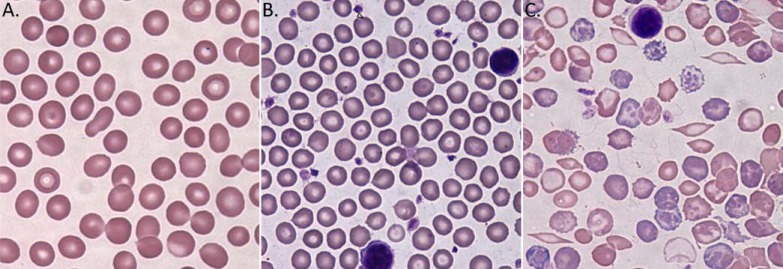
Peripheral blood smeer at 100x. (A) C57BL/6 mouse (B) TH3+/C57BL/6 mouse (C) Berkeley mouse. Wright Giemsa staining of Peripheral blood smear at 100x. The image acquisition system consisted of a Nikon Eclipse 80i microscope (Nikon Inc., Melville, NY) Nikon 100x oil immersion objective, Evolution TM MP color camera (Media Cybernetics, Inc., Bethesda, MD) and Image-Pro Plus V 5.1 software (Media Cybernetics).
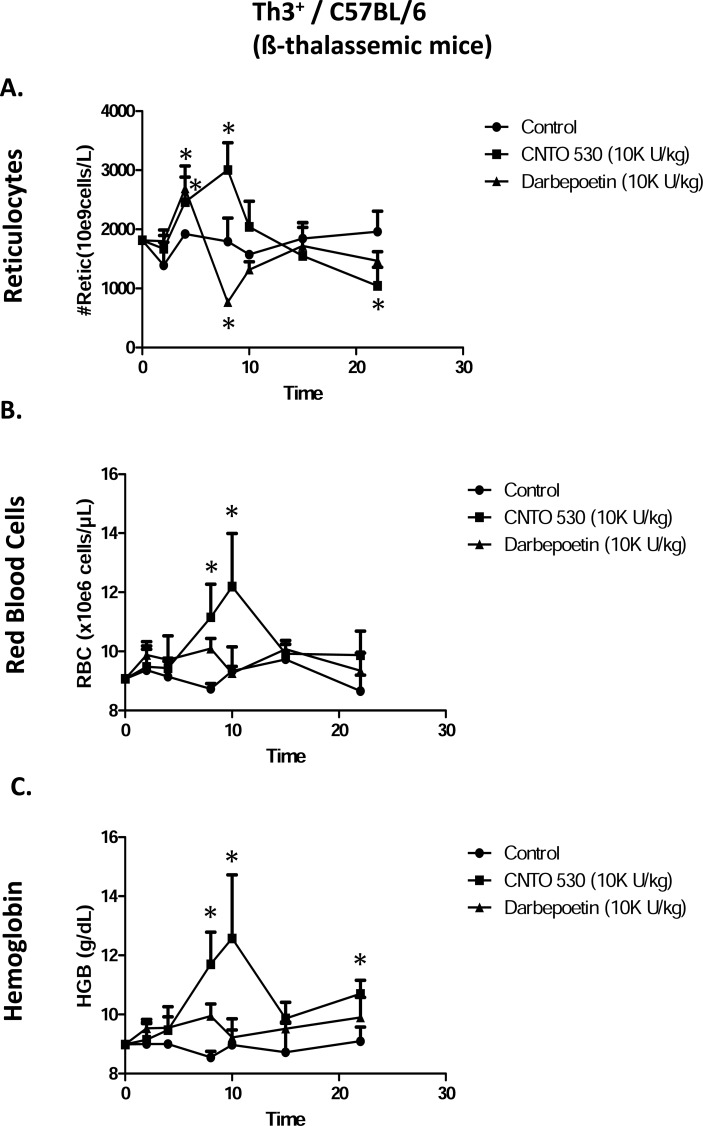
In β-thalassemic mice, compared to darbepoetin-α, CNTO 530 caused a greater and longer-lived increase in reticulocytes in Th3+/C57BL/6 mice (Fig. 2a). Again, as seen in normal mice, the longer duration of reticulocytosis was reflected in a greater and longer-lived increase in RBC (Fig. 2b) and Hgb (Fig. 2c).
Fig. (2).
Comparative effects of CNTO 530 and Darbepoetin-α on (A) Reticulocytes, (B) RBC, and (C) Hgb in normal C57BL/6 (control) mice. Asterisk (*) indicates statistically greater than control, ANOVA with Bonferroni or ANOVA on Ranks, Dunn’s correction, (P<0.05).
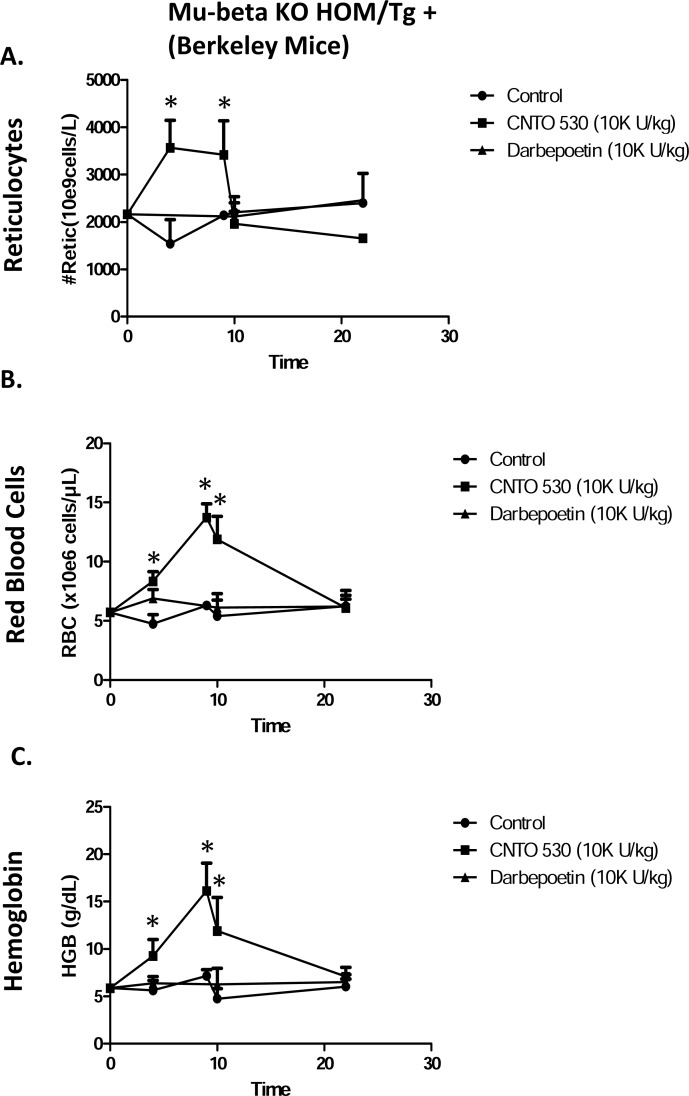
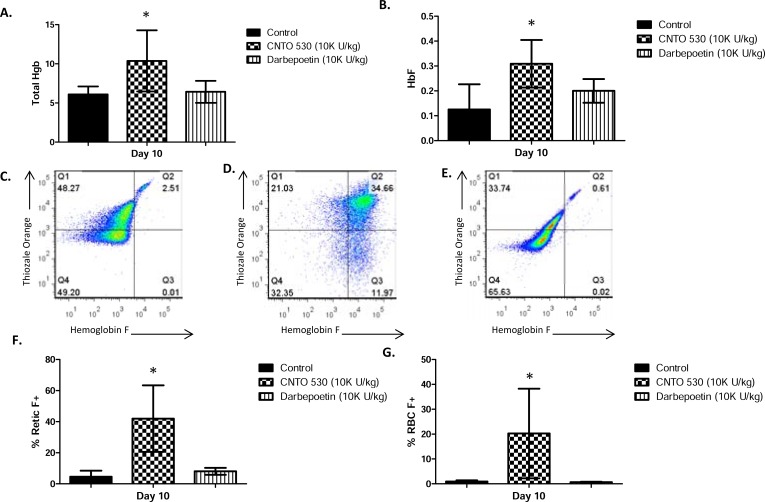
In sickle cell mice, CNTO 530 caused an increase in reticulocytes (Fig. 3a). In contrast, darbepoetin-α had no effect on reticulocytes in these mice. The increase in reticulocytes in the CNTO 530 treated mice was reflected in an increase RBC (Fig. 3b) and Hgb (Fig. 3c). Again, in contrast, darbepoetin-α had no effect on these parameters. To determine the effects of CNTO 530 and darbepoetin-α on expression of HbF, blood samples collected 10 days after dosing with 10,000 U/kg were analyzed by ion exchange chromatography. There was a statistically significant increase total Hgb (Fig. 4a) and in expression of HbF (Fig. 4b) in the CNTO 530 treated but not the darbepoetin-α treated mice. These blood samples were also analyzed by flow cytometry for enumeration of HbF+ reticulocytes and RBC. The gating strategy for measuring F+ reticulocytes and F+ RBC in sickle cell mice is shown in Fig. 4c (control), Fig. 4d (CNTO 530 treated), and Fig. 4e (darbepoetin-α). The effects of CNTO 530 on %F+ reticulocytes and %F+ RBC are shown in (Fig. 4f and 4g). CNTO 530, but not darbepoetin-α, caused a statistically significant increase in % F reticulocytes and %F+ RBC.
Fig. (3).
Comparative effects of CNTO 530 and Darbepoetin-α on (A) Reticulocytes, (B) RBC, and (C) Hgb in ß-thalassemic mice. Asterisk (*) indicates statistically greater than control, ANOVA with Bonferroni or ANOVA on Ranks, Dunn’s correction, (P<0.05).
Fig. (4).
Comparative effects of CNTO 530 and Darbepoetin- α on (A) Reticulocytes, (B) RBC, and (C) Hgb in Berkeley mice. Asterisk (*) indicates statistically greater than control, ANOVA with Bonferroni or ANOVA on Ranks, Dunn’s correction, (P<0.05).
DISCUSSION
In this report, murine models of ß-thalassemia and SCA have been characterized and the comparative effects of a novel erythropoietin receptor agonist fusion protein and darbepoetin-α described. Although both test articles were active in normal mice at the doses tested, their effects were attenuated in the models and only CNTO 530 increased RBC and Hgb. CNTO 530 also increased expression of HbF in the SCA mice. Although the increase in HbF was small, in SCA, patients with HbF levels lower than 8-9% are more anemic than those with HbF levels higher than 8-9% [12] suggesting that relatively small increases in expression of HbF may be beneficial.
Prior to embarking on the pharmacodynamic experiments, the anemia in Th3+/C57Bl/6 and Berkeley mice was characterized. Originally described by Yang et al. [9], Th3+/C57BL/6 mice are heterozygous for a deletion of both the beta-1 and beta-2 globin gene. As shown in (Table 1), these mice showed a decrease in RBC, Hgb, Hct, MCV and MCH and an increase in HDW. Examination of peripheral blood smears showed Howell-Jolly bodies, target cells, and moderate anisocytosis. Additionally, the mice were splenomegalic. WBC indices were modestly elevated while platelet indices were unremarkable. These findings are similar to those described in human ß-thalassemia intermedia/major [4]. Berkeley mice were originally described by Pászty et al. [10]. These mice are knocked out for murine alpha and beta globin and are transgenic for human alpha, beta(sickle) and gamma globin genes. Thus, they express human hemoglobin A (HbAsickle) (or sickle hemoglobin, HbS) and can also express human HbF. As shown in (Table 1), these mice showed a marked reticulocytosis, decreased RBC, Hgb and Hct and decreased MCH. Examination of peripheral blood smears showed numerous target cells, fragmented red cells, and sickle cells. In addition, the mice were splenomegalic and WBC and platelet indices were markedly affected. These findings are similar to those described in human SCA [5]. Thus, Th3+/C57Bl/6 and Berkeley mice were considered good models to test the effects of CNTO 530.
In embryonic and fetal life, alternate forms of Hgb, HbE and HbF are expressed. These forms of Hgb are composed of α-type globin chains and the embryonic (epsilon-globin) or fetal (gamma-globin) chains [13]. Although these forms of Hgb predominate in embryonic and fetal life, respectively, they are sequentially down regulated and HbE is normally not expressed and HbF is expressed at low levels postnatally. In Berkeley mice, the human fetal Gγ- and Aγ-globin genes were inserted as a 39kb Kpn1 fragment (with the human adult δ and βS globin genes) in their normal genomic context [10]. As reported by Pászty et al. [10], newborn Berkeley mice expressed appreciable amounts of human HbF and trace amounts of HbE but as adults HbF and HbE were not detected. In contrast, in our study trace amounts of HbF were detectable in control adult Berkeley mice using ion exchange chromatography. Increased expression of HbF is considered important as these forms of Hgb can alleviate anemia in β-thalassemia and SCA and may decrease sickle crisis [14, 15]. Erythropoietin receptor agonists either alone, or more commonly in combination with hydroxy urea have been shown to have beneficial activity in ß-thalassemia and SCA, [16-21]. This is probably, at least in part, through increased expression of HbF [22-25], but others have failed to find an effect of rhEPO on HbF expression [26].
The mechanism by which CNTO 530 increases HbF in Berkeley mice is unknown. As reviewed by Mahjan et al. [27], although control of gamma globin gene expression has been the subject of intensive study the normal control mechanisms remain incompletely understood. Moreover, as reviewed by Mabaera et al. [14], neither the molecular mechanisms nor target molecules for any of the over 70 pharmacologic agents that can increase expression of HbF have been verified. Regarding EPO-R agonists, in addition to its well known growth factor, anti-apoptotic and differentiation effects on early erythroid precursors [28, 29], EPO has also been shown to express a number of other effects including cytoprotective [30] and improved iron and glucose metabolism [31, 32].
Although CNTO 530 utilizes the same receptor and signal transduction systems as epoetin-α [2], the EMP-1 pharmacophore of CNTO 530 engages EPO-R in a manner distinct from epoetin-α[33]. Thus, CNTO 530 may have novel effects beyond its function as a long-lived EPO-R agonist. This hypothesis is supported by the work of Sathyanarayana et al. [34]_ENREF_34 who showed that CNTO 530 increased expression of podocalyxin X by erythroblasts and caused a sustained expansion of a KitnegCD71highTer119neg erythroid progenitor pool in murine bone marrow. Thus, the effects of CNTO 530 in β-thalassemic and SCA mice may depend on a unique engagement of the EPO-R resulting in pharmacologic effects beyond stimulation of erythropoiesis.
CONCLUSIONS
In conclusion, this single dose pharmacodynamic study has shown that CNTO 530 is active in murine models of β-thalassemia and SCA. CNTO 530 showed superior activity compared to darbepoetin-a and therefore may have other beneficial effects in addition to being a long-lived EPO-R agonist. Thus, testing CNTO 530 in β-thalassemia and SCA may be warranted.
Fig. (5).
Ion Exchange Chromatographic Analysis of comparative effects of CNTO 530 and darbepoetin-α on Hgb from Berkeley mice on Day 10. (A) Total Hgb and (B) HbF. Flow cytometric analysis of HbF+ retics and RBC in Berkeley mice on Day 10. Representative results are shown for control and each treatment. Retics were gated based on staining with thizole orange (Quadrants 1 and 2). HbF+ retics (Quadrant 2) and RBC (Quadrant 3) were gated based on staining for HbF. Numbers in corners are mean fluorescence intensity (MFI). (C) PBS Control (D) CNTO 530 (E) Darpepoetin-α. Comparative effects of CNTO 530 on (F) retics stained for HbF and (G) RBC stained for HbF.
ACKNOWLEDGEMENTS
The authors thank Patricia Rafferty for her excellent technical expertise.
DISCLOSURE STATEMENT
This work was supported by Centocor R&D, a Division of JJPRD LLC and Centocor R&D discovered CNTO 530. All authors with the exception of Denise Chroscinski are employees of Centocor R&D and hold an equity stake in Johnson & Johnson, the parent of Centocor R&D.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur. J. Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 2.Bugelski PJ, Capocasale RJ, Makropoulos D, Marshall D, Fisher PW, Lu J, Achuthanandam R, Spinka-Doms T, Kwok D, Graden D, Volk A, Nesspor T, James IE, Huang C. CNTO 530: molecular pharmacology in human UT-7EPO cells and pharmacokinetics and pharmacodynamics in mice. J. Biotechnol. 2008;134(1-2):171–80. doi: 10.1016/j.jbiotec.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DL, Farrell FX, Barbone FP, McMahon FJ, Tullai J, Hoey K, Livnah O, Wrighton NC, Middleton SA, Loughney DA, Stura EA, Dower WJ, Mulcahy LS, Wilson IA, LKJ Identification of a 13 amino acid peptide mimetic of erythropoietin and description of amino acids critical for the mimetic activity of EMP1. Biochemistry. 1998;37:3699–3710. doi: 10.1021/bi971956y. [DOI] [PubMed] [Google Scholar]
- 4.Cao A, Galanello R. Beta-thalassemia. Genet. Med. 2010;12(2):61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg MH. Pathophysiology of sickle cell disease. Baillieres Clin. Haematol. 1998;11(1):163–184. doi: 10.1016/s0950-3536(98)80074-7. [DOI] [PubMed] [Google Scholar]
- 6.Ballas S, Marcolina M. Determinants of red cell survival and erythropoietic activity in patients with sickle cell anemia in the steady state. Hemoglobin. 2000;24(4):277–286. doi: 10.3109/03630260008993134. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi C, Schechter A, Rodgers G. Sickle cell disease pathophysiology. Baillieres Clin. Haematol. 1993;6(1):57–91. doi: 10.1016/s0950-3536(05)80066-6. [DOI] [PubMed] [Google Scholar]
- 8.Al-Khatti A, Veith RW, Papayannopoulou T, Fritsch EF, Goldwasser E, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis by erythropoietin in baboons. N. Engl. J. Med. 1987;317(7):415–420. doi: 10.1056/NEJM198708133170704. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for β-thalassemia. Proc. Natl. Acad. Sci.USA. 1995;92:11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pászty C, Brion C, Manci E, Witkowska H, Stevens M, Mohandas N, Rubin E. Transgenic knockout mice with exclusively human hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 11.Morin L, Barton E. Improved separation of fetal hemoglobin by mini-colum chromatography. Clin. Chem. 1987;33(2):288–289. [PubMed] [Google Scholar]
- 12.Croizat H, Nagel R. Circulating cytokines response and the level of erythropoiesis in sickle cell anemia. Am. J. Hematol. 1999;60(2):105–115. doi: 10.1002/(sici)1096-8652(199902)60:2<105::aid-ajh4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Testa U. Fetal hemoglobin inducers for treatment of hemoglobinopathies. Ann. Hematol. 2009;88(6):505–528. doi: 10.1007/s00277-008-0637-y. [DOI] [PubMed] [Google Scholar]
- 14.Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, Lowrey CH. A cell stress signaling model of fetal hemoglobin induction: what doesn't kill red blood cells may make them stronger. Exp. Hematol. 2008;36(9):1057–1072. doi: 10.1016/j.exphem.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Rachmilewitz E A, Aker M. The role of recombinant human erythropoietin in the treatment of thalassemia. Ann. N. Y. Acad., Sci. 1998;850:129–138. doi: 10.1111/j.1749-6632.1998.tb10470.x. [DOI] [PubMed] [Google Scholar]
- 16.el-Hazmi MA, al-Momen A, Warsy AS, Kandaswamy S, Huraib S, Harakati M, al-Mohareb F. The pharmacological manipulation of fetal haemoglobin: trials using hydroxyurea and recombinant human erythropoietin. Acta Haemato. 1995;93(2-4):57–61. doi: 10.1159/000204112. [DOI] [PubMed] [Google Scholar]
- 17.Loukopoulos D, Voskaridou E, Stamoulakatou A, Papassotiriou Y, Kalotychou V, Loutradi A, Cozma G, Tsiarta H, Pavlides N. Hydroxyurea therapy in thalassemia. Ann. NY. Acade. Sci. 1998;850:120–128. doi: 10.1111/j.1749-6632.1998.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 18.Breymann C, Fibach E, Visca E, Huettner C, Huch A, Huch R. Induction of fetal hemoglobin synthesis with recombinant human erythropoietin in anemic patients with heterozygous beta-thalassemia during pregnancy. J. Matern. Fetal Med. 1999;8(1):1–7. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<1::AID-MFM1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Little JA, McGowan VR, Kato GJ, Partovi KS, Feld JJ, Maric I, Martyr S, Taylor JGT, Machado RF, Heller T, Castro O, Gladwin MT. Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica. 2006;91(8):1076–1083. [PMC free article] [PubMed] [Google Scholar]
- 20.Perrine SP. Fetal globin stimulant therapies in the beta-hemoglobinopathies: principles and current potential. Pediatr. Ann. 2008;37(5):339–346. doi: 10.3928/00904481-20080501-10. [DOI] [PubMed] [Google Scholar]
- 21.Schettler V, Wieland E. A case report of darbepoetin treatment in a patient with sickle cell disease and chronic renal failure undergoing regular hemodialysis procedures that induce a dose-dependent extension of blood transfusion intervals. Ther. Apher. Dial. 2009;13(1):80–82. doi: 10.1111/j.1744-9987.2009.00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Stamatoyannopoulos G, Umemura T, al-Khatti A, Spadaccino E, Abels RI, Fritsch EF, Papayannopoulou T. Modulation of HBF production by erythropoietin. Prog. Clin. Biol. Res. 1989;316B:269–280. [PubMed] [Google Scholar]
- 23.Nagel RL, Vichinsky E, Shah M, Johnson R, Spadacino E, Fabry M E, Mangahas L, Abel R, Stamatoyannopoulos G. F reticulocyte response in sickle cell anemia treated with recombinant human erythropoietin: a double-blind study. Blood. 1993;81(1):9–14. [PubMed] [Google Scholar]
- 24.Rodgers GP, Dover GJ, Uyesaka N, Noguchi CT, Schechter AN, Nienhuis AW. Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease. N. Engl. J. Med. 1993;328 (2):73–80. doi: 10.1056/NEJM199301143280201. [DOI] [PubMed] [Google Scholar]
- 25.Bourantas K, Economou G, Georgiou J. Administration of high doses of recombinant human erythropoietin to patients with beta-thalassemia intermedia: a preliminary trial. Eur. J. Haematol. 1997;58(1):22–25. doi: 10.1111/j.1600-0609.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg MA, Brugnara C, Dover GJ, Schapira L, Charache S, Bunn HF. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N. Engl. J. Med. 1990;323(6):366–372. doi: 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan M, Karmakar S, Weissman S. Control of beta globin genes. J. Cell. Biochem. 2007;102:801–810. doi: 10.1002/jcb.21507. [DOI] [PubMed] [Google Scholar]
- 28.Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18(7):1176–1199. doi: 10.1038/sj.leu.2403383. [DOI] [PubMed] [Google Scholar]
- 29.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15(3):146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Joyeux-Faure M. Cellular protection by erythropoietin: new therapeutic implications? J. Pharmacol. Exper. Ther. 323(3):759–762. doi: 10.1124/jpet.107.127357. [DOI] [PubMed] [Google Scholar]
- 31.Allegra V, Mengozzi G, Martimbianco L. Early and late effects of erythropoietin on glucose metabolism in maintenance hemodialysis patients. Am. J. Nephrol. 1996;16:304–308. doi: 10.1159/000169014. [DOI] [PubMed] [Google Scholar]
- 32.Robach P, Recalcati S, Girelli D, Gelfi C, Aachmann-Andersen N, Thomsen J, Norgaard A, Alberghini A, Campostrini N, Castagna A, Viganò A, Santambrogio P, Kempf T, Wollert K, Stephane Moutereau S, Carsten Lundby C, Gaetano Cairo G. Alterations of systemic and muscle iron metabolism in human subjects treated with low-dose recombinant erythropoietin. Blood. 2009;113:6707–6715. doi: 10.1182/blood-2008-09-178095. [DOI] [PubMed] [Google Scholar]
- 33.Livnah O, Johnson D, Stura E, Farrell F, Barbone F, You Y, Liu K, Goldsmith M, He W, Krause C, Pestka S, Jolliffe L, Wilson I. An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat. Struc. Biol. 1998;5:993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- 34.Sathyanarayana P, Houde E, Marshall D, Volk A, Makropoulos D, Emerson C, Pradeep A, Bugelski P J, Wojchowski D M. CNTO 530 functions as a potent EPO mimetic via unique sustained effects on bone marrow proerythroblast pools. Blood. 2009;113(20):4955–4962. doi: 10.1182/blood-2008-08-172320. [DOI] [PMC free article] [PubMed] [Google Scholar]