Abstract
Background
gonorrhoeae, a sexually transmitted disease caused by Neisseria gonorrhea for which humans are the only natural host. The causative organism is highly adapted to the genital tract and often causing asymptomatic and undetected infection in females in which Acquisition of gonococcal infection late in pregnancy can adversely affect labor and delivery as well as the well-being of the fetus. The aims of this study were to determine the prevalence and drug susceptibility pattern of Neisseria gonorrhea among symptomatic women in Hawassa Referral Hospital.
Methods
A cross-sectional study was conducted from December 1 2010 to February 30, 2011 at Hawassa Referral Hospital. All women who visited gynecology outpatient department (OPD) with suspected gonococcal infection were included. Endocervical swab was collected by the attending physician. The presence of gonorrhea was confirmed by culture, gram staining and biochemical tests. Antimicrobial sensitivity test was performed using disc diffusion method and the result was interpreted accordingly.
Results
Of the total 215 cases examined, 11 (5.1%) were confirmed to have gonococcal infection. Although not statistically significant, most of the cases 5/11 (45.5%) were in age group of 20–24 years and the identified organism had low level susceptibility to quinolones (ciprofloxacin 55%, ofloxacin 64% & lomefloxacin 64%).
Conclusion
Despite low rates of gonorrhea infection, it is important to focus on high-risk populations (reproductive age group) because of the great physical and emotional costs of the disease. A high resistance for quinolones, the commonly used antibiotics was observed for this laboratory-based diagnosis is recommended.
Keywords: Neisseria gonorrhea, drug susceptibility, symptomatic women, Southern Ethiopia
Gonorrhoeae is a sexually transmitted disease caused by the bacteria Neisseria gonorrhea for which humans are the only natural host (1). The advent of sulfonamide in 1936 and penicillin in 1943 antibiotic therapy for the treatment of gonococcal infection led to a rapid decrease in gonorrhea prevalence. Then since the beginning of the 20th century, peaks of reported cases of gonorrhea occurred during World Wars I and II and following the “sexual liberation” of the late 1960s and early 1970s (2). In the late 1980s, with the onset of the HIV epidemic and a coincident widespread use of barrier contraceptives, the incidence of gonococcal infection again declined (1).
In the absence of a national gonococcus screening program, little is known about the prevalence of gonococcal infection in women of reproductive age group in Ethiopia. To our knowledge the last gonococcal study in reproductive age group of women in Ethiopia was done before 20 years (3). The STI surveillance system in the country is weak. The Integrated Disease Surveillance Team of the Ministry of Health in 2005 reported that there is under reporting of STI cases including gonorrhea in most part of the country. Except for the adult prevalence of HIV (4.7%) and syphilis (1.8%) there is no national estimate of other STIS including gonorrhea. This is because the pattern of reporting from health institutions is not uniform. Some health institutions report using syndrornic approach while others use etiologic approach (4).
The drug resistance varies greatly among countries. Therefore having prevalence's data as well as the drug susceptibility pattern within consecutive year is important especially for gonorrhea, the highly drug resistant bacteria (5). There is no up to date data about the prevalence and resistance pattern of gonorrhea in Ethiopia even if it is often incomplete due to; clinical presentation not specific enough for diagnosis based solely on symptoms also in lack of proper reporting mechanisms. The major objective of this study was to assess the prevalence of Neisseria gonorrhea and their antimicrobial susceptibility patterns among symptomatic women attending a gynecology outpatient department in Hawassa referral hospital.
Subjects and Method
Study Design and Area: A prospective cross-sectional study was conducted from December 1, 2010 to February 30, 2011 at Gynecology outpatient department (OPD) and Microbiology Laboratory of Hawassa Referral Hospital, Hawassa, Ethiopia.
Sample size was calculated based on the highest prevalence of gonorrhea estimate, 5–15% for all Africa and we used the highest 15% for this study(6). Expected margin of error (d) was 0.05 and confidence interval (z) was 95%. The calculated sample size was 215 (10% non-response rate inclusive) (7). All women of reproductive age group who attended gynecology out patient at Hawassa Referral Hospital with suspected STIs were included in the study.
Women of reproductive age group (15–44 years) with any one of the sign and symptom for STIs such as pain during sexual intercourse, a painful or burning sensation when urinating and abnormal vaginal discharge were included. Others with symptoms indicating development of Pelvic Inflammatory Disease (PID) like cramps and pain, bleeding between menstrual periods, vomiting, and fever were also included in the study.
Women who have no sign and symptom for STIs, women on recent antibiotic treatment, and those who were outside the reproductive age group were excluded from the study. Clinical examination was done by physician to all patients who were attending gynecological OPD.
All relevant data were obtained by attending physician and was transferred to the questionnaire prepared for this study. Two swabs were collected from each patient from endocervical canal by the physician one for gram stain and the other for culture. The samples were immediately delivered and inoculated to appropriate media in Microbiology Laboratory of Hawassa Referral Hospital. Amies transport media (Oxoid, Basingstoke, and Hampshire, UK, England) was used at times of delay.
While one of the two swabs taken from individual patient was used for gram stain the other was inoculated on to nonselective chocolate agar and selective agar modified Thayer-Martin medium (Oxoid, Basingstoke, and Hampshire, UK, England). Some fastidious strains, such as the arginine-, hypoxanthine-and uracil-requiring strains, are more susceptible to the concentrations of vancomycin or trimethoprim used in the selective media which can grow in nonselective chocolate agar. The inoculated plates were incubated at 37°C in a moist atmosphere enriched with CO2 5% using candle jar. N. gonorrhoeae produces small raised, grey shiny colonies on modified Thayer-Martin medium after overnight incubation (8).
Neisseria gonorrhea is differentiated from other Neisseria species, Moraxella species, Kingella species and other commensals based on the production of acid from glucose only and not from maltose, lactose, sucrose and fructose. Accordingly, the carbohydrate utilization test was done using API NH identification kit strips (Oxoid, Basingstoke, and Hampshire, UK, England). In general all positive cultures were identified by their characteristic appearance on the media, Gram staining reaction and confirmed by the pattern of biochemical reactions using the standard method.
Antimicrobial susceptibility was assessed by using Kirby-Bauer disk diffusion test, according to NCCLS (9). Gonococcal specimens were sub cultured from the selective primary medium to a chocolate agar to obtain a pure culture of the specimen. From a pure culture of 3–5 selected colonies of bacteria were transferred to a tube with a straight wire and prepared a suspension in 2.5 ml normal saline and incubated at 37°C until the turbidity of the suspension become adjusted to a McFarland 0.5. Sterile swab was used to distribute the bacteria evenly over the entire surface of chocolate agar. The susceptibility to the following antimicrobial agents (Oxoid) were assessed: penicillin (P 10 IU), tetracycline (TE 30µg), ciprofloxacin (CIP 5µg), ceftriaxone (CRO 30µg), cefixime (CFM 5µg), Cefoxitin (FOX 30µg), cefpodoxime (CPD 10µg), spectinomycin (SPT 100µg). The criteria used to select the antimicrobial agents tested were based on their availability and frequent prescriptions for the management of gonococcal infection.
Standard reference strain of Neisseria gonorrhoeae ATCC 49226 was used as recommended by the Clinical and Laboratory Standards Institute (CLSI) for QC of susceptibility testing of gonococcal isolates. Reference strain from the EHNRI laboratory stock was also used as a quality control throughout the study for culture and antimicrobial susceptibility testing.
Data entry and analysis was done using SPSS for Windows version 16.0. Prevalence rate was calculated for the positive cases of examined subjects and separately by age groups. Logistic regression and Fisher exact test analysis were used to estimate adjusted odds ratios. The level of significance was set at 0.05 in order to consider a p-value <0.05 as indicator of a statistically significant difference with 95% confidence.
This research project was approved by the Department of Medical Microbiology, Parasitology and Immunology Research and Ethical Review Committee, Faculty of Medicine; Addis Ababa University. Official permission from the study site was obtained. Written informed consent was obtained from study participants. The laboratory test results were given for participants and proper treatment was also delivered for positive individuals.
Results
Socio-Demographic Characteristics of The Study Population: Out of 1342 patients visited the Hawassa University Referral Hospitals gynecology OPD between December 1, 2010 and February 30, 2011, a total of 215(16.0%) patients were included in the study. On their visit, patients were interviewed and examined clinically. One hundred sixty three (75.8%) of the 215 cases were from urban and 52 (24.2%) from rural. The age ranged from 15 to 44 years with a mean of 25.51 years ± 7.45. Most of the study subjects, 118 (54.9%) were married, 72 (33.5%) were students in occupation, 92(44.2%) Protestants in religion and 68(33.5%) Sidama by ethnic group (Table 1).
Table 1.
Description of the demographic data of 215 patients investigated for gonococcal infection at Hawassa University Referral Hospitals, Hawassa, Ethiopia (Dec2010– Feb 2011).
| Socio-demographic characteristics | Category | Frequency | Percent |
| Age- group | 15–19 | 50 | 23.3 |
| 20–24 | 71 | 33 | |
| 25–29 | 34 | 15.8 | |
| 30–34 | 25 | 11.6 | |
| 35–39 | 23 | 10.7 | |
| 40–44 | 12 | 5.6 | |
| Address | Urban | 163 | 75.8 |
| Rural | 52 | 24.2 | |
| Marital status | Single | 81 | 37.7 |
| Married | 118 | 54.9 | |
| Divorced | 9 | 4.2 | |
| Widowed | 7 | 3.3 | |
| Occupation | Student | 72 | 33.5 |
| Gov.employees | 48 | 22.3 | |
| Farmers | 42 | 19.5 | |
| House wife | 18 | 8.4 | |
| Daily workers | 13 | 6 | |
| Merchants | 11 | 5.1 | |
| Ethnic group | Others | 11 | 5.1 |
| Sidama | 72 | 33.5 | |
| Amhara | 42 | 19.5 | |
| Oromo | 38 | 17.7 | |
| Wolayta | 33 | 15.3 | |
| Gurage | 11 | 5.1 | |
| Tigre | 10 | 4.2 | |
| Others | 9 | 4.2 |
®= Kembata, Yem, Silte, Gedio. Etc, Ω = Employee In Private Sector, No Occupation
Overall Prevalence of Gonococcal Infection:
Among 215casess, 11 (5.1%) were confirmed to have gonococcal infection. Of the 11 patients who were positive for gonococcal infection, 6(54.5%) were from rural, and 5(45.5%) were from urban setting. There is significant statistical association with living area (p= 0.026) and the odds of having gonorrhea infection for women living in rural was four-times higher than urban counter parts (OR = 4.12, (95% CI, 1.203, 14.121). Gonococcal infection was observed in 6/11 (54.5%) of married women and 5/11 (45.5%) of students by occupation. Of the 25 pregnant women included in the study, one (4.0%) was positive for Neisseria gonorrhoeae. Five of the 11 patients who were confirmed to have gonococcal infection were in age group of 20-24 years though not statistically significant (p>0.05) (Table 2 and fig 2).
Table 2.
Association between prevalence of gonococcal infection and demographic characteristics of 215 patients investigated for gonococcal infections at Hawassa University Referral Hospital, Hawassa, Ethiopia (Dec 2010–Feb 2011).
| Socio-demographic characteristics |
Category | NG | No NG | OR(95%CI) | Fisher's Exact Test |
| Age- group | 15–19 | 3 | 47 | 1 | 0.894 |
| 20–24 | 5 | 66 | 1.187 (0.27, 5.21) | ||
| 25–29 | 2 | 32 | 0.979 (0.155 , 6.195) | ||
| 30–34 | 0 | 25 | 0.000 (0.000) | ||
| 35–39 | 1 | 22 | 0.712 (0.070, 7.240) | ||
| 40–44 | 0 | 12 | 0.000 (0.000) | ||
| Address | Urban | 5 | 158 | 1 | 0.026 |
| Rural | 6 | 46 | 4.122(1.203, 14.121) | ||
| Marital status | Single | 5 | 76 | 1 | 0.565 |
| Married | 5 | 113 | 0.673 (0.188, 2.403) | ||
| Divorced | 1 | 8 | 1.900(0.197, 18.341) | ||
| Widowed | 0 | 7 | 0.000 (0.000) | ||
| Occupation | Student | 5 | 67 | 1 | 0.644 |
| Gov. employees |
1 | 47 | 0.285(0.032, 2.520) | ||
| Farmers | 2 | 40 | 0.670(0.124, 3.616) | ||
| House wife | 2 | 16 | 1.67(0.298, 9430) | ||
| Daily workers |
1 | 12 | 1.11(0.120,10.418) | ||
| Merchants | 0 | 11 | - | ||
| Religion | Others | 0 | 11 | - | |
| Protestant | 4 | 92 | 1 | 0.188 | |
| Orthodox | 3 | 53 | 2.314(0.499, 10.738) | ||
| Muslim | 2 | 36 | 1.704 (0.273, 10.623 | ||
| Catholic | 2 | 8 | 7.667(1.113, 52.796) | ||
| Others | 0 | 15 | 0 | ||
| Ethnic group | Sidama | 4 | 68 | 1 | 0.995 |
| Amhara | 3 | 39 | 1.308 (0.278, 6.148) | ||
| Oromo | 2 | 36 | 0.944 (0.165, 5.407) | ||
| Wolayta | 2 | 31 | 1.097 (0.191, 6.310) | ||
| Gurage | 0 | 11 | 0 | ||
| Tigre | 0 | 10 | 0 | ||
| Macroscopic examination of swab | Others | 0 | 9 | 0 | |
| Muco purulent |
8 | 101 | 1 | 0.042 | |
| Bloody swab |
3 | 87 | 2.409 (0.619, 9.378) | ||
| Whitish | 0 | 27 | 0 |
NG = Neisseria gonorrhoeae, No NG = no Neisseria gonorrhoeae, ®= Kembata, Yem, Silte, Gedio. Etc, Ω = Employee In Private Sector, No Occupation
The macroscopic examination of swab showed that 8/11 (72.7) positives were from mucopurulent swab and 3/11 were from bloody swab, however, no statically significance association was observed (OR =2.409, 95% CI (0.619, 9.378)).
Bacteriologic Examination: Of the 215 endocervical specimens examined by Gram stain, 21 (9.8%) were positive for either intra cellular or extra cellular gram negative diplococcic (GNDC). Among the 21 gram stain positive for GNDC, 19 were showed growth on media (Table 3).
Table 3.
Gram stain, Culture and Biochemical tests result of patients investigated for gonococcal infections at Hawassa University Referral Hospitals, Hawassa, Ethiopia (Dec2010–Feb 2011).
| Test | Gram stain |
Growth in chocolate |
Growth in MTM |
Oxidase test |
Oxidation of glucose only in API NH kit |
Isolated Neisseria gonorrhoeae |
| Positive | 21 | 23 | 16 | 15 | 11 | 11 |
| Negative | 194 | 192 | 199 | 8 | 4 | 204 |
| Total | 215 | 215 | 215 | 23 | 15 | 215 |
*MTM = modified Thayer-Martin medium, *API NH = Analytical profile index for identification of neisseriae and hemophilia
Of the 215 endocervical specimens cultured on chocolate and MTM, 16(7.4%) were positive on MTM and 23(10.7%) were positive on chocolate. Oxidase tests were done for all culture positives and for colony resembling Neisseria gonorrhea in chocolate agar even in the absence of growth in MTM. Among the 23 Oxidase tested, 15 (65.2%) were Oxidase positive and further gram staining was done from the colony and finally the confirmatory biochemical tests were done. In general, from a total of 16 culture positive on MTM and from 15 of Oxidase positives only 11 were isolated as Neisseria gonorrhea by further biochemical tests (Oxidation or utilization of carbohydrates) and reported as Neisseria gonorrhea (Table 4).
Table 4.
Antimicrobial Susceptibility Patterns of Neisseria gonorrhoeae from patients who visited gynecologic OPD at Hawassa University Hospitals, Hawassa, Ethiopia (Dec2010–Feb 2011)
| Organism | CRO | FOX | CIP | SPT | CFM | CPD | CTX | OFX | LOM | P | TE | |
| NG n=11 |
S | 11(100%) | 9(82%) | 6(55%) | 9(82%) | 11(100%) | 10(91%) | 10(91%) | 7(64%) | 7(64%) | 0 | 0 |
| I | - | 2(18%) | 3(27%) | 2(18%) | - | - | - | 2(18%) | 3(27%) | 2(18%) | 5(45%) | |
| R | - | 0 | 2(18%) | 0 | - | - | - | 2(18%) | 1(9%) | 9(82%) | 6(55%) |
S= Sensitive, I=Intermediate, R=Resistant, P: Penicillin, TE: Tetracycline, CIP: Ciprofloxacin CRO: Ceftriaxone; CTX: cefotaxime; FOX: Cefoxitin; CFM: cefixime; CPD: cefpodoxime; OFX: ofloxacin; LOM: lomefloxacin; SPT: spectinomycin
Antimicrobial Susceptibility Testing: The susceptibility patterns of isolated bacteria (n=11) was done against 11 antimicrobial agents by the agar disc diffusion technique. The sensitivity of gonococcal isolates ranges from 100% to Ceftriaxone and cefixime to 0 % to Penicillin and Tetracycline. The lowest susceptibility was observed for penicillin and Tetracycline. No resistance was found to Ceftriaxone and cefixime. However, low level of susceptibility to quinolones (ciprofloxacin 55.0%, ofloxacin 64.0% & lomefloxacin 64.0%), recommended in the national protocol as first-line antibiotics for gonorrhea treatment was observed. There was decreased susceptibility to spectinomycin as well (82%). As shown in Table 4, most of the isolates haven't shown multiple drug resistance 9/11 (81.8%) and none of the isolates were sensitive to all antibiotics. In this finding high level of resistance (82%) to Penicillin and (55%) to Tetracycline was observed.
Discussion
Gonococcal infection has a disproportionate impact on the health of women. In women, it is often chronic, presenting with vague or no symptoms, but may lead to severe complications such as chronic pelvic inflammatory disease, ectopic pregnancy, and infertility. Because of the lack of diagnostic and treatment facilities, limited opportunity for seeking medical care, and poor health-care-seeking behavior. The impact of gonococcal infection on ill health tends to be more severe among women (17).
In Ethiopia, twenty years back the prevalence of Neisseria gonorrhea was done on women attending gynecologic, obstetric and family planning clinics to correlate the serological diagnosis of gonorrheae with clinical evidence of pelvic infection. So comparison with results from this study is not easy as the methodology of the studies were unrelated (3).
The overall prevalence of Gonococcal infection among reproductive age group of women in this study is almost similar with findings from Laos 3.7% (10) and it is within the range of sub-Saharan African estimated report which is 2–15% (6).
The prevalence in this study is higher compared to other reports like Jordan 2.2% (11) and Vietnam 0.7% (12). The likely reason might be due to lack of differential diagnosis which can lead to increase number of untreated patient. As resistance was developed for most of the drugs ordered in syndromic management and consequently increase rate of transmission also lead to drug resistance.
On the other hand, the prevalence of gonococcal infection in our study was lower compared southern Mozambique (13), India (14) and Nigeria (15). This Variation In the prevalence of gonococcal infections in different area might offer an explanation for this difference. Besides these, today's treatment is almost universal, making the rigorous exclusion of gonorrhoeae, up to one-third of female gonorrhoeae contacts eventually found to be negative (16). Also it is known gonorrhoeae and drug resistance vary greatly among countries and in regions even in sub region of the developing world, because of sociodemographic factors, the treatment algorithm and the way the case diagnosed and treated varies in every region.
In our study, patients who came from rural areas had 4 fold increase risk of developing infections. This is because they lack treatment facilities, limited opportunities for seeking medical care and as they have poor health-careseeking behavior are less likely to be diagnosed, and treated effectively for gonococcal infection. Although socio-demographic factors have great influence in the prevalence of STDs in which most studies confirmed (18), in our finding the distribution of gonococcal infection to most sociodemographic factors have no statistical significant association (p>0.05).
Regarding age group, there is no statistically significant difference in the frequency of gonococcal infections among different age groups but, the highest prevalence was observed in age group 20–24 years. This might be because of the sexual active age groups are at risk of STIs and the unsafe sex practice might be higher in rural area where in our study the dominating study groups were living. The other reason for the highest burden among the young women could be due to the fact that young is at greater disadvantage due to the absence of information necessary for early recognition of disease symptoms (19). The other possible explanation, they might have practice of unsafe sex or they might be victim for rape, even though, our questionnaire hasn't addressed these factors are important for acquiring the infection.
The high level of resistance to penicillin and tetracycline found in our study has been widely reported throughout the world, USA (20), Australia (18) and Romania (21) due to emergence of penicillin resistant beta-lactamase producing strains. There was no resistance to ceftriaxone and cefixime. The possible explanation for this might be these drugs are expensive, not intensively used and not easily available outside the hospitals beyond this these drugs are newer compared to the others. The absence of resistance to third-generation cephalosporines (cefixime and ceftriaxone) in our study make these drugs excellent choices as first-line treatment.
According to syndromic case management principle set by Ministry of health (22) the drugs (ciprofloxacin, Tetracycline and spectinomycin) prescribed for patients suspected for gonococcal infections have shown resistance. Low level of susceptibility to quinolones (ciprofloxacin, ofloxacin & lomefloxacin), recommended in the national protocol as first-line antibiotics for gonorrhea treatment was almost similar with other studies like, USA (20) and Australia (18). And this is also in agreement with study done in other part of Ethiopia where most of the isolates were resistant to commonly used antibiotics (23, 24). This may be because of the intensive use of antimicrobial agent, easy availability and indiscriminate use of these drugs outside the hospitals, and many antibiotics are available over the counter for Self-medication.
The cephalosporin drugs; Cefixime, Ceftriaxone and cefpodoxime were effective antibiotics for the treatment of Neisseria gonorrhea which are responsible to cause endocervical infections. This might be because these agents are expensive and not commonly used. This is in agreement with study done in USA (25), Australian (18) and Romania (21). However, the present study showed a high level of resistance to ciprofloxacin compared to the study in Central African Republic, Cameroon, and Madagascar (26). It will be real that Scientists are worried gonorrhea will soon become untreatable with these antibiotics (25). This leads to conclusion that if the problem won't be attended to on time, it will become very difficult to treat the infection.
In conclusion despite low rates of gonorrhea infection, it is important to focus on high-risk populations (reproductive age group) because of the great physical and emotional costs of the disease. Future studies should focus on identifying behavioral or environmental factors to address differences in predictors within groups. Future studies to assess the resistance trends in Ethiopia and to allow timely revision of treatment protocols are highly recommended.
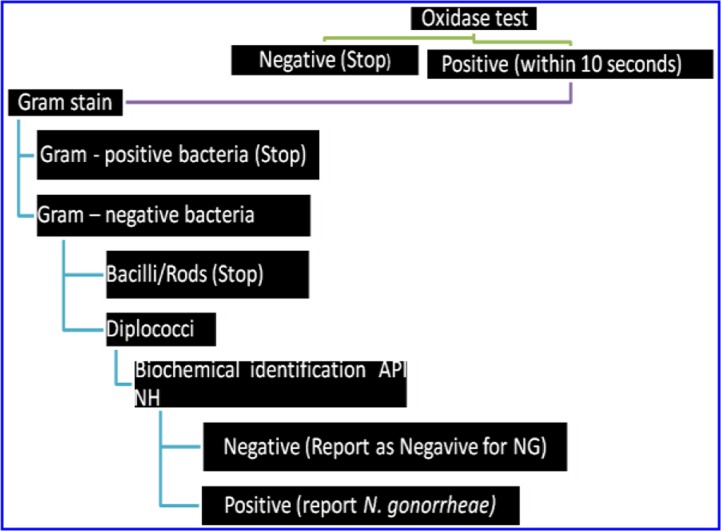
Figure 1.
Flow chart of Laboratory identification of bacteria
Acknowledgements
We wish to thank the staff of gynecology OPD for their support in identifying the target patients with careful clinical examination and sample collection also for their dedicated follow up and treatment of infected women. We thank EHNRI especially Mr. Surafel who supplied us control strains and other kits.
We greatly acknowledge the financial assistance of Addis Ababa University for covering the research.
We gratefully acknowledge Mr. Getahun Hailemeskel, head of Department of medical laboratory of Hawassa Referral Hospital and other staff of Hawassa referral hospital for their unreserved technical support and in facilitating good working environment.
References
- 1.Knapp, Rice R J. Neisseria and Branhamella. Principles and practice of infectious disease. 3rd ed. Churchill Livingstone, New York: 1995. pp. 324–340. [Google Scholar]
- 2.Aral S O, Holmes KK. Epidemiology of sexual behavior and sexually transmitted diseases. Sexually transmitted diseases. 3rd ed. New York: McGraw Hill; 1999. pp. 39–76. [Google Scholar]
- 3.Duncan ME, Reimann K, Tibaux G, Pelzer A, Mehari L, Lind I. Seroepidemiological study of gonorrhoea in Ethiopian women. Genitourin Med. 1991;67(6):493–497. doi: 10.1136/sti.67.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO, author. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections Overview and Estimates. 2005. http://www.who.int/reproductivehealth/publications/rtis/9789241502450/en/index.html. [PubMed] [Google Scholar]
- 5.WHO, author. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in WHO Western Pacific and South East Asian regions. Commun Dis Intell. 2010;34(1):1–7. http://www.ncbi.nlm.nih.gov/pmc/articles/PM C1195885/ [PubMed] [Google Scholar]
- 6.Gerbase A, Rowley TJ, Heymann LHD, et al. Global prevalence estimates of selected curable STDs. J Sex Transm Infect. 1998;74(suppl 1):S12–S16. [PubMed] [Google Scholar]
- 7.Wayne WD. Determination of sample sizes: Estimation. Biostastics for analysis in health sciences. 6th ed. 1998. pp. 180–181. [Google Scholar]
- 8.Janda WM, Knapp JS. Neisseria and Moraxella catarrhalis Manual of Clinical Microbiology. 8th edition. Washington: American Society Microbiology; 2003. pp. 585–608. [Google Scholar]
- 9.NCCLS. National Committee for Clinical Laboratory Standards, author. Performance standards of antimicrobial susceptibility. NCCLS approved standard. 2002. M 100 - 59. [Google Scholar]
- 10.Sihavong, Amphoy MD, Phouthavane, Traykhouane Reproductive Tract Infections among Women Attending a Gynecology Outpatient Department in Vientiane, Lao. J Sex Transm Dis. 2007;34(10):791–795. doi: 10.1097/01.olq.0000260918.82625.fd. [DOI] [PubMed] [Google Scholar]
- 11.Mahafzah AM, Al-Ramahi MQ, Asa'd AM, El-Khateeb MS. Prevalence of sexually transmitted infections among sexually active Jordanian females. J Sex Transm Dis. 2008;35(6):607–610. doi: 10.1097/OLQ.0b013e3181676bbd. [DOI] [PubMed] [Google Scholar]
- 12.Lan Pham Thi, Lundborg Cecilia Stålsby, Mogren Ingrid, Phuc Ho Dang, Chuc Nguyen Thi Kim. Reproductive tract infections in women seeking abortion in Vietnam. BMC Infect Dis. 2009;9(1):1–9. doi: 10.1186/1471-2334-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menéndez C, Castellsagué X, Renom M, et al. Prevalence and risk factors of sexually transmitted infections and cervical neoplasia in women from a rural area of southern Mozambique. Infect Dis Obstet Gynecol. 2010;11:60–93. doi: 10.1155/2010/609315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divekar AA, Gogate AS, Shivkar LK, Gogate S, Badhwar VR. Disease prevalence in women attending the STD clinic in Mumbai, India. Int J STD AIDS. 2000;11(1):45–48. doi: 10.1258/0956462001914896. [DOI] [PubMed] [Google Scholar]
- 15.Jatau, Galadima M, Odama LE, Kwaga Prevalence and antimicrobial susceptibility of Neisseria gonococcal isolated from patients in various locations of Kaduna state. Nigeria Nigerian journal of surgical research. 2003;5(1):50–56. [Google Scholar]
- 16.Barlow D, Nayyar K, Phillips I, Barrow J. Diagnosis of gonorrhoea in women. Br J Vener Dis. 1976;52(5):326–328. doi: 10.1136/sti.52.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson D, Abdool Karim SS, Harrison A, et al. Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Health Organ. 1999;77:22–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Bates J, Murphy D, Hicks V, et al. Annual report of the Australian Gonococcal Surveillance Programme. Commun Dis Intell. 2009;33(3):268–274. [PubMed] [Google Scholar]
- 19.Gross kurth, Mayaud P, Mosha F. Asymptomatic gonorrhoea and chlamydial infection in rural Tanzania. British Medical Journal. 1996;312:277–280. doi: 10.1136/bmj.312.7026.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, author. Gonococcal Isolate Surveillance Project (GISP), Annual Report. Atlanta, GA: U.S. Department of Health and Human Services; 2001. [2001]. Available at http://www.cdc.gov/std/gonorrhea/arg/gisp/d efault.htm. [Google Scholar]
- 21.Filipiuc S, Nicolae O, Luncă C, Iancu LS. The monitoring of Neisseria gonorrhoeae infection incidence and its resistance in Suceava County. Med Nat Iasi. 2009;113(4):1238–1242. [PubMed] [Google Scholar]
- 22.MOH, author. National guidelines for the management of sexually transmitted infections using the syndromic approach for Healthcare Facilities in Ethiopia [online] 2009. [ http://www.esog.org.et/gbv.pdf]
- 23.Tadesse A, Mekonnen A, Kassu A, Asmelash T. Antimicrobial sensitivity of Neisseria gonorrhoea in Gondar, Ethiopia. East Africa Med J. 2001;78(5):259–261. doi: 10.4314/eamj.v78i5.9050. [DOI] [PubMed] [Google Scholar]
- 24.Meless H, Abegaze B. Drug susceptibility of Neisseria isolates from patients attending clinics for sexually transmitted diseases in Addis Ababa, Ethiopian. East Afr Med J. 1997;74(7):447–449. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention, author. Guidelines for Treatment of Sexually Transmitted Diseases. Atlanta, Georgia: US Department of Health and Human Services; 2006. Nov, Sexually transmitted disease treatment guidelines, surveillance, 2005. [online] (MMWR 1998;47 [No. RR-1]) [Google Scholar]
- 26.Cao V, Ratsima E, Van Tri D, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae strains. Sex Transm Dis. 2008;35(11):941–945. doi: 10.1097/OLQ.0b013e31818318d8. [DOI] [PubMed] [Google Scholar]