Abstract
TNF-related apoptosis-inducing ligand (TRAIL) is a promising cytokine for killing tumor cells. However, a number of studies have demonstrated that different cancer cells resist TRAIL treatment and, moreover, TRAIL can promote invasion and metastasis in resistant cells. Here we report that TRAIL rapidly activates caspase-8 in a panel of non-small-cell lung carcinomas (NSCLCs). Adenocarcinomas derived from the lung in addition to high caspase-8 expression are characterized by increased expression of DR4 compared with adjacent non-neoplastic tissues. Blocking DR4 or lowering caspase-8 expression significantly reduced apoptosis in NSCLC cell lines, indicating the importance of DR4 and signifying that higher levels of caspase-8 in lung adenocarcinomas make them more susceptible to TRAIL treatment. Despite rapid and robust initial responsiveness to TRAIL, surviving cells quickly acquired resistance to the additional TRAIL treatment. The expression of cellular-FLIP-short (c-FLIPS) was significantly increased in surviving cells. Such upregulation of c-FLIPS was rapidly reduced and TRAIL sensitivity was restored by treatment with cycloheximide. Silencing of c-FLIPS, but not c-FLIP-long (c-FLIPL), resulted in a remarkable increase in apoptosis and significant reduction of clonogenic survival. Furthermore, chelation of intracellular Ca2+ or inhibition of calmodulin caused a rapid proteasomal degradation of c-FLIPS, a significant increase of the two-step processing of procaspase-8, and reduced clonogenicity in response to TRAIL. Thus, our results revealed that the upregulation of DR4 and caspase-8 expression in NSCLC cells make them more susceptible to TRAIL. However, these cells could survive TRAIL treatment via upregulation of c-FLIPS, and it is suggested that blocking c-FLIPS expression by inhibition of Ca2+/calmodulin signaling significantly overcomes the acquired resistance of NSCLC cells to TRAIL.
Keywords: TRAIL, DR4, c-FLIPS, calcium, calmodulin, lung adenocarcinoma
Chemo- and radiotherapy are two main regimens that are used for the treatment of lung cancers. Both these treatments primarily engage the mitochondrial apoptotic pathway. In contrast to small-cell lung carcinomas (SCLCs) that respond well to chemo- and radiotherapy, non-small-cell lung carcinomas (NSCLCs) are quite resistant to treatment. Thus, considering the poor responsiveness of NSCLC cells to conventional therapy, alternative pathways that trigger cell death might be beneficial for efficient killing of these tumors. In contrast to conventional therapeutic drugs, which trigger the DNA-damage response, TNF-related apoptosis-inducing ligand (TRAIL) binds to the surface death receptors (DRs), leading to the recruitment of caspase-8 and Fas-associated death domain (FADD), and formation of death-inducing signaling complex (DISC), where caspase-8 is autocatalytically processed and activates apoptotic signaling. Recruitment of cellular-FLICE inhibitory protein (c-FLIP) blocks the processing and activation of procaspase-8 and -10 at the DISC level, and inhibits cell death mediated by the DR4, DR5, CD95, and TNF-R1 DRs. To date, three c-FLIP isoforms, including c-FLIP-long (c-FLIPL), c-FLIP-short (c-FLIPS), and c-FLIPR, have been identified at the protein level1, 2, 3 and the increased expression of both FLIPL and c-FLIPS isoforms in some lung tumors was reported.4
A number of recent clinical trials in phases Ib and II demonstrate safety and efficiency of using TRAIL-related apoptogens (the recombinant ligand or agonist monoclonal antibodies) that trigger TRAIL receptors in combinatory treatment of advanced NSCLCs with conventional therapeutic drugs paclitaxel, carboplatin, and bevacizumab.5, 6, 7 However, in contrast to these publications, several other reports demonstrate that instead of inhibition of tumor growth in cells resistant to TRAIL-mediated apoptosis, this compound can promote tumor progression, invasion, and metastasis.8, 9, 10, 11, 12 Thus, questions concerning the mechanisms of the specific killing of different cancer cells with TRAIL and, more importantly, the promotion of survival of cancer cells in response to TRAIL treatment remain to be resolved.
We have previously reported that a number of SCLC cells are resistant to TRAIL treatment owing to the lack of caspase-8 expression, and restoration of this protease is sufficient to sensitize these cancer cells to TRAIL13. However, several studies have demonstrated that even in tumors expressing caspase-8 and all other components essential for DISC formation, TRAIL monotherapy is inefficient, probably due to high survival rates of tumor cells in response to this treatment. Therefore, different combinations of TRAIL with drugs were proposed to improve the apoptotic response.14 In this study, using a panel of NSCLC cell lines, we demonstrate that most of these cells quickly and efficiently die upon TRAIL treatment, and apoptotic response in all studied cell lines is dependent on the expression of DR4. Importantly, we found that in comparison to non-neoplastic tissues, lung adenocarcinomas express higher levels of DR4 and caspase-8, suggesting that TRAIL will preferentially kill these tumor cells. Nevertheless, using an in vitro model we demonstrate that in response to TRAIL, the surviving cells rapidly upregulate c-FLIPS and become resistant to the additional TRAIL treatment. In addition, we established that blockage of the Ca2+/calmodulin signaling pathway rapidly decreases the stability of c-FLIPS protein expression in NSCLC cells, which suggests that inhibition of this pathway could be a promising way for the efficient elimination of NSCLC cells in response to TRAIL treatment.
Results
Expression of DISC components and apoptotic cell death in NSCLC cells upon treatment with TRAIL
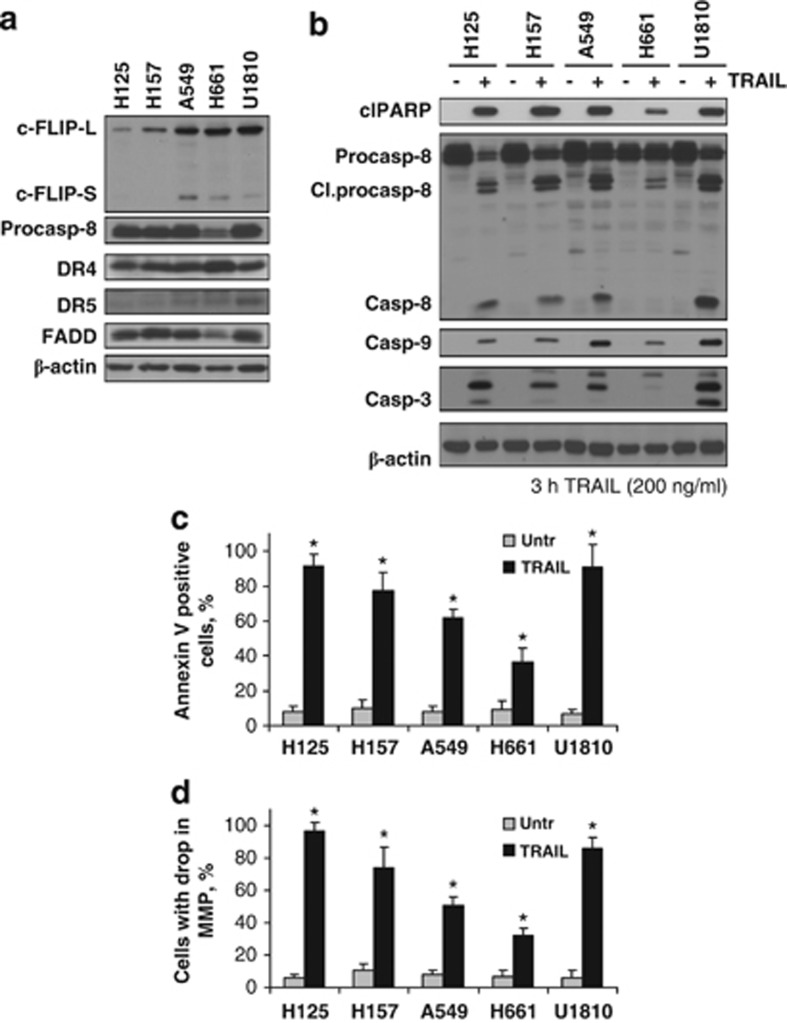
A number of studies have shown that activation of the TRAIL receptor pathway is a promising therapeutic strategy to eradicate selectively NSCLCs. Nevertheless, the resistance of cells to TRAIL-induced cell death occurs in most cases and is believed to be related to downstream factors. To evaluate susceptibility to treatment of NSCLC cells with TRAIL, expression of the key proteins involved in its signaling was analyzed in a panel of NSCLC cells (H125, H157, A549, H661, and U1810). The expression of procaspase-8, DR4 and DR5, and FADD, as well as c-FLIPL and c-FLIPS isoforms were examined by western blot analysis (Figure 1a). All cell lines exhibited relatively high levels of the proteins essential for DISC formation. In addition, both c-FLIPS and c-FLIPL levels were significantly higher in three out of five studied cell lines (A659, H661, and U1810). Despite relatively high levels of c-FLIPL expression, two cell lines, H125 and H157, completely lacked the expression of its short isoform (Figure 1a). Importantly, the majority of cell lines had very low (A549, H661, and U1810) or undetectable (H125 and H157) endogenous levels of DR5, whereas DR4 was expressed at high levels in all cell lines (Figure 1a).
Figure 1.
Expression of DISC components and apoptotic response in NSCLC cells upon treatment with TRAIL. (a) Expression of c-FLIPS, procaspase-8, DR4 and DR5, and FADD in a panel of NSCLC cells. (b) TRAIL-mediated activation of caspase cascade in NSCLC cells. NSCLC cells were treated with TRAIL (3 h, 200 ng/ml) and processing of procaspase-8 and formation of active forms of caspase-9 and -3 and specific cleavage (Cl) of PARP-1 were analyzed by immunoblot. (c and d) NSCLC cells were treated with TRAIL (24 h, 100 ng/ml) and MMP was assessed using TMRE staining. Apoptotic cell death was measured by Annexin V staining. Error bars represent S.E. *P<0.05
Further, we analyzed NSCLC cell lines for their sensitivity to TRAIL-mediated apoptosis. Treatment with TRAIL (3 h, 200 ng/ml) caused pronounced processing of caspase-8 and -3, as well as massive cleavage of poly(ADP)ribose polymerase (PARP)-1 in a panel of NSCLC cell lines (Figure 1b). Annexin V-based cell death assay showed that TRAIL efficiently killed 40% to over 90% of cells within 24 h of treatment (Figure 1c and Supplementary Figure 1). In addition, such treatment engaged the mitochondrial pathway and resulted in the cleavage of caspase-9 (Figure 1b). The drop of mitochondrial membrane potential (MMP) was observed in more than 40% of cells 24 h after treatment with TRAIL (Figure 1d), indicating that mitochondria signaling contributes to the TRAIL-induced cell death. Overall, these data demonstrate that NSCLC cell lines possess high sensitivity to apoptosis induction by TRAIL.
DR4 mediates apoptosis of NSCLC cells in response to TRAIL treatment
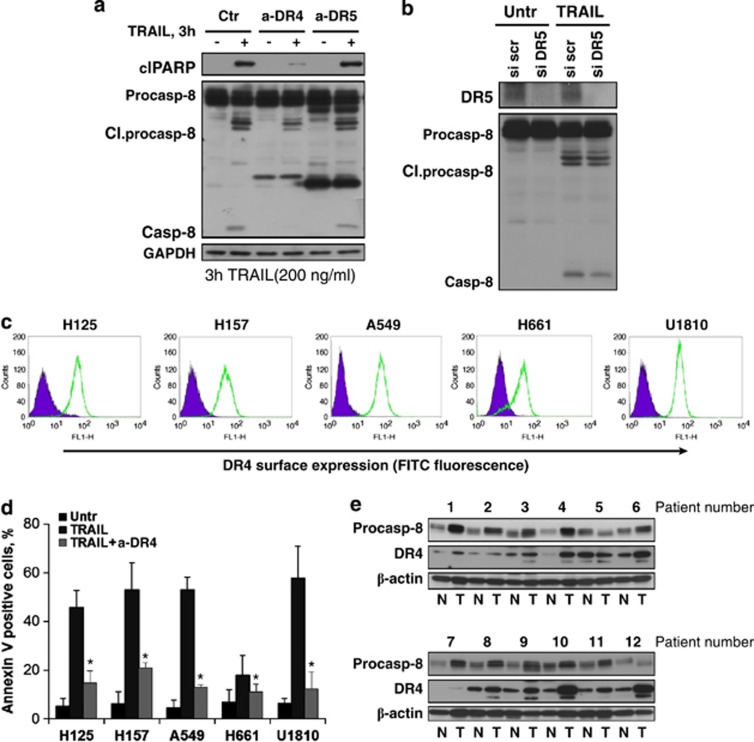
As TRAIL can induce apoptotic signaling via either of two DD-containing receptors, DR4 and DR5, we aimed to clarify which of them is essential for the transmission of the death signal upon TRAIL binding. Antagonizing antibodies directed against DR4 and DR5 were used in the A549 cell line to block receptors before treatment with TRAIL. Notably, blockage of DR4 but not DR5 resulted in a significant reduction in the TRAIL apoptotic response as assessed by a decrease in processing of caspase-8 and cleavage of PARP-1 (Figure 2a). In addition, silencing of DR5 did not affect caspase-8 activation (Figure 2b). Flow cytometry analysis revealed that DR4 was highly expressed at the cell surface in all studied cell lines (Figure 2c). Blockage of DR4 (but not DR5) almost completely abrogated apoptosis in all NSCLC cell lines in response to TRAIL treatment (Figure 2d and Supplementary Figure 2). To further validate the importance of DR4, its expression as well as expression of caspase-8 (which is often not expressed in SCLC cells) was analyzed in clinical samples. Western blot analysis revealed that several adenocarcinomas derived from the lung had much higher expression of both DR4 and caspase-8 compared with adjacent non-neoplastic tissues (Figure 2e). These data signify that DR4 but not DR5 determines the sensitivity of selected NSCLC cells to TRAIL-induced apoptosis. Importantly, high expression of DR4 and caspase-8 in lung adenocarcinomas could predispose susceptibility of these tumors to TRAIL-mediated therapy.
Figure 2.
DR4 mediates apoptosis of NSCLC cells in response to TRAIL treatment. (a) DR4 and DR5 in A549 cells were blocked using specific antagonizing antibodies for 30 min before TRAIL treatment (3 h, 200 ng/ml). Processing of procaspase-8 and cleavage (Cl) of PARP were analyzed by immunoblot. (b) DR5 was silenced in A549 cells using specific small interfering RNA (siRNA), and 48 h post-siRNA transfection, cells were treated with TRAIL (3 h, 200 ng/ml). (c) Expression of surface DR4 assessed by flow cytometry as described in Materials and Methods section. Filled histograms represent antibody control and green histograms represent staining with DR4 antibody. (d) Surface DR4 in a panel of NSCLC cells was blocked using a neutralizing antibody for 30 min prior TRAIL treatment (3 h, 200 ng/ml). Cell death was analyzed by Annexin V staining using flow cytometry. (e) Expression of procaspase-8 and DR4 in lung adenocarcinomas and adjacent non-neoplastic tissues was analyzed by immunoblot. Error bars represent S.E. *P<0.05. Ctr, control; a-DR, antagonizing DR4, or DR5 antibody; FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; N, normal tissue; T, tumor tissue; scr, scrambled; Untr, untreated
NSCLC cells that survived TRAIL treatment become resistant to its additional administration
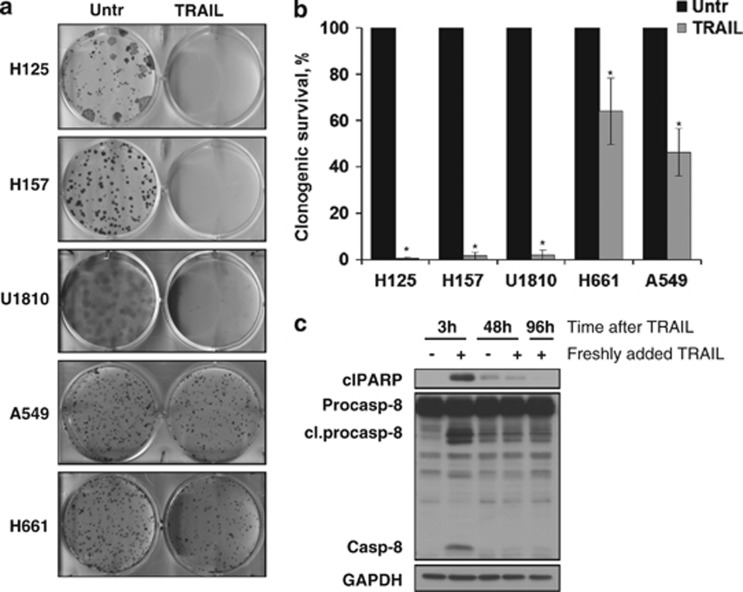
Although TRAIL efficiently triggered apoptosis in NSCLC cell lines, we observed that a number of cells survived this treatment. To further determine the response of these cells to TRAIL for long-term assessment of cell viability, the clonogenic assay has been performed. Using this assay, we found that only a small number of H157 and U1810 cells formed colonies, whereas more than 40% of cells in H661 and A549 cell populations survived TRAIL treatment (Figures 3a and b). Thus, despite an initial response, a significant amount of A549 and H661 cells retained the ability to survive and recovered after treatment with TRAIL. Moreover, using A549 cells we found that no caspase-8 activation and PARP-1 cleavage was detected when these cells were additionally treated with TRAIL at 48 and 96 h after initial treatment (Figure 3c).
Figure 3.
Survival of NSCLC cells in response to TRAIL treatment. (a and b) Cells were seeded in 6-well plates, and 24 h after the seeding, cells were treated with TRAIL (200 ng/ml). At 14 days after treatment, cells were fixed in paraformaldehyde and colonies were counted. (c) A549 cells were repeatedly treated for 3 h with TRAIL (200 ng/ml). At 48 and 96 h after the initial treatment, fresh TRAIL (200 ng/ml) was added for 3 h and cells were collected. Procaspase-8 processing and PARP-1 cleavage was analyzed by immunoblot. Error bars represent S.E. *P<0.05. Cl, cleaved; Unt, untreated
Upregulation of c-FLIPS is essential for clonogenic survival of NSCLC cells treated with TRAIL
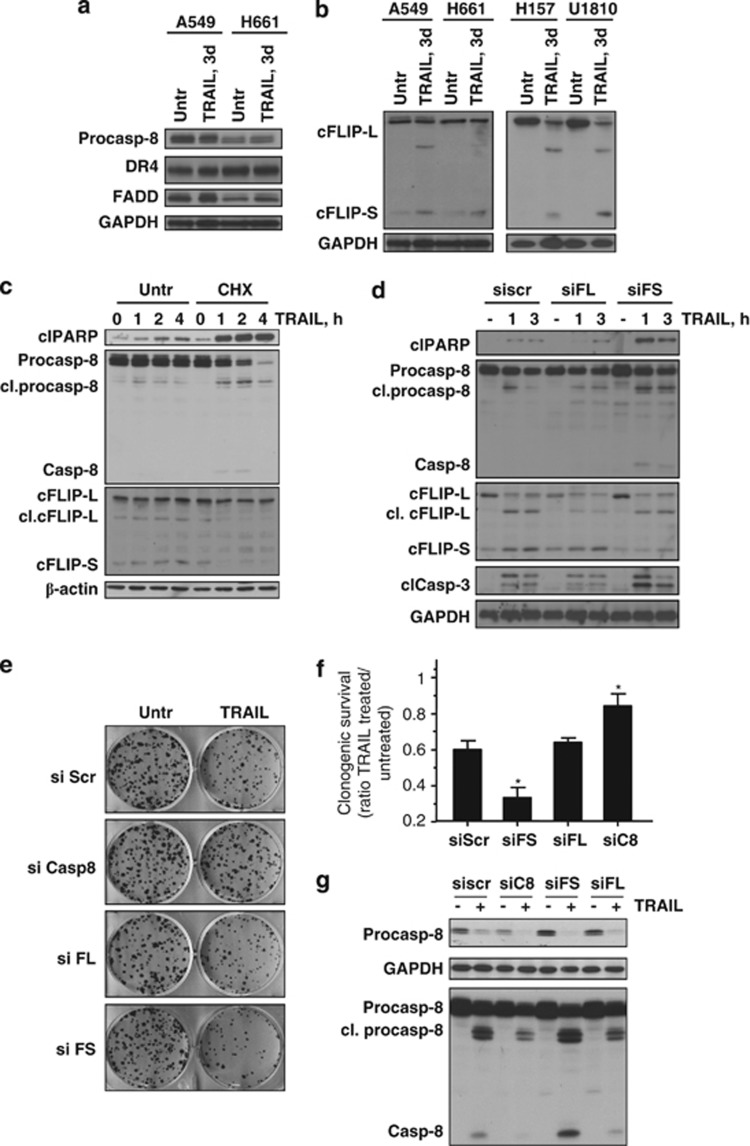
Our observation that only the NSCLC cells, which have high expression of c-FLIPS protein, were able to survive in clonogenic assay following TRAIL treatment suggested its possible contribution to survival. To clarify the molecular mechanism of acquired TRAIL resistance, the effect of prolonged treatment with TRAIL on the expression of main components involved in its signaling pathway was examined. Incubation of A549 and H661 cells in the presence of TRAIL (3 days) did not affect the expression of essential DISC components, such as caspase-8, DR4, and FADD (Figure 4a). However, the expression of c-FLIPS was significantly increased in response to TRAIL, which correlated with the survival of A549 and H661 cells. We decided to wash away dead cells and measure the level of c-FLIPS in three other NSCLC cell lines 3 days after incubation with TRAIL. Only one out of these cell lines (H125) completely died within 3 days. However, in the case of H157 and U1810 cell lines, enough cells survived to measure the expression of c-FLIPS. Although c-FLIPS protein expression was undetectable before TRAIL administration in these two cell lines, its expression was drastically increased in cells that survived TRAIL treatment (Figure 4b). Importantly, the level of c-FLIPL was not upregulated and even slightly reduced in some TRAIL-treated cells, most likely due to its proteolytic cleavage as documented by the appearance of its p43 fragment (Figure 4b).
Figure 4.
Upregulation of c-FLIPS is essential for clonogenic survival of NSCLC cells treated with TRAIL. (a and b) NSCLC cells were treated with TRAIL (3 days, 200 ng/ml) and DISC components were analyzed by immunoblot. (c) A549 cells that survived 3 days treatment with TRAIL were untreated (Untr) or treated with CHX (10 μg/ml) simultaneously with freshly added TRAIL (200 ng/ml). Processing of procaspase-8, expression of c-FLIPS, and formation of cleaved (Cl) PARP-1 were detected by immunoblot. (d) The effect of silencing of c-FLIPL and c-FLIPS on TRAIL-mediated activation of procaspase-8 and -3, and cleavage of PARP-1. (e and f) Clonogenic survival of A549 cells transfected with small interfering (si)RNAs targeting c-FLIPL, c-FLIPS, and procaspase-8. At 24 h after siRNA transfection, cells were seeded in 6-well plates, and 24 h after the seeding, cells were treated with TRAIL (200 ng/ml). Cell colonies were fixed and counted 14 days after the treatment. Ratio of the number of colonies in TRAIL-treated divided by the number of colonies in TRAIL-untreated samples for each specific siRNA is presented as bars in (f). (g) Processing of procaspase-8 in A549 cells transfected with siRNAs targeting procaspase-8, c-FLIPL, and c-FLIPS, and treated with TRAIL (200 ng/ml, 2 h). Cells from the same transfection experiment were used for clonogenic assay. Error bars represent S.E. *P<0.05. FADD, Fas-associated protein with death domain; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; scr, scrambled
As the expression of c-FLIPs is tightly regulated at the protein level, A549 cells that survived TRAIL treatment were subjected to additional incubation with TRAIL in the presence of the protein biosynthesis inhibitor cycloheximide (CHX) (10 μg/ml). Consequently, in the presence of CHX, the protein level of c-FLIPS (but not c-FLIPL) was rapidly reduced, which is in an agreement with a much shorter lifespan of c-FLIPS. Reduction of c-FLIPS was followed by the activation of caspase-8 and cleavage of PARP-1 (Figure 4c). To prove the role of different c-FLIP isoforms in cell death, A549 cells were transfected with siRNAs targeting either c-FLIPL or c-FLIPS, followed by treatment with TRAIL (200 ng/ml, 3 h). Downregulation of c-FLIPL had no effect on apoptotic response induced by TRAIL, whereas silencing of c-FLIPS resulted in a significant increase in caspase-8 and -3 activation, as well as PARP-1 cleavage upon treatment with TRAIL (Figure 4d and Supplementary Figure 3). Furthermore, A549 cells were examined for clonogenic survival after transfection with siRNAs targeting c-FLIPL, c-FLIPS, or caspase-8. No decrease in clonogenic survival was observed in cells after silencing of c-FLIPL, whereas partial reduction of caspase-8 expression inhibited TRAIL-induced loss in clonogenic survival (Figures 4e–g). In contrast, when A549 cells were transfected with siRNA against c-FLIPS, a significant reduction in survival following TRAIL exposure was observed (Figures 4e and f). Overall, these data indicate that upregulation of c-FLIPS results in complete inhibition of TRAIL cytotoxicity and is absolutely required to confer survival of NSCLC cells in response to TRAIL.
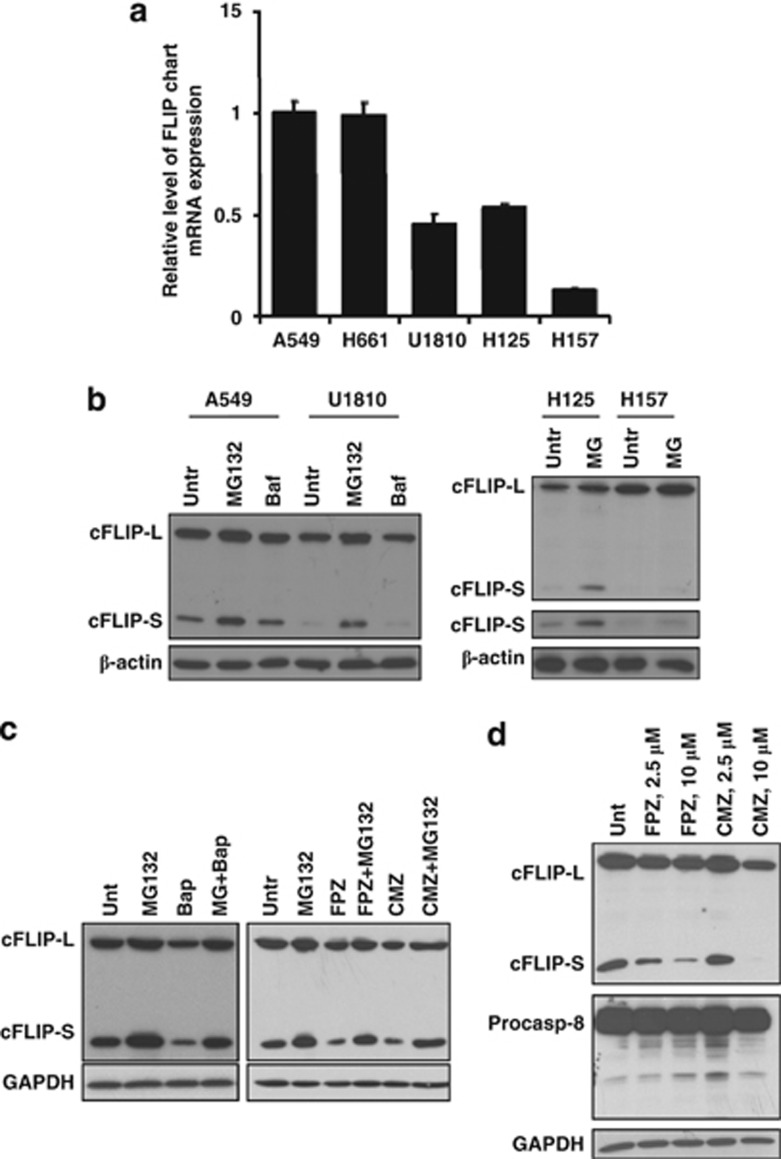
Difference in c-FLIPS mRNA expression and regulation of its degradation in NSCLC cells
To further establish the mechanisms responsible for different expression of c-FLIPS in a panel of NSCLC, we characterized the expression level of c-FLIPS mRNA in selected cell lines. c-FLIPS mRNA expression was significantly lower in U1810, H125, and H157 cells compared with A549 and H661, correlating with its protein expression (Figure 5a). Nevertheless, even cell lines with undetectable level of c-FLIPS protein (H125 and H157) expressed c-FLIPS mRNA, indicating that the low level of protein expression could be due to accelerated degradation rather than decreased protein synthesis of c-FLIPS. As c-FLIP degradation is catalyzed proteolysis within proteasomes, the cells were treated with the specific proteasome inhibitor MG132 (5 μM, 3 h) to assess the degradation rate of c-FLIPS in cells with different levels of its mRNA expression. MG132 application resulted in the inhibition of c-FLIPS degradation and its accumulation in cells expressing high and low levels of c-FLIPS mRNA (Figure 5b). Notably, inhibiton of lysosomal degradation by bafilomycin A1 (200 nM, 3 h) did not induce c-FLIPS protein accumulation (Figure 5b). These data indicate that the protein level of c-FLIPS is differentially regulated by the ubiquitin–proteasome pathway in NSCLC cells and in the presence of TRAIL lung cancer cells might upregulate c-FLIPS, leading to blockage of caspase-8 activation and apoptosis.
Figure 5.
Difference in c-FLIPS mRNA expression and regulation of its degradation in NSCLC cells. (a) The expression of c-FLIPS in NSCLC cells was analyzed by quantitative real-time polymerase chain reaction (PCR). (b) NSCLC cells were untreated (Untr) or treated with either inhibitor of proteasomal proteolysis MG132 (5 μM, 3 h) or bafilomycin A1 (Baf) (200 nM, 3 h). The expression of c-FLIPS was analyzed by immunoblot. (c) A549 cells were treated with MG132 (5 μM, 3 h) alone or in combination with Ca2+ chelator BAPTA-AM (10 μM, 3 h) or the inhibitors of calmodulin, CMZ (10 μM, 3 h) and FPZ (10 μM, 3 h). The expression of c-FLIPS was analyzed by immunoblot. (d) A549 cells were treated with calmodulin inhibitors and c-FLIPS and procaspase-8 expression were analyzed by immunoblot. Error bars represent S.E. GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Inhibition of Ca2+/calmodulin signaling rapidly decreases the expression of c-FLIPS, facilitates processing of caspase-8, and reduces survival of NSCLC cells in response to TRAIL
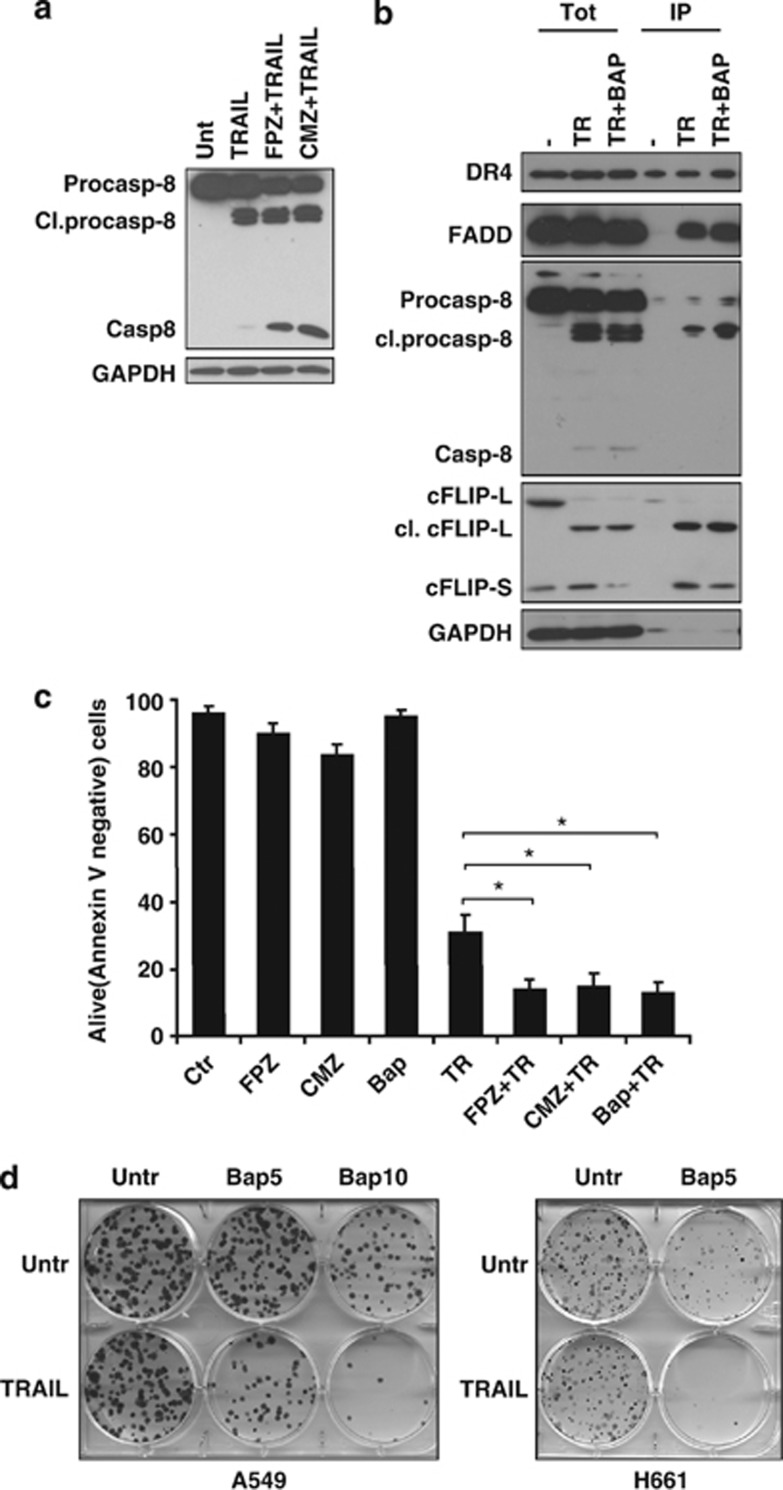
It has been reported that calcium signaling is impaired in lung cancer and the level of calmodulin is significantly increased compared with normal lung tissues. It is assumed that calmodulin has an important role in proliferation of lung cancer cells.15 Importantly, we observed that the protein level of c-FLIPS substantially decreased when A549 cells were treated with cytosolic Ca2+ chelator BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester) (10 μM, 3 h; Figure 5c). Furthermore, inhibition of proteasomal degradation with MG132 significantly restored the level of c-FLIPS protein, indicating the importance of calcium signaling in the blockage of c-FLIPS degradation in proteasomes (Figure 5c). Similarly, application of the inhibitor of calmodulin-regulated enzymes, calmidazolium chloride (CMZ, 10 μM, 3 h), as well as the potent irreversible inactivator of calmodulin, fluphenazine-N-2-chloroethane (FPZ, 10 μM, 3 h), resulted in a rapid and pronounced decrease of the protein level of c-FLIPS in A549, and the effect on c-FLIPS expression was attenuated in the presence of MG132 (Figure 5c). Importantly, downregulation of c-FLIPS was not accompanied by the activation of caspase-8 (Figure 5d).
Further, to establish whether blockage of Ca2+/calmodulin signaling will affect the sensitivity of NSCLC to TRAIL-induced cell death, A549 cells were pretreated with calmodulin inhibitors (10 μM, 1 h), followed by treatment with TRAIL (200 ng/ml, 2 h). Preincubation with either CMZ or FPZ resulted in a significant increase in caspase-8 processing, indicating their important contribution to TRAIL-induced apoptosis (Figure 6a). Immunoprecipitation analysis revealed that intracellular calcium chelation reduced the level of c-FLIPS and was clearly associated with higher processing of caspase-8 at the DISC (Figure 6b). Importantly, blockage of Ca2+/calmodulin signaling also considerably reduced the survival of NSCLC cells in response to TRAIL (200 ng/ml, 24 h) treatment measured by Annexin V staining (Figure 6c). These data were further supported by the results of the clonogenic assay. Thus, pretreatment with BAPTA-AM (5 or 10 μM, 30 min), followed by treatment with TRAIL (200 ng/ml, 3 h), resulted in a tremendous decrease in survival of NSCLC cells (Figure 6d), indicating that blockage of Ca2+/calmodulin signaling significantly reduces survival of NSCLC cells in response to TRAIL administration due to pronounced downregulation of c-FLIPS.
Figure 6.
Inhibition of Ca2+/calmodulin signaling reduces survival of NSCLC cells in response to TRAIL. (a) A549 cells were pretreated (1 h) with calmodulin inhibitor CMZ (10 μM) or FPZ (10 μM), followed by 2-h treatment with TRAIL (200 ng/ml). Processing of procaspase-8 was analyzed by immunoblot. (b) A549 cells were pretreated with calcium chelator BAPTA-AM (10 μM, 1 h), followed by 1-h treatment with biotinylated TRAIL (500 ng/ml). Protein complexes were precipitated using streptavidin agarose as described in Materials and Methods section. Biotinylated TRAIL was also added to untreated cell lysate at 500 ng/ml. DR4, FADD, c-FLIPS, and procaspase-8 cleavage were detected by immunoblot using specific antibodies. (c) A549 cells were pretreated with calmodulin inhibitors FPZ and CMZ or calcium chelator BAPTA-AM (10 μM, 1 h). To avoid toxicity, cells were washed and treated for an additional 24 h with TRAIL (200 ng/ml). Cell death was analyzed by flow cytometry using Annexin V staining. (d) The effect of calcium chelator BAPTA-AM on clonogenic survival of A549 and H661 cells. Cells were either untreated (Untr) or pretreated with BAPTA-AM (10 μM, 1 h), followed by TRAIL treatment (200 ng/ml, 3 h). Then, cells were washed and incubated for an additional 14 days and a clonogenic assay was performed as described above. Error bars represent S.E. *P<0.05. Cl, cleaved; FADD, Fas-associated protein with death domain; IP, immunoprecipitation; GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Discussion
Our findings demonstrate that most of NSCLC lines express high levels of caspase-8, FADD, and surface DR4, and that stimulation of these cells with TRAIL leads to their rapid apoptotic response. Furthermore, blockage of surface DR4 in NSCLC cells significantly reduces TRAIL-mediated apoptosis. Importantly, increased expression of DR4 was found in clinical samples obtained from patients with lung adenocarcinomas compared with non-neoplastic tissues, suggesting a correlation between expression of this DR and the response of these tumors to TRAIL treatment. Our idea that DR4 has a significant contribution to TRAIL-mediated apoptosis could be also supported by recent clinical studies demonstrating the efficiency of combinatory treatment of advanced NSCLCs with a DR4 agonist monoclonal antibody and carboplatin and paclitaxel or previously treated with platinum-based compounds.16 However, other clinical trials using the monoclonal agonist antibody that binds DR5 in patients with NSCLCs are also ongoing.7 Interestingly, our previous publication demonstrated that, in contrast to NSCLCs, most of the SCLC cell lines investigated (four out of five) do not express DR4 at the surface and the molecular mechanisms involved in DR4 surface expression in SCLC cells should be further investigated. The apoptotic response to TRAIL treatment in SCLC is mediated via DR5,17 supporting the notion that different approaches should be used in the treatment of SCLCs and NSCLCs. It should be noted that epigenetic silencing of DR4 was found in several tumor types, including melanomas, gliomas, and ovarian carcinomas, while DR5 expression in these tumors was not affected.18, 19, 20 Using various cancer cell models, several studies revealed different contributions of TRAIL DRs: DR5 contributes to TRAIL-mediated cell death in colon and breast cancer cell lines;21 however, chronic lymphocytic leukemia cells exhibit apoptotic signaling via DR4.22 On the basis of accumulated evidence from our and other groups, it seems that increased expression of DR4 might be a specific DR target in NSCLC cells.
Another important finding of our study is that treatment with TRAIL leads to massive processing of c-FLIPL and is accompanied by the upregulation of c-FLIPS expression. Using immunoprecipitation of DISC, we found that c-FLIPS and the cleaved form of FLIPL are present in a complex with DR4 and FADD. Interestingly, it was reported that procaspase-8 expression is increased in NSCLC and concomitant silencing of both c-FLIPL and c-FLIPS isoforms induced death of these cells via the TRAIL DR4 and DR5, but was not dependent on ligation of the receptors by TRAIL.4 In our experiments, single silencing of either c-FLIPL or c-FLIPS by itself did not cause any significant effect on apoptotic cell death. Furthermore, the obtained data demonstrate that c-FLIPL has no major impact on TRAIL-mediated apoptosis and clonogenic survival. In contrast to c-FLIPS, which has two death effector domains (DED), the FLIPL isoform in addition to DED domains also contains a caspase-like domain where the active-center cysteine residue is substituted by a tyrosine residue and both these forms potently inhibit apoptosis induced by DRs.1 Previously, it was also reported that c-FLIPL at physiologically relevant levels upon stimulation of CD95 enhanced procaspase-8 activation through heterodimerization at DISC and promoted apoptosis, while a decrease of its expression resulted in apoptosis inhibition.23 A more recent report described a mathematical model of CD95-induced apoptosis where c-FLIPL has a proapoptotic role only upon strong receptor stimulation or in the presence of high amounts of one of the short c-FLIP isoforms, c-FLIPS or c-FLIPR.24 This study also demonstrated that in contrast to the dual functions of c-FLIPL, overexpression of its short c-FLIPS and c-FLIPR isoforms blocked procaspase-8 cleavage at the DISC even after long stimulation of the CD95 receptor. Taken together, these reports support our findings that TRAIL-mediated upregulation of c-FLIPS blocked the processing of caspase-8 at DISC and promoted survival of NSCLC cells.
Previously, it was demonstrated that CHX sensitizes T cells to CD95-mediated apoptosis. Such treatment only slightly reduced c-FLIPL; however, c-FLIPS became almost undetectable, suggesting that c-FLIPS rather than c-FLIPL, confers resistance of T cells to CD95-mediated death in the context of immune responses.25 Stimulation of TCR/CD3 in human peripheral blood T lymphocytes mediated upregulation of c-FLIPS, which inhibited CD95-dependent apoptosis by blocking DISC activity.26 Another group has demonstrated that activated T cells upregulate the CD95 ligand; however, despite the expression of CD95 receptor, they remain resistant to apoptosis due to upregulation of c-FLIPS by the calcineurin/NFAT pathway.27 Moreover, c-FLIPS expression might also reduce cancer cell death,28, 29 which signifies the importance of our findings that upregulation of c-FLIPS in NSCLC cells in response to TRAIL is involved in the maintenance of survival. Furthermore, our data also demonstrate that the expression of c-FLIPS (but not c-FLIPL) is rapidly downregulated upon administration of CHX to the survived population of TRAIL-treated NSCLC cells, which strongly correlates with caspase-8 activation and execution of apoptosis. In fact, the more important finding is that downregulation of c-FLIPS correlates with clonogenic survival of NSCLC cells.
It has been shown that rapid turnover of c-FLIPS is determined by its unique C-terminal tail.30 Here we observed that inhibition of proteasomal, but not lysosomal, degradation is involved in c-FLIPS stabilization in NSCLC cells. Thus, even short (3 h) inhibition of proteasomal degradation in NSCLC cells with an undetectable level of c-FLIPS protein dramatically increased its expression/stabilization.
Potential involvement of Ca2+/calmodulin signaling in the regulation of c-FLIP expression has also been suggested. Thus, CAMK-II regulated c-FLIPL expression through the regulation of its stabilization by phosphorylation.31 Another publication demonstrated that protein-kinase-C-mediated S193 phosphorylation selectively affected the stability of the short c-FLIP and determined the sensitivity of cells to death-receptor-mediated apoptosis.32 Keeping in mind the few observations that the calmodulin level is significantly higher in lung cancers and its level correlates with the hystopathological grading and tumor staging,15 we inhibited Ca2+/calmodulin signaling and were able to demonstrate its involvement in the stability of c-FLIPS protein and survival of NSCLC cells in response to TRAIL administration. Using the inhibitor MG132, we confirmed that inhibition of Ca2+/calmodulin signaling promotes proteasomal degradation of c-FLIPS, but we were not able to detect phosphorylation of c-FLIPS using a phospho-(Ser/Thr)-specific antibody and, therefore, further studies are required to reveal the molecular mechanisms of its stabilization by Ca2+/calmodulin signaling. Similarly, the specific ubiquitin-ligase involved in ubiquitination and degradation of c-FLIPS needs to be determined. In addition, another study demonstrated a Ca2+-dependent direct interaction between calmodulin and c-FLIPL; however, no c-FLIPS was found in this complex.33 Thus, one can assume that – similar to the interaction with c-FLIPL – calmodulin might act together and inhibit the activity of other proteins, such as a specific ubiquitin ligase involved in the degradation of c-FLIPS in proteasomes. In this way, expression of calmodulin could predispose survival of NSCLC cells in response to TRAIL treatment.
Importantly, our observations demonstrate that besides reduction of procaspase-8, chelation of intracellular Ca2+ or inhibition of calmodulin significantly increases the amount of its active p18 domain. The formation of p18 caspase-8 domain resulted from the second-step cleavage of procaspase-8,34 suggesting that blockage of Ca2+/calmodulin signaling in response to DR4-mediated apoptosis also facilitates the second step of procaspase-8 activation or promotes stabilization of its active form.
Taken together, we demonstrate increased DR4 expression and its importance for the sensitivity of NSCLC cells to TRAIL treatment. Furthermore, we reveal that upregulation of c-FLIPS in response to TRAIL is a potential mechanism in the prevention of the apoptotic response and clonogenic survival of NSCLC cells. We propose that facilitation of proteasomal degradation of c-FLIPS in NSCLC cells by inhibition of Ca2+/calmodulin signaling is sufficient to reduce drastically survival of NSCLC cells in response to TRAIL treatment. Thus, specific inhibitors of calmodulin or calcium channels that reduce intracellular calcium levels might potentially be used persisting NSCLC cell survival in response to TRAIL treatment.
Materials and Methods
Cell culture and treatments
Human lung carcinoma A549 (ATCC; Manassas, VT, USA, CCL-185), H661 (ATCC; HTB-183), H157 (ATCC; CRL-5802), H125 (ATCC; CRL-5801), and U1810 cell lines (from the collection at the Uppsala University, Uppsala, Sweden) were cultured in RPMI 1640 (Sigma, Stockholm, Sweden) with 10% heat-inactivated fetal bovine serum, glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml) (all obtained from Gibco, Paisley, UK) at 37 °C, 5% CO2, and 95% humidity. Cells were seeded 24 h before treatment for the indicated time periods with TRAIL. Cells were treated with inhibitors of calmodulin (CMZ and FPZ), calcium chelator BAPTA-AM, protease inhibitor MG132, and inhibitor of proteosynthesis CHX as specified in the text.
Clinical material
Surgically resected specimens were collected from patients with lung adenocarcinomas at the Clinical Oncology Research Institute, NN Blokhin Russian Cancer Research Center during the period 2007–2009. After surgical removal, the tumor specimens were frozen and stored in liquid nitrogen. All patients signed informed consent forms according to the legal institutional guidelines and ethical permission. The tumor clinic morphological stages were determined according to the standard tumor TNM classification systems of the International Union Against Cancer (edition 7).
Clonogenic assay
For the clonogenic assay, cells were seeded in 6-well plates, and 24 h after seeding, cells were treated as described in the text and grown for an additional 14 days. Colonies were washed in PBS, fixed with 4% paraformaldehyde (20 min, RT), and stained with crystal violet solution (0.4% (w/v)).
Surface TRAIL receptor expression
The level of surface expressed TRAIL receptors was detected by flow cytometry as described previously. Briefly, after washing with PBS, cells were incubated (30 min, 4 °C) with primary antibody: anti-DR4 (Diaclone, Besancon, France; clone B-N36) antibody. After washing two times, cells were incubated (30 min, 4 °C) with secondary antibody (AlexaFluor488-conjugated donkey anti-rabbit IgG; Molecular Probes, Eugene, OR, USA). Then, cells were washed two times and stained (15 min, 4 °C) with 7-AAD (1 μg/ml; Molecular Probes) and analyzed using flow cytometry (FACScan; Becton Dickinson, San Jose, CA, USA). 7-AAD-negative cells were subjected to receptor analysis (Cell Quest software, San Jose, CA, USA). The results are expressed as histograms and related to appropriate controls lacking the specific primary antibody.
Immunoprecipitation of DISC
Ligand affinity precipitations of TRAIL/TRAIL-R complexes were performed using biotinylated TRAIL. A total of 5 × 106 cells were treated with bio-TRAIL (500 ng/ml, 2 h), washed two times with ice-cold PBS, and lysed in 500 μl buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, and 1% NP-40. The resulting protein complexes were precipitated from the lysates by incubation (4 h) with 30 μl streptavidin beads (Sigma) at 4 °C. To control for protein association to non-stimulated receptors, bio-TRAIL was added to the untreated lysates at 500 ng/ml. Streptavidin beads were washed five times and proteins eluted by the addition of 15 μl 2 × Laemmli buffer. Samples were boiled and expression of proteins was determined by western blot.
Annexin V/PI staining and assessment of mitochondrial membrane potential by FACS
Annexin V/PI double-staining was carried out using an Annexin-V-FLUOS Staining Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions.
For the assessment of mitochondrial membrane potential, cells were washed in PBS, incubated for 20 min at 37 °C with 25 nM of tetramethylrhodamine ethyl ester perchlorate (TMRE; Molecular Probes; T-669) in PBS, and subsequently analyzed by flow cytometry (FACScan; Becton Dickinson). Cells were analyzed by flow cytometry (FACScan; Becton Dickinson), and data were evaluated using the Cell Quest software.
RNA interference
Cells were seeded onto 6-well plates in growth media without antibiotics. Non-targeting siRNA, anti-caspase-8, anti-FLIPL, and anti-FLIPS siRNAs (all from Dharmacon, Lafayette, CO, USA) were diluted in 100 μl of OPTI-MEM (Gibco) and mixed with 5 μl of INTERFERin siRNA Transfection Reagent (Polyplus-transfection, Illkirch, France). After 10 min of incubation, the complexes were added to the cells. The final concentration of siRNA in the medium was 50 nM. At 24 h after transfection, the medium was replaced and treatments were administered. Otherwise, cells were re-seeded and the desired treatments were applied 24 h after the seeding.
Immunoblotting
Cells were lysed using Roche buffer, supplemented with protease inhibitor cocktail (Roche). The protein concentration was determined using the BCA protein assay (Pierce, Rockford, IL, USA). The samples were mixed with Laemmli buffer, boiled for 5 min, subjected to SDS-PAGE, and blotted onto nitrocellulose membrane (Bio-Rad, Munich, Germany), which was blocked for 1 h with 5% non-fat milk in PBS and probed with primary antibodies diluted in PBS containing 2% BSA (Sigma) and 0.05% Tween-20 (Sigma). The following antibodies were used: mouse anti-cleaved PARP-1, anti-cleaved caspase-3, anti-cleaved caspase-9 (all obtained from Cell Signaling Technology, Danvers, MA, USA), anti-DR4, anti-DR5 (Sigma), anti-GAPDH (Trevigen, Gaithersburg, MD, USA), rabbit anti-actin (Sigma; A2066), anti-c-FLIP and anti-caspase-8 (provided by Drs. I Lavrik and P Krammer), and anti-FADD (BD Biosciences, San Jose, CA, USA). The recognized proteins were detected using horseradish peroxidase-labeled secondary antibodies: anti-goat IG, anti-mouse IgG (Pierce), anti-rabbit IgG (Pierce), and an enhanced chemiluminescence kit (Amersham, Amersham Bioscience, Freiburg, Germany).
Real-time quantitative-PCR
Reversed-transcribed cDNA from the samples were used as templates. FLIPS (FS-1, 5′-GGGCCGAGGCAAGATAAGCAAGG-3′ and FS-2, 5′-TCAGGACAATGGGCATAGGGTGT-3′) and 18S ribosomal RNA (18S-1, 5′-CGCTACTACCGATTGGATGGTT-3′ and 18S-2, 5′-AGTCAAGTTCGACCGTCTTCTC-3′) primers (Invitrogen, Stockholm, Sweden) were designed to match the target cDNA sequence. In all, 20 ng of the reversed-transcribed cDNA template was mixed with SYBR Green PCR Master Mix (Applied Biosystems, Stockholm, Sweden) and amplified using a 7500 Real-Time PCR System (Applied Biosystems), with the following program: 40 cycles, with each cycle consisting of a denaturation step at 95 °C for 15 s and an annealing/extension step at 60 °C for 1 min. mRNA expression levels of each gene in the treated cells are presented as the fold-increase relative to untreated cells, after normalization against 18S RNA.
Statistical analysis
All the results are expressed as means±S.E. Statistical evaluation was performed using an unpaired t-test.
Acknowledgments
We thank Dr. Inna Lavrik and Prof. Peter Krammer for providing us with anticaspase-8 and c-FLIP antibodies. This study was supported by grants from the Swedish and Stockholm Cancer Societies, the Swedish Research Foundation, the Swedish Childhood Cancer Society, the European Union (Chemores and Apo-Sys), and the Russian Ministry of High Education and Science (11.G34.31.0006). VOK and OVS were supported by the Swedish Institute, Karolinska Institutet and Lindhes Advokatbyrå AB.
Glossary
- DISC
death-inducing signaling complex
- DR
death receptor
- FADD
Fas-associated death domain
- c-FLIP
FLICE-like inhibitory protein
- NSCLC
non-small-cell lung carcinoma
- PARP
poly(ADP)ribose polymerase
- TRAIL
TNF-related apoptosis-inducing ligand
- CHX
cycloheximide
- CMZ
calmidazolium chloride
- FPZ
fluphenazine-N-2-chloroethane
- BAPTA-AM
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Raschellá
Supplementary Material
References
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Redmond KM, McLaughlin KM, Crawford N, Gately K, O'Byrne K, et al. Procaspase 8 overexpression in non-small-cell lung cancer promotes apoptosis induced by FLIP silencing. Cell Death Differ. 2009;16:1352–1361. doi: 10.1038/cdd.2009.76. [DOI] [PubMed] [Google Scholar]
- Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010;28:1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Torres JMS, Diaz-Padilla I, Links M, Reguart N, Boyer M, et al. Safety and efficacy of AMG 655 in combination with paclitaxel and carboplatin (PC) in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol 20092719048(Meeting abstract). [Google Scholar]
- Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- Ehrenschwender M, Siegmund D, Wicovsky A, Kracht M, Dittrich-Breiholz O, Spindler V, et al. Mutant PIK3CA licenses TRAIL and CD95L to induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death Differ. 2010;17:1435–1447. doi: 10.1038/cdd.2010.36. [DOI] [PubMed] [Google Scholar]
- Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–G136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- Fingas CD, Blechacz BR, Smoot RL, Guicciardi ME, Mott J, Bronk SF, et al. A smac mimetic reduces TNF related apoptosis inducing ligand (TRAIL)-induced invasion and metastasis of cholangiocarcinoma cells. Hepatology. 2010;52:550–561. doi: 10.1002/hep.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi HC, Chen SL, Liao CJ, Liao CH, Tsai MM, Lin YH, et al. Thyroid hormone receptors promote metastasis of human hepatoma cells via regulation of TRAIL. Cell Death Differ. 2012;19:1802–1814. doi: 10.1038/cdd.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskyy VO, Surova OV, Vaculova A, Zhivotovsky B. Combined inhibition of DNA methyltransferase and histone deacetylase restores caspase-8 expression and sensitizes SCLC cells to TRAIL. Carcinogenesis. 2011;32:1450–1458. doi: 10.1093/carcin/bgr135. [DOI] [PubMed] [Google Scholar]
- Pintzas A, Zhivotovsky B, Linardopoulos S, Martinou JC, Lacal JC, Robine S, et al. Sensitization of (colon) cancer cells to death receptor related therapies: a report from the FP6-ONCODEATH research consortium. Cancer Biol Ther. 2012;13:458–466. doi: 10.4161/cbt.19600. [DOI] [PubMed] [Google Scholar]
- Liu GX, Sheng HF, Wu S. A study on the levels of calmodulin and DNA in human lung cancer cells. Br J Cancer. 1996;73:899–901. doi: 10.1038/bjc.1996.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61:82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Vaculova A, Kaminskyy V, Jalalvand E, Surova O, Zhivotovsky B. Doxorubicin and etoposide sensitize small cell lung carcinoma cells expressing caspase-8 to TRAIL. Mol Cancer. 2010;9:87. doi: 10.1186/1476-4598-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SI, Cheriyath V, Jacobs BS, Reu FJ, Borden EC. Reversal of methylation silencing of Apo2L/TRAIL receptor 1 (DR4) expression overcomes resistance of SK-MEL-3 and SK-MEL-28 melanoma cells to interferons (IFNs) or Apo2L/TRAIL. Oncogene. 2008;27:490–498. doi: 10.1038/sj.onc.1210655. [DOI] [PubMed] [Google Scholar]
- Elias A, Siegelin MD, Steinmuller A, von Deimling A, Lass U, Korn B, et al. Epigenetic silencing of death receptor 4 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in gliomas. Clin Cancer Res. 2009;15:5457–5465. doi: 10.1158/1078-0432.CCR-09-1125. [DOI] [PubMed] [Google Scholar]
- Horak P, Pils D, Haller G, Pribill I, Roessler M, Tomek S, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005;3:335–343. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]
- Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–782. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010;190:377–389. doi: 10.1083/jcb.201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz I, Weyd H, Krueger A, Baumann S, Fas SC, Krammer PH, et al. Resistance of short term activated T cells to CD95-mediated apoptosis correlates with de novo protein synthesis of c-FLIPshort. J Immunol. 2004;172:2194–2200. doi: 10.4049/jimmunol.172.4.2194. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Muller WW, Krueger A, Schmitz I, Krammer PH. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J Immunol. 2000;165:6293–6300. doi: 10.4049/jimmunol.165.11.6293. [DOI] [PubMed] [Google Scholar]
- Ueffing N, Schuster M, Keil E, Schulze-Osthoff K, Schmitz I. Up-regulation of c-FLIP short by NFAT contributes to apoptosis resistance of short-term activated T cells. Blood. 2008;112:690–698. doi: 10.1182/blood-2008-02-141382. [DOI] [PubMed] [Google Scholar]
- Bin L, Li X, Xu LG, Shu HB. The short splice form of Casper/c-FLIP is a major cellular inhibitor of TRAIL-induced apoptosis. FEBS Lett. 2002;510:37–40. doi: 10.1016/s0014-5793(01)03222-7. [DOI] [PubMed] [Google Scholar]
- Salon C, Eymin B, Micheau O, Chaperot L, Plumas J, Brambilla C, et al. E2F1 induces apoptosis and sensitizes human lung adenocarcinoma cells to death-receptor-mediated apoptosis through specific downregulation of c-FLIP(short) Cell Death Differ. 2006;13:260–272. doi: 10.1038/sj.cdd.4401739. [DOI] [PubMed] [Google Scholar]
- Poukkula M, Kaunisto A, Hietakangas V, Denessiouk K, Katajamaki T, Johnson MS, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005;280:27345–27355. doi: 10.1074/jbc.M504019200. [DOI] [PubMed] [Google Scholar]
- Yang BF, Xiao C, Roa WH, Krammer PH, Hao C. Calcium/calmodulin-dependent protein kinase II regulation of c-FLIP expression and phosphorylation in modulation of Fas-mediated signaling in malignant glioma cells. J Biol Chem. 2003;278:7043–7050. doi: 10.1074/jbc.M211278200. [DOI] [PubMed] [Google Scholar]
- Kaunisto A, Kochin V, Asaoka T, Mikhailov A, Poukkula M, Meinander A, et al. PKC-mediated phosphorylation regulates c-FLIP ubiquitylation and stability. Cell Death Differ. 2009;16:1215–1226. doi: 10.1038/cdd.2009.35. [DOI] [PubMed] [Google Scholar]
- Pawar PS, Micoli KJ, Ding H, Cook WJ, Kappes JC, Chen Y, et al. Calmodulin binding to cellular FLICE-like inhibitory protein modulates Fas-induced signalling. Biochem J. 2008;412:459–468. doi: 10.1042/BJ20071507. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19:36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.