Abstract
Neuroinflammation is a common feature of acute neurological conditions such as stroke and spinal cord injury, as well as neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis. Previous studies have demonstrated that acute neuroinflammation can adversely affect the survival of neural precursor cells (NPCs) and thereby limit the capacity for regeneration and repair. However, the mechanisms by which neuroinflammatory processes induce NPC death remain unclear. Microglia are key mediators of neuroinflammation and when activated to induce a pro-inflammatory state produce a number of factors that could affect NPC survival. Importantly, in the present study we demonstrate that tumor necrosis factor α (TNFα) produced by lipopolysaccharide-activated microglia is necessary and sufficient to trigger apoptosis in mouse NPCs in vitro. Furthermore, we demonstrate that microglia-derived TNFα induces NPC apoptosis via a mitochondrial pathway regulated by the Bcl-2 family protein Bax. BH3-only proteins are known to play a key role in regulating Bax activation and we demonstrate that microglia-derived TNFα induces the expression of the BH3-only family member Puma in NPCs via an NF-κB-dependent mechanism. Specifically, we show that NF-κB is activated in NPCs treated with conditioned media from activated microglia and that Puma induction and NPC apoptosis is blocked by the NF-κB inhibitor BAY-117082. Importantly, we have determined that NPC apoptosis induced by activated microglia-derived TNFα is attenuated in Puma-deficient NPCs, indicating that Puma induction is required for NPC death. Consistent with this, we demonstrate that Puma-deficient NPCs exhibit an ∼13-fold increase in survival as compared with wild-type NPCs following transplantation into the inflammatory environment of the injured spinal cord in vivo. In summary, we have identified a key signaling pathway that regulates neuroinflammation induced apoptosis in NPCs in vitro and in vivo that could be targeted to promote regeneration and repair in diverse neurological conditions.
Keywords: apoptosis, neuroinflammation, neural precursor cells, microglia
The persistence of neural precursor cells (NPCs) in distinct niches of the adult brain and spinal cord suggests the potential for regeneration in the affected nervous system.1 Indeed, numerous studies have reported evidence of increased neurogenesis in animal models of cerebral ischemia, epilepsy, and spinal cord injury (SCI), as well as in models of neurodegenerative disease including Alzheimer's, Parkinson's, and Huntington's disease.2 However, the hostile environment of the injured or degenerating nervous system is known to be detrimental to the survival of NPCs and newborn neurons thereby limiting the capacity for regeneration and repair.3, 4
Neuroinflammation is a common feature of many acute and chronic neurological conditions.5 Neuroinflammatory processes can have both beneficial and detrimental effects on neurogenesis in the affected nervous system depending on the nature and duration of the inflammatory response.2, 6 Microglia cells are the innate immune cells of the central nervous system and are primary regulators of neuroinflammatory responses.7 During brain injury, microglia cells become activated and produce a number of anti- and pro-inflammatory factors that can modulate neurogenesis.2 On the one hand, it has been reported that microglia can produce growth factors and chemokines that can promote the proliferation and recruitment of NPCs to sites of injury.8, 9 On the other hand, microglia can also produce pro-inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6 as well as reactive oxygen species that can inhibit neurogenesis and induce NPC apoptosis.10, 11, 12, 13 TNFα can signal through its cognate receptors TNFR1 and TNFR2 to promote cell survival or cell death.14, 15 Moreover, depending on the cellular context, TNFα can induce cell death via caspase-8-mediated apoptosis or RIPK1-mediated necroptosis.16, 17 However, the mechanism by which TNFα signaling affects NPC survival has not been defined.
The Bcl-2 gene family consists of pro-apoptotic and pro-survival members that interact physically and functionally to regulate apoptosis.18 In response to apoptotic stimuli, the Bcl-2 family members Bax and/or Bak oligomerize in the mitochondria and induce membrane permeabilization, leading to the release of factors such as cytochrome-c and Smac/Diablo that promote caspase activation and apoptotic cell death.19, 20 Bax/Bak activation requires the actions of a third group of Bcl-2 family proteins known as the BH3-domain-only subfamily that promote apoptosis by binding to and neutralizing pro-survival Bcl-2 proteins such as Bcl-2, Bcl-XL, and Mcl-1.21 Several BH3-domain only proteins have been identified and specific members can be activated through transcriptional mechanisms and/or post-translational mechanisms.22 For example, the BH3-only family member Puma is known to be regulated through p53-mediated transcriptional activation whereas the BH3-only protein Bid is regulated primarily through proteolytic cleavage.23, 24, 25 The existence of multiple BH3-domain only proteins and activation pathways is thought to underlie the cell type and stimulus-specific nature of apoptosis regulation. Importantly, the Bcl-2 family proteins involved in the regulation of NPC apoptosis induced by neuroinflammatory conditions has not been investigated and is the focus of this study.
The nuclear factor κB (NF-κB) family of transcription factors are ubiquitously expressed and can be activated by a diverse array of stimuli.26 NF-κB complexes can regulate the expression of genes that either promote survival or cell death depending on the cellular milieu.27, 28 In the present study, we demonstrate that TNFα produced by lipopolyssacharide-activated microglia induces NPC apoptosis via a mechanism involving the NF-κB-dependent transcriptional induction of the BH3-only family member Puma. Furthermore, we demonstrate that Puma plays a critical role in regulating NPC apoptosis induced by activated microglia in vitro and in the neuroinflammatory environment of the injured spinal cord in vivo.
Results
Soluble factors released by activated microglia induce NPC apoptosis
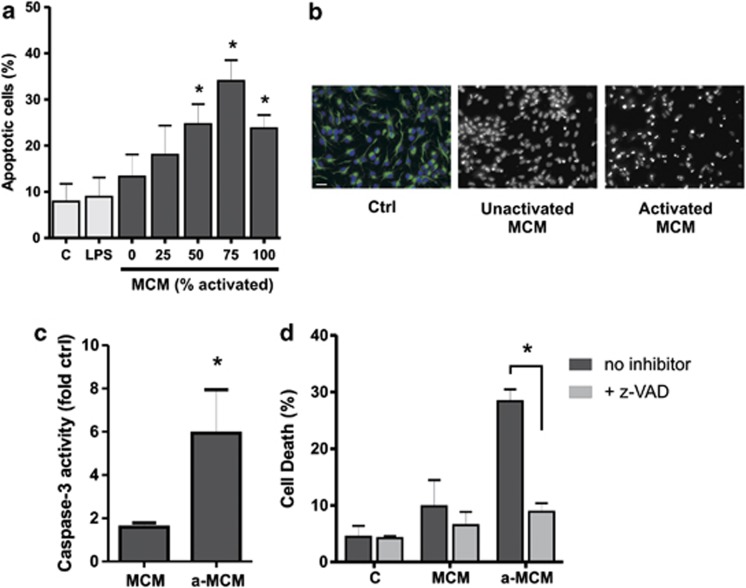
To investigate the mechanism by which microglia cells trigger NPC apoptosis, we utilized the EOC-20 mouse microglia cell line as a homogeneous and renewable source of microglia cells.29 We first examined whether EOC-20 microglia cells when activated by the bacterial endotoxin lipopolysaccharide (LPS) to induce a pro-inflammatory phenotype secrete factors that promote NPC apoptosis. To examine the effects of microglia-derived factors on NPC survival, culture media was removed from adherent NPCs and replaced with unconditioned stem cell media or conditioned stem cell media from either unactivated microglia or LPS-activated microglia and apoptosis was assessed by Hoechst 33342 staining. As shown in Figures 1a and b, the fraction of apoptotic cells was increased in NPCs cultured in LPS-activated microglia conditioned stem cell media (MCM) in a concentration-dependent manner (Figures 1a and b). In contrast, NPC apoptosis was not increased by conditioned media (CM) from unactivated microglia or unconditioned media directly supplemented with LPS (Figures 1a and b). Consistent with this, NPCs treated with CM from activated microglia but not unactivated microglia exhibited a significant increase in caspase-3-like activity (Figure 1c). Furthermore, cell death induced by activated microglia conditioned media (MCM) as assessed by Live/Dead assay was markedly reduced in the presence of the pan-caspase inhibitor z-VAD-FMK consistent with a predominate role of apoptotic cell death in these conditions (Figure 1d). Taken together, these findings indicate that soluble factors released from activated microglia can trigger apoptosis in NPCs.
Figure 1.
LPS-activated microglia release soluble factors that induce NPC apoptosis. NPCs were cultured for 2 days in stem cell media and then media was replaced with either unconditioned stem cell media (c) or microglia conditioned stem cell media from unactivated microglia (0% MCM) or LPS-activated microglia (25–100% MCM). Activated microglia conditioned media was left undiluted (100%) or diluted to 75%, 50%, or 25% with unactivated microglia conditioned media. NPCs maintained in unconditioned stem cell media and directly treated with LPS (10 ng/ml) are labeled as LPS. (a) NPCs were stained at 72 h with Hoechst 33342 and the fraction of apoptotic cells was determined by examining nuclear morphology (n=4, *P<0.05). (b) Representative images of Hoechst-stained NPCs maintained in unconditioned stem cell media (Ctrl), or treated with conditioned media from unactivated microglia (MCM) or LPS-activated microglia (a-MCM) for 72 h. Note the marked increase in NPCs exhibiting chromatin condensation and pyknotic/fragmented nuclei following incubation in conditioned media from LPS-activated microglia. Nestin immunostaining (green) demonstrates that the vast majority of NPCs remain in an undifferentiated state. Scale bar, 20 μm. (c) Protein extracts were obtained from NPCs at 72 h and assayed for caspase-3-like activity. Caspase-3 activity in NPCs treated with microglia conditioned media is reported as fold increase over that in NPCs cultured in unconditioned stem cell media (n=3, *P<0.05). (d) NPCs were cultured in unconditioned stem cell media (c), conditioned media from unactivated microglia (MCM) or conditioned media from LPS-activated microglia (a-MCM) in the presence of the pan-caspase inhibitor z-VAD-FMK (100 μM) or DMSO as a vehicle control. The fraction of dead (ethidium positive) NPCs was determined by Live/Dead assay at 48 h (n=4, *P<0.05)
TNFα released from activated microglia induces NPC apoptosis
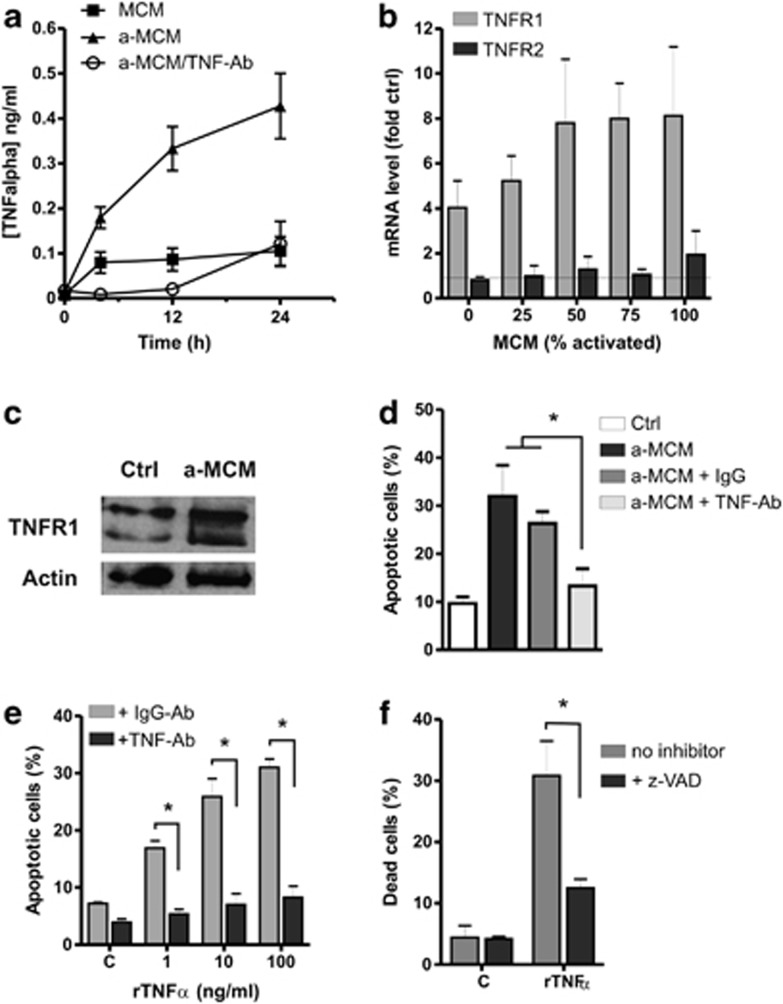
Activated microglia release a variety of soluble factors including reactive oxygen/nitrogen species, chemokines, and both pro- and anti-inflammatory cytokines.7 ELISA analysis of CM from LPS-stimulated microglia revealed a significant increase in TNFα levels as well as several other pro-inflammatory factors in CM from LPS-stimulated microglia as compared with unactivated microglia (Figure 2a, data not shown). TNFα is a potent pro-inflammatory cytokine that has been reported to affect neurogenesis13, 30, 31, 32, 33 and exerts its effects on cells by activating two receptor subtypes on target cell membranes: TNF receptor-1 (TNFR1), and TNF receptor-2 (TNFR2).14 We examined the expression of TNFR1 and TNFR2 in NPCs treated with MCM by qRT-PCR and western blot. NPCs exposed to MCM exhibited a marked increase in Tnfr1 mRNA levels (Figure 2b) as well as a corresponding increase in TNFR1 protein levels (Figure 2c). In contrast, Tnfr2 mRNA levels were not altered by treatment with MCM (Figure 2b) and TNFR2 protein was not detectable in NPCs (data not shown). Given this increase in TNFR1 expression, we examined whether TNFα produced by activated microglia contributes to NPC apoptosis. To address this, we pre-treated MCM with a TNFα-neutralizing antibody or IgG antibody as a control and then examined NPC apoptosis. The efficacy of the antibody to neutralize TNFα in MCM was verified by ELISA (Figure 2a). As shown in Figure 2d, NPC apoptosis induced by activated MCM was significantly reduced in the presence of the TNFα-neutralizing antibody but not IgG control antibody. Furthermore, we found that addition of recombinant TNFα (rTNFα) was sufficient to induce NPC apoptosis and that this could be attenuated by the addition of the TNFα-neutralizing antibody or the pan-caspase inhibitor zVAD-FMK (Figures 2e and f). Taken together, these results indicate that TNFα is a key mediator of NPC apoptosis induced by activated microglia.
Figure 2.
Activated microglia derived TNFα induces NPC apoptosis. (a) Conditioned stem cell media from unactivated microglia (MCM) or LPS-activated microglia (a-MCM) was collected at the indicated times and incubated in the presence or absence of TNFα-neutralizing antibody (10 μg/ml) and then assayed for TNFα levels by ELISA (n=4). (b) RNA was harvested from NPCs incubated with increasing concentrations of activated microglia conditioned media (MCM) for 24 h and TNFR1 and TNFR2 mRNA levels were determined by qRT-PCR. TNFR1/2 mRNA levels in NPCs incubated in microglia conditioned media are reported as fold increase over that in NPCs incubated in unconditioned media (n=3). (c) NPCs were incubated in unconditioned media (c) or activated microglia conditioned media (a-MCM) for 24 h and TNFR1 protein levels were determined by western blot. Representative blot from three independent experiments is shown. (d) NPCs were cultured in unconditioned stem cell media (c) or LPS-activated microglia conditioned media (a-MCM) in the presence of TNFα-neutralizing antibody or IgG control antibody (10 μg/ml) and the fraction of apoptotic cells was determined by Hoechst 33342 staining at 72 h (n=5, *P<0.05). (e) NPCs were treated with the indicated concentrations of recombinant TNFα (rTNF) in the presence of TNFα-neutralizing antibody or IgG control antibody (10 μg/ml) and the fraction of apoptotic cells was determined by Hoechst staining at 72 h (n=4, *P<0.05). (f) NPCs were treated with rTNFα (10 ng/ml) in the presence or absence of the pan-caspase inhibitor zVAD-FMK (100 μM) and the fraction of dead (ethidium positive) cells was determined by Live/Dead assay at 72 h (n=4, *P<0.05)
Activated microglia/TNFα induces NPC apoptosis via a Bax-mediated mitochondrial pathway
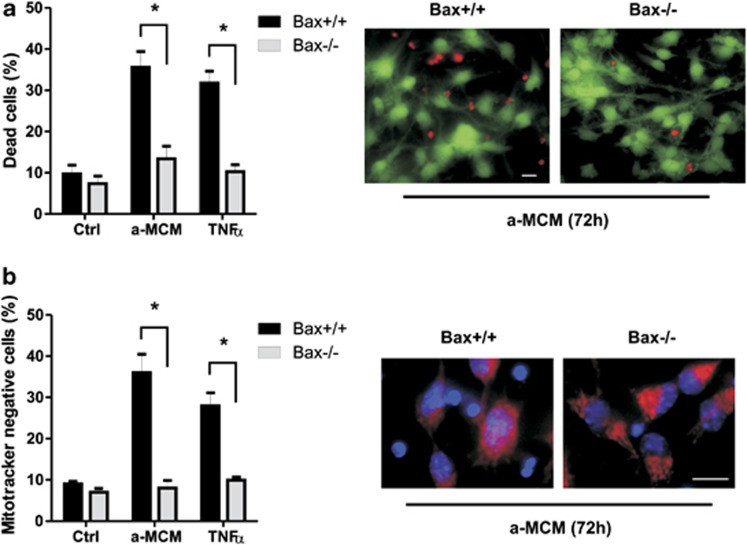
Depending on the cell type, TNFα has been reported to trigger apoptosis through either a mitochondrial-dependent pathway or a mitochondrial-independent pathway involving direct activation of caspase-3 by caspase-8.34 The pro-apoptotic Bcl-2 family proteins Bax and Bak are known to be essential regulators of mitochondrial mediated apoptotic pathways.20 Therefore, to determine whether microglia-derived TNFα induces NPC apoptosis via a mitochondrial pathway, we examined survival in NPCs derived from Bax-deficient mice and wild-type littermates by Live/Dead assay. As shown in Figure 3, NPC death induced by either LPS-activated MCM or rTNFα was markedly reduced in Bax-deficient NPC cultures. Furthermore, Mitotracker Red staining revealed that both a-MCM and rTNFα treatments caused mitochondrial depolarization in a significant portion of wild-type NPCs but not Bax-null NPCs (Figure 3b). These results suggest that activated microglia-derived TNFα induces NPC apoptosis primarily through a mitochondrial-dependent pathway regulated by the Bcl-2 protein family.
Figure 3.
Activated microglia/TNFα-induced NPC apoptosis via a Bax-mediated mitochondrial pathway. NPCs derived from Bax+/+ and Bax−/− embryos were maintained in unconditioned stem cell media (c) or treated with LPS-activated microglia conditioned media (a-MCM) or recombinant TNFα (10 ng/ml). (a) Cell death was assessed at 72 h by Live/Dead assay for 72 h and is reported as the percentage of dead (ethidium positive) cells (n=4, *P<0.05). Representative images of Live/Dead staining of Bax+/+ and Bax−/− NPCs treated with activated microglia conditioned media (a-MCM) for 72 h are shown. Scale bar, 20 μm. (b) Mitochondrial membrane potential was assessed at 72 h by MitoTracker Red staining and the percentage of MitoTracker negative NPCs is reported (n=4, *P<0.05). Representative images of MitoTracker Red and Hoechst 33342 staining in Bax+/+ and Bax−/− NPCs treated with activated microglia conditioned media (a-MCM) for 72 h are shown. Scale bar, 20 μm
Activated microglia-derived TNFα induces Puma expression via an NF-κB-dependent pathway
BH3-only proteins are known to play a key role in regulating Bax activation with distinct family members being activated in a cell type and stimulus-specific manner.18 The BH3-only protein Bid has previously been implicated in death receptor mediated apoptotic pathways.23, 35 It has been proposed that when engaged, death receptors such as Fas and TNFR recruit and activate caspase-8, which can then cleave Bid into its active, truncated form tBid. Therefore, we examined tBid production in NPCs treated with LPS-activated MCM. While we could detect tBid in NPCs treated with activated MCM, the exposure time required to detect tBid was much longer than that for full-length Bid, suggesting that the amount of tBid produced was very modest (Figure 4a). Consistent with this, activated MCM did not cause an appreciable decrease in the level of full-length Bid. Furthermore, tBid production did not appear to be related to TNFα (or cell death) as it was not blocked by the TNFα-neutralizing antibody (Figure 4a) and was not detected in NPCs treated with rTNFα (data not shown). Therefore, we examined the expression levels of other BH3-only family members and interestingly found that Puma protein levels were consistently increased in response to both activated MCM and rTNFα treatment (Figure 4a). Furthermore, we found that neutralization of TNFα blocked the induction of Puma in response to MCM (Figure 4a). Since Puma expression is generally regulated at the transcriptional level, we examined Puma mRNA levels by quantitative RT-PCR. As shown in Figure 4b, Puma mRNA levels were not affected in CM from unactivated microglia but were markedly induced by activated MCM. Furthermore, the increase in Puma mRNA observed in response to activated MCM was significantly reduced in the presence of the TNFα-neutralizing antibody (Figure 4c). These results indicate that activated microglia-derived TNFα promotes Puma induction in NPCs.
Figure 4.
Activated microglia derived TNFα induces the expression of the pro-apoptotic Bcl-2 family member Puma. (a) NPCs were incubated in unconditioned stem cell media (C), unactivated microglia conditioned media (MCM) or LPS-activated microglia conditioned media (a-MCM) in the presence or absence of TNFα-neutralizing antibody (10 μg/ml). NPCs were harvested after 48 h and protein extracts were subjected to SDS-PAGE and immunoblotted for Bid/tBid, Puma or Actin as a loading control. Representative blots from three independent experiments are shown. It should be noted that much longer exposure times were required to detect tBid relative to full-length Bid. (b) RNA was harvested from NPCs treated with increasing concentrations of LPS-activated microglia conditioned media (MCM) for 24 h and Puma mRNA levels were determined by qRT-PCR. Puma mRNA levels in NPCs treated with MCM is reported as fold increase over NPCs incubated in unconditioned media (n=4). (c) NPCs were cultured in unconditioned stem cell media or treated with LPS-activated microglia conditioned media (a-MCM) in the presence of TNFα-neutralizing antibody or IgG control antibody (10 μg/ml)for 24 h and Puma mRNA levels were determined by qRT-PCR. Puma mRNA levels in NPCs treated with microglia conditioned media (+/− antibody) is reported as fold increase over NPCs incubated in unconditioned media (n=4, *P<0.05)
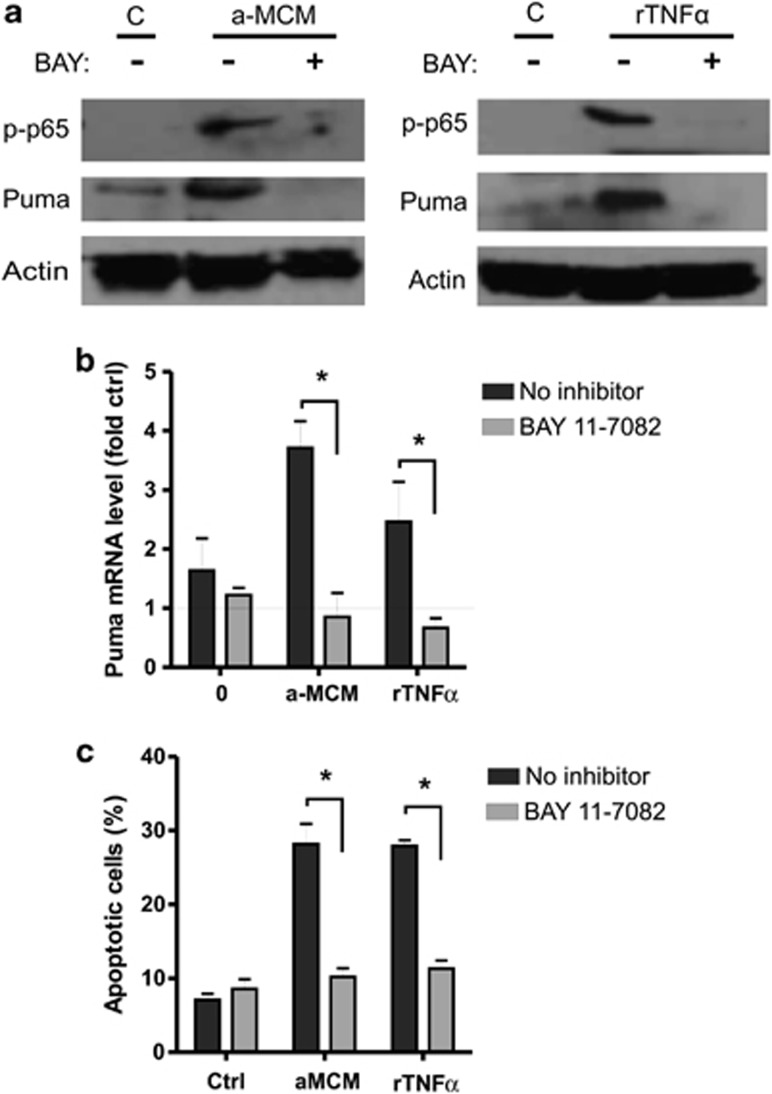
NF-κB is a well-known mediator of the cellular response elicited by TNFα signaling and has been shown to regulate the transcription of both pro-survival and pro-apoptotic genes depending on the cellular context.27, 28 Therefore, we investigated whether activated MCM induces Puma expression in NPCs via an NF-κB-dependent mechanism. To test this, we used the pharmacological inhibitor BAY-117082 that inhibits NF-κB activation by blocking cytokine induced phosphorylation of IκB-α, which binds to NF-κB subunits keeping them sequestered in an inactive state in the cytoplasm.36 As an indicator of NF-κB activity, we examined protein levels of serine-536 phosphorylated (active) p65 subunit. As shown in Figure 5a, phosphorylated-p65 protein levels were increased in NPCs following treatment with either MCM or rTNFα and this was abrogated in the presence of BAY-117082. Importantly, we found that NF-κB inhibition attenuated Puma induction in response to activated MCM and rTNFα (Figures 5a and b). Consistent with this, BAY-117082 also significantly reduced MCM and rTNFα induced apoptosis in NPCs (Figure 5c). Taken together, these results suggest that NF-κB regulates Puma induction and NPC apoptosis induced by activated microglia-derived TNFα.
Figure 5.
Activated microglia-derived TNFα induces Puma expression in NPCs via an NF-κB-dependent pathway. (a) NPCs were cultured in unconditioned stem cell media (C) or treated with LPS-activated microglia conditioned media (a-MCM) or rTNFα (10 ng/ml) in the presence of the NF-κB inhibitor BAY-117082 (10 μM) or DMSO (0.1%) as a vehicle control. NPCs were harvested at 48 h and protein extracts were subjected to SDS-PAGE and immunoblotted for phoshphorylated-p65 (Ser536), Puma, and Actin as a loading control. Representative images from three independent experiments are shown. (b) NPCs were treated with LPS-activated microglia conditioned media (a-MCM) or rTNFα (10 ng/ml) in the presence or absence of the NF-κB inhibitor BAY-117082 (10 μM) for 24 h and Puma mRNA levels were determined by qRT-PCR. Puma mRNA levels are reported as fold increase over untreated controls (n=4, *P<0.05). (c) NPCs were treated with activated microglia conditioned media (a-MCM) or rTNFα (10 ng/ml) in the presence or absence of BAY-117082 (10 μM) and the fraction of apoptotic cells was determined at 48 h by Hoechst 33342 staining (n=4, *P<0.05)
Puma is required for NPC apoptosis induced by neuroinflammation in vitro and in vivo
We next examined whether Puma is necessary for microglia/TNFα induced NPC apoptosis. To address this, we compared apoptotic frequencies in NPCs derived from Puma−/− mice and their wild-type littermates following treatment with CM from unactivated microglia or increasing concentrations of CM from LPS-activated microglia. As shown in Figures 6a and b, apoptosis induced by activated MCM was markedly reduced in Puma−/− NPCs as compared with wild-type NPCs. Similarly, we found that rTNFα induced apoptosis was attenuated in Puma-deficient NPCs (Figure 6c). Taken together, these results indicate that Puma is required for the induction of NPC apoptosis by activated microglia-derived TNFα.
Figure 6.
Puma is required for activated microglia/TNFα-induced NPC apoptosis. (a) NPCs derived from Puma+/+ and Puma−/− embryos were maintained in unconditioned stem cell media (C) or treated with increasing concentrations of LPS-activated microglia conditioned media (MCM) and the fraction of apoptotic cells was determined by Hoechst staining at 72 h (n=5, *P<0.05, **P<0.01). (b) Representative images of Hoechst staining in Puma+/+ and Puma−/− NPCs treated for 72 h with LPS-activated microglia conditioned media (a-MCM). Scale bar, 20 μm. (c) Puma+/+ and Puma−/− NPCs were treated with the indicated concentrations of rTNFα and the fraction of apoptotic cells was determined by Hoechst staining at 72 h (n=5, *P<0.05)
SCI induces an inflammatory response associated with the infiltration of microglia and peripheral immune cells and is associated with elevated levels of a variety of inflammatory cytokines including TNFα.37, 38, 39 Furthermore, several studies have reported that inflammatory processes in the injured spinal cord markedly reduce the survival of transplanted NPCs.40, 41 As we have identified Puma as a key regulator of microglia induced NPC death in vitro, we next examined whether Puma plays an important role in regulating NPC death in the inflammatory environment of the injured spinal cord in vivo. To address this, we first bred Puma−/− mice with transgenic mice ubiquitously expressing enhanced green fluorescent protein (EGFP) under control of the chicken β-actin promoter (ACTB–EGFP). Puma+/−/EGFP+ progeny were then bred to generate Puma+/+/EGFP+ and Puma−/−/EGFP+ embryos from which EGFP-labeled NPCs were harvested and expanded for transplantation. K1-15–EGFP transgenic mice that express EGFP exclusively in keratinocytes were used as recipient mice for NPC transplants to avoid potential immunogenic responses to EGFP expression by transplanted NPCs. K15–EGFP mice received a contusion injury at the T7–8 segment of the spinal cord, and 1 week after injury, the mice received an intraspinal injection of equal numbers of NPCs isolated from either Puma−/−/EGFP mice or Puma+/+/EGFP mice. Three weeks after SCI, mice were euthanized and the number of surviving EGFP+ NPCs remaining in the lesioned spinal cords of mice transplanted with Puma+/+/EGFP+ or Puma−/−/EGFP+ NPCs was evaluated. As shown in Figures 7a and b, a substantial number of transplanted Puma−/−/EGFP+ NPCs remained in the lesioned region of the cord and the majority of the engrafted cells were located at the lesion epicenter. In contrast, transplanted Puma+/+/EGFP+ NPCs were rarely detected at the lesion epicenter and only a few were found at the interface between the lesion epicenter and the spared neural tissue (Figures 7c and d). Indeed, the number of engrafted Puma−/− NPCs remaining in the lesioned spinal cord was ∼13-fold greater than that of Puma+/+ NPCs (3828±1219 versus 297±149; Figure 7e). These results suggest that similar to the situation in vitro, Puma plays a prominent role in regulating NPC survival during neuroinflammation in vivo.
Figure 7.
Transplanted Puma-deficient NPCs exhibit increased survival in the injured mouse spinal cord. K15–EGFP mice received a contusion injury at the T7–8 segment of the spinal cord. One week after injury, animals received an intraspinal injection of NPCs isolated from either Puma+/+/EGFP mice (3 × 104 cells) or Puma−/−/EGFP mice (3 × 104 cells) at the lesion site. Three weeks following injury, mice were killed and spinal cord sections spanning the lesion site were prepared and immunostained with EGFP antibody and visualized by DAB reaction and hematoxylin counterstain. Representative images of EGFP-stained sections from the lesion site of spinal cord injured mice transplanted with Puma−/−/EGFP NPCs (a, b) or Puma+/+/EGFP NPCs (c, d). Scale bars, 100 and 10 μm (insets). (e) The number of EGFP+ cells remaining in the injured spinal cord of mice transplanted with Puma+/+/EGFP NPCs (n=6 mice) and Puma−/−/EGFP NPCs (n=6 mice) was counted in 25 sections/animal and the data are reported as the mean±S.E.M. (n=6, *P<0.05)
In summary, we have determined that TNFα produced by activated microglia induces NPC apoptosis via the NF-κB-mediated induction of the pro-apoptotic Bcl-2 family member Puma. Furthermore, we demonstrate that Puma plays a key role in the regulation of NPC survival during neuroinflammatory responses both in vitro and in the injured nervous system in vivo.
Discussion
Neuroinflammation is a common feature of acute neurological injuries as well as many chronic neurodegenerative conditions and several studies have demonstrated that neuroinflammatory processes can induce apoptosis in NPCs and immature neurons.11, 12, 13, 42 Microglia are key regulators of neuroinflammation and depending on the nature of their activation can produce anti-inflammatory and/or pro-inflammatory factors and exert either beneficial or detrimental effects on neurogenesis.2, 6 Previous studies have demonstrated that microglial cells stimulated with LPS to induce a pro-inflammatory response secrete factors that induce NPC apoptosis although the underlying mechanisms were not examined.10, 13 Importantly, while previous studies have suggested a correlation between TNFα and NPC death, our study is the first to directly implicate TNFα as a key determinant in microglia induced NPC apoptosis. Specifically, we demonstrate that neutralization of microglia-derived TNFα blocks NPC apoptosis induced by activated MCM and that rTNFα is sufficient to induce NPC death. Consistent with our findings, it has been reported that acutely activated microglia that produce high levels of pro-inflammatory cytokines such as TNFα induce NPC apoptosis whereas chronically activated microglia that produce low levels of TNFα and increased levels of anti-inflammatory factors do not induce significant apoptosis.10 In another study, it was shown that microglia activated in the presence of the anti-inflammatory factor IL-4 resulted in decreased TNFα production and instead promoted neurogenesis.43 Interestingly, we also found that expression of TNFR1, but not TNFR2, is upregulated in NPCs exposed to activated MCM but that this is not mediated by TNFα as it is not blocked by TNFα neutralization. This suggests that TNFR1 induction is triggered by additional microglia-derived factors that may contribute to NPC death. One interesting possibility is IL-6 as Monje et al.13 previously reported that IL-6 contributes to the anti-neurogenic effects of LPS-activated microglia. Interestingly, Iosif and colleagues44, 45 have reported that TNFR1 knockout mice exhibit increased neurogenesis in models of epileptic seizure and cerebral ischemia, although NPC death was not assessed in these in vivo contexts. While it is likely that TNFR1 induction potentiates the apoptotic response of NPCs this is yet to be formally tested.
Depending on the cell type death receptors such as TNFR, Fas and Trail can trigger apoptosis via a caspase-8-dependent mechanism, resulting in direct caspase-3 activation or through an indirect pathway involving Bax/Bak-dependent mitochondrial permeabilization.15 We have determined that microglia-derived TNFα as well as rTNFα induce NPC apoptosis predominately via a Bax-dependent mitochondrial pathway. Specifically, we demonstrate that CM from activated microglia or rTNFα treatment induces mitochondrial depolarization and caspase-3 activation in NPCs and that this is attenuated in Bax-deficient NPCs. BH3-domain-only Bcl-2 family proteins are known to regulate Bax activation in a cell type and stimulus-specific manner.21, 22 It has previously been reported that in certain cell types, TNFα can trigger apoptosis through caspase-8-mediated cleavage of the BH3-only family member Bid.23, 35 However, we have found that NPCs exposed to activated MCM exhibited only a very modest increase in tBid levels and that this was not blocked by the anti-TNF-neutralizing antibody. Furthermore, we did not detect an increase in tBid in NPCs treated with rTNFα. NPCs appear to express full-length Bid, thus, the lack of Bid cleavage suggests that caspase-8 is not efficiently activated by TNFα in NPCs although the reason for this is not clear. These results suggest that tBid is not likely required for activated microglia/TNFα induced apoptosis in NPCs. On the other hand, we found that NPCs exposed to CM from activated microglia exhibited a marked increase in the expression of the BH3-only family member Puma that appears to be mediated by TNFα as it is suppressed by a TNFα-neutralizing antibody. Consistent with this, we found that Puma expression was also induced in NPCs treated with rTNFα. Importantly, we have demonstrated that Puma-deficient NPCs are remarkably resistant to apoptosis induced by activated MCM and rTNFα, indicating that Puma induction is required for cell death.
We further examined the importance of this cell death pathway in an in vivo model of SCI known to involve a marked inflammatory response including extensive microglia activation and elevated levels of pro-inflammatory cytokines such as TNFα.39 Previous studies have demonstrated that the majority of stem cells transplanted into the injured spinal cord undergo cell death.40, 41 Importantly, we have determined that 3 weeks after transplantation, the number of Puma-deficient NPCs remaining in the injured spinal cord was >13-fold higher than that of wild-type NPCs. These results are consistent with the high propensity of NPCs to undergo apoptotic cell death in a neuroinflammatory environment and emphasizes the importance of Puma activation in mediating NPC cell death in vivo.
Puma expression can be induced by diverse apoptotic stimuli and is typically regulated through transcriptional mechanisms.24 Pro-inflammatory cytokines including TNFα are known to activate NF-κB family transcription factors.26 Indeed, we found that NPCs exposed to activated MCM exhibited a marked increase in p65 phosphorylation consistent with NF-κB activation. Importantly, we demonstrated that the NF-κB inhibitor BAY-117082 significantly reduced Puma induction and NPC apoptosis, indicating that NF-κB is a key mediator of Puma induction in NPCs exposed to activated MCM. Similar to our findings, it has recently been demonstrated that pro-inflammatory cytokines including TNFα can induce Puma expression via an NF-κB-mediated pathway in colorectal cancer cells and islet β-cells.46, 47, 48 NF-κB has been reported to promote survival or cell death depending on the context.27, 28 The protective effects of NF-κB have been attributed to its ability to induce the expression of anti-apoptotic factors such as Bcl-2, Bcl-XL, and cIAP2.26 Conversely, NF-κB has been shown to promote apoptosis in certain conditions by inducing the expression of pro-apoptotic proteins such as death receptor-5, Fas/Fas ligand, and p53.27 Several studies have suggested that p65 and p53 can co-operate to activate the expression of pro-apoptotic genes and promote cell death.49, 50 Puma was originally identified as a p53 responsive gene;24, 25 therefore, it is possible that p53 and NF-κB could act together to promote Puma induction in NPCs exposed to microglia-derived pro-inflammatory factors.
In summary, we have identified a key signaling pathway that regulates neuroinflammation induced apoptosis in NPCs that could potentially be targeted to promote regeneration and repair in diverse injury and neurodegenerative conditions.
Materials and Methods
Animals
Mice carrying a targeting null mutation for Bax were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and were genotyped as previously described.51 Mice carrying a targeted null mutation for Puma were generated and maintained on a C57/BL6 background in the laboratory of Dr. Andreas Strasser (WEHI, Victoria, Australia) and genotyping of these mice was performed as previously described.52 K1-15–EGFP and ACTB–EGFP transgenic mice that express EGFP under the control of the mouse keratin complex-I gene 15 promoter and the chicken β-actin promoter, respectively, were obtained from Jackson Laboratories. For the transplantation experiments transgenic ACTB–EGFP mice were crossed with Puma−/− mice to generate Puma+/−/EGFP mice. These progeny were then crossed to generate Puma+/+/EGFP and Puma−/−/EGFP littermates from which NPCs were harvested and expanded for spinal cord transplantation experiments. Timed pregnant wild-type CD1 mice were purchased from Charles River Laboratories (Sherbrooke, QC, Canada).
NPC culture
NPCs were dissociated from the striatum of E13.5 mice and grown as neurospheres for 7 days in neural stem cell media consisting of DMEM-F12 containing 𝒟-glucose (6 mg/ml), ℒ-glutamine (2 mM), penicillin/streptomycin, insulin (20 μg/ml), apotransferrin (100 μg/ml), progesterone (0.02 nM), putrescine (20 nM), sodium selenite (30 nM), heparin (0.3 nM), and bFGF (10 ng/ml) as previously described.53 Neurospheres were then dissociated into a single cell suspension using the NeuroCult chemical dissociation kit (Stem Cell Technologies, Vancouver, BC, Canada) and plated on dishes coated with poly-ℒ-ornithine and laminin (Sigma, Oakville, ON, Canada).
Microglia cell culture and preparation of MCM
The mouse micrgolial cell line EOC-20 was obtained from the American Type Culture Collection (ATCC CRL-2469, Manassas, VA, USA). Cells were maintained at 37 °C and 5% CO2 in DMEM supplemented with 10% fetal bovine serum, 0.5% penicillin/streptomycin, 4 mM ℒ-glutamine, and 20% conditioned medium from bone-marrow-derived Ladmac cells (ATCC CRL-2420) as a source of colony stimulating factor-1. To prepare MCM, EOC-20 cells were grown to 60% confluence at which point their media was removed and replaced with neural stem cell media (lacking bFGF and heparin). To activate microglia, NPC media was supplemented with 10 ng/ml LPS (Sigma) for 24 h. MCM was collected, centrifuged and filtered through a 0.2-μm filter to remove cells and debris. MCM was then supplemented with 10 ng/ml bFGF and 0.3 nM heparin and immediately used for NPC cultures. In the indicated experiments, LPS was added to non-activated MCM or unconditioned stem cell media immediately before adding to NPC culture. LPS-activated MCM was added to NPCs either undiluted (100%) or in indicated experiments diluted with CM from unactivated microglia to yield 25, 50, or 75% activated MCM.
NPC treatments and TNFαneutralization experiments
NPCs were treated with MCM or recombinant mouse TNFα (rTNFα; R&D Systems, Minneapolis, MN, USA) 2 days after plating as a monolayer. In the indicated experiments, the pan-caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone (z-VAD-FMK, Santa Cruz Biotech, Santa Cruz, CA, USA) or the NF-κB inhibitor BAY-117082 (Santa Cruz Biotech) was added to NPC cultures simultaneously with the switch to MCM or rTNFα treatment. For TNFα neutralization experiments, MCM was supplemented with 10 μg/ml anti-mouse TNFα/TNFSF1A (R&D Systems) or 10 μg/ml normal goat IgG as a control and incubated for 1 h before adding to NPC cultures.
Cell death assays
NPC apoptosis was assessed by examining nuclear morphology in Hoechst 33342-stained cells as previously described.54 NPCs were stained with 1 μg/ml Hoechst 33342 (Sigma) and the fraction of cells exhibiting an apoptotic nuclear morphology characterized by chromatin condensation and/or apoptotic bodies, was determined. In certain experiments, NPC death was determined by Live/Dead assay according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA) to account for apoptotic and non-apoptotic cell death. Briefly, NPCs were stained with Calcein-AM (2 μM) and ethidium homodimer (4 μM) for 20 min and the fraction of live (Calcein-AM positive) and dead (ethidium positive) cells was scored. NPCs were visualized by fluorescence microscopy (IX70, Olympus, Richmond Hill, ON, Canada) and images were captured with a CCD camera (Q-imaging, Burnaby, BC, Canada) and Northern Eclipse software (Empix Imaging, Mississauga, ON, Canada). Images were captured and scored by an observer blinded to the treatment. A minimum of 500 cells from five randomly selected fields were analyzed for each treatment and data represent the mean and S.E.M. from a minimum of four independent experiments.
Determination of mitochondrial depolarization by Mitotracker Red staining
Mitochondrial membrane potential was assessed using the potentiometric dye Mitotracker Red as per the manufacturer's instructions (Molecular Probes Inc., Eugene, OR, USA). Mitotracker Red is selectively incorporated into mitochondria with an intact transmembrane potential and loss of mitochondrial staining can be used as indicator of mitochondrial depolarization. NPCs were incubated with Mitotracker Red (50 nM) for 30 min at 37 °C and counterstained with Hoechst 33342. Images were captured as described above and the fraction of Mitotracker Red positive and negative cells relative to the total cell number (Hoechst labeled) was scored. Data are presented as the fraction of cells exhibiting mitochondrial depolarization (Mitotracker Red negative). A minimum of 500 cells from five randomly selected fields were analyzed for each treatment and data represent the mean and S.E.M. from a minimum of four independent experiments.
Caspase-3-like activity assay
NPCs were harvested in lysis buffer (1 mM KCl, 10 mM HEPES, pH 7.4, 1.5 mM MgCl2, 1 mM PMSF, 5 μg/ml leupeptin, 2 μg/ml aprotinin, and 10% glycerol) and 10 μg of protein was used in caspase-3-like activity assay as previously described.55 Briefly, protein samples were added to caspase reaction buffer (25 mM HEPES (pH 7.4), 10 mM DTT, 10% sucrose, 0.1% CHAPS, and 10 μM N-acetyl-Asp-Glu-Val-Asp-(7-amino-4-trifluoromethyl coumarin (Ac-DEVD-AFC)) and fluorescence produced DEVD-AFC cleavage was measured on a SpectraMax M5 fluorimeter (excitation 400 nm, emission 505 nm) over a 1-h interval. Caspase-3-like activity is reported as the ratio of the fluorescence output in NPCs cultured in MCM to NPCs cultured in unconditioned stem cell media.
TNFα ELISA
Conditioned stem cell media from microglia either left unstimulated or stimulated with 10 ng/ml LPS (Sigma) was collected at 0, 4, 12, or 24 h. TNFα levels were detected using the Quantikine ELISA kit (R&D Systems) as per the manufacturer's instructions. Briefly, CM samples were added to microplates pre-coated with mouse polyclonal TNFα antibody. Following incubation and washes to remove unbound TNFα, an enzyme-linked mouse polyclonal antibody was added. The addition of the substrate yields a colorimetric product and the absorbance (450 nm) was measured using a microplate reader. Samples were assayed in duplicate and TNFα concentrations were determined from a standard curve using SoftmaxPro software (Molecular Devices, Sunnyvale, CA, USA).
Quantitative real-time RT-PCR
RNA was isolated using Trizol reagent as per the manufacturer's instructions (Invitrogen) and 10 ng of RNA was used in one-step Sybr green reverse transcription (RT)-PCR (QuantiFast, Qiagen, Mississauga, ON, Canada). RT-PCR was carried out on a Chromo4 system (MJ Research Bio-Rad, Mississauga, ON, Canada) and changes in gene expression were determined by the Δ(ΔCt) method using S12 transcript for normalization as previously described.54 Data are reported as fold increase in mRNA levels in treated samples relative to corresponding untreated control cells for each transcript. All PCR's exhibited high amplification efficiency (>90%) and the specificity of PCR products was confirmed by sequencing. Primer sequences used for gene-specific amplification are available on request.
Western blot analysis
To prepare whole-cell lysates, NPCs were incubated in lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8, 1 mM EDTA, 1 μM DTT, and protease and phosphatase inhibitor cocktail (Invitrogen) for 20 min on ice and soluble extract was recovered by centrifugation. Protein concentration was determined by BCA assay (Pierce, Rockford, IL, USA) and 40 μg of protein was separated on 12.5% SDS-PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked for 1 h in TBS-T (10 mM Tris, 150 mM NaCl, 0.05% Tween-20), followed by overnight incubation in primary antibodies to Bid/tBid (R&D Systems), Puma, phospho(ser536)-p65 (Cell Signaling Technology, Danvers, MA, USA), Tnfr1 or Actin (Santa Cruz Biotech) in TBS-T containing 5% skim milk. Membranes were washed with TBS-T and incubated for 1 h with the appropriate HRP-conjugated secondary antibodies and developed by enhanced chemiluminescence system according to the manufacturer's instructions (ThermoScientific, Ottawa, ON, Canada).
Mouse model of SCI and NPC transplantation
All protocols for these experiments were approved by the University of Western Ontario Animal Care Committee in accordance with the policies established in the Guide to Care and Use of Experimental Animals prepared by the Canadian Council on Animal Care. A total of 12 adult female C57B6-Kr15–EGFP mice weighing 18–22 g were anesthetized with Ketamine (100 mg/kg) and xylazine (10 mg/kg) and subjected to SCI as previously described.56 Kr15–EGFP mice that express EGFP exclusively in hair follicle bulge cells were used in these experiments to avoid a potential immunogenic response induced by transplantation of EGFP expressing NPCs. Briefly, the vertebral column was stabilized at T6 and T10 and a laminectomy was performed by exposing the dura matter at spinal cord level T7–8. Spinal cord contusion was performed using the Infinite Horizon Impactor (Precision Systems and Instrumentation, Fairfax, VA, USA) with 50 kdyn and 1 s dwelling time. After surgery, mice were kept at 37 °C and closely monitored. Urine was expressed manually twice per day, and mice were checked daily to monitor their overall health. NPC transplantation was performed 7 days after SCI. The dura was incised with the tip of an injection needle exposing the surface of the injured spinal cord. Puma+/+/EGFP NPCs (3 × 104 cells/3 μl, six mice) or Puma−/−/EGFP NPCs (3 × 104 cells/3 μl, six mice) were injected into the cord at the lesion site using a spinal stereotaxic frame by means of a glass pipette (tip diameter 100 μm) configured to a 10-μl Hamilton syringe. Each SCI mouse was transplanted with NPCs derived from an independent Puma−/−/EGFP or Puma+/+/EGFP donor mouse (n=6 for each genotype).
Immunohistochemistry
At 21 days after injury, mice were transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). The T2–L1 vertebral segments, which included the site of the contusion injury, were removed and processed for cryo-sectioning and immunohistochemistry as previously described.56 Transverse sections (16 μm) of spinal cord were serially collected and mounted on glass slides. Every 10th section (25 sections out of 250 sections from each cord), spanning ∼4 mm in length across the lesion site (∼2 mm caudal to ∼2 mm rostral to the lesion epicenter), was stained for EGFP expression. The slides were incubated with rabbit anti-EGFP antibody (1 : 300; Invitrogen) in a humidified chamber at 4 °C overnight and the signal was visualized by a peroxidase-DAB reaction (Zymed, Carlsbad, CA, USA) and hematoxylin counterstain. The immunostained sections were examined using an Olympus epifluorescence microscope (BX51) and the total number of EGFP+ NPCs in the 25 sections/animal was counted.
Data analysis
Data are reported as mean and standard error of the mean. The n value represents the number of independent experiments and/or number of mice from which independent NPC cultures were prepared. Data were analyzed by one-way ANOVA followed by Tukey's post-hoc test and differences were considered significant at P<0.05. All statistical analyses were conducted using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA).
Acknowledgments
This work was supported by grant funding to SPC from the Canadian Institutes of Health Research and the Krembil Foundation to SPC and AB.
Glossary
- ACTB
β-actin
- Bax
Bcl-2-associated X protein
- Bak
Bcl-2 homologous antagonist killer
- Bcl-2
B-cell lymphoma 2
- Bcl-Xl
B-cell lymphoma extra large
- bFGF
basic fibroblast growth factor
- BH3
Bcl-2 homology domain 3
- Bid
BH3 interacting domain death agonist
- EGFP
enhanced green fluorescent protein
- IgG
immunoglobulin G
- LPS
lipopolysaccharide
- Mcl-1
myeloid cell leukemia protein 1
- MCM
microglia conditioned media
- NF-κB
nuclear factor κB
- NPC
neural precursor cell
- Puma
p53 upregulated modulator of apoptosis
- RIPK-1
receptor interacting serine/threonine protein kinase 1
- SCI
spinal cord injury
- TNFα
tumor necrosis factor-α
- TNFR
tumor necrosis factor receptor
The authors declare no conflict of interest.
Footnotes
Edited by G Raschellá
References
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Mohapel P, Weber E, Bahr B, Blomgren K, Lindvall O. Caspase-mediated death of newly formed neurons in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2002;16:1463–1471. doi: 10.1046/j.1460-9568.2002.02202.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Cacci E, jmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden BT, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Walker WS, Gatewood J, Olivas E, Askew D, Havenith CE. Mouse microglial cell lines differing in constitutive and interferon-gamma-inducible antigen-presenting activities for naive and memory CD4+ and CD8+ T cells. J Neuroimmunol. 1995;63:163–174. doi: 10.1016/0165-5728(95)00146-8. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187:171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- van Raam BJ, Salvesen GS. Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta. 2012;1824:113–122. doi: 10.1016/j.bbapap.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Conti A, Cardali S, Genovese T, Di PR, La RG. Role of inflammation in the secondary injury following experimental spinal cord trauma. J Neurosurg Sci. 2003;47:89–94. [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di PR, Muia C, Esposito E, et al. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Lee SI, Kim BG, Hwang DH, Kim HM, Kim SU. Overexpression of Bcl-XL in human neural stem cells promotes graft survival and functional recovery following transplantation in spinal cord injury. J Neurosci Res. 2009;87:3186–3197. doi: 10.1002/jnr.22149. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kim KN, An SS, Pennant WA, Kim HJ, Gwak SJ, et al. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant. 2011;20:837–849. doi: 10.3727/096368910X539083. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Germano CM, Cunha DA, Ortis F, Vanderwinden JM, Marchetti P, et al. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem. 2010;285:19910–19920. doi: 10.1074/jbc.M110.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K, Mitsuhashi M, Ishiyama K, Nair I, Rawson J, Todorov I, et al. mRNA of the pro-apoptotic gene BBC3 serves as a molecular marker for TNF-alpha-induced islet damage in humans. Diabetologia. 2011;54:2056–2066. doi: 10.1007/s00125-011-2183-8. [DOI] [PubMed] [Google Scholar]
- Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–1202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, et al. Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem. 2004;279:27549–27559. doi: 10.1074/jbc.M313435200. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Cregan SP, Maclaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, et al. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci. 1999;19:7860–7869. doi: 10.1523/JNEUROSCI.19-18-07860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der KD. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, et al. Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007;27:12989–12999. doi: 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, Fortin A, Maclaurin JG, Callaghan SM, Cecconi F, Yu SW, et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Geremia N, Bao F, Pniak A, Rossoni M, Brown A. Schwann cell coculture improves the therapeutic effect of bone marrow stromal cells on recovery in spinal cord-injured mice. Cell Transplant. 2011;20:1065–1086. doi: 10.3727/096368910X544906. [DOI] [PMC free article] [PubMed] [Google Scholar]