Abstract
Background
Mixed chimerism is an effective approach for tolerance induction in transplantation. Strategies to achieve mixed chimerism with relatively low toxicity have significantly expanded the clinical use of chimerism.
Methods
Allogeneic bone marrow transplants were performed between B6 (H2b) and BALB/c (H2d) mice. Recipient B6 mice were nonmyeloablatively conditioned with anti-αβ-TCR, anti-CD154, or rapamycin alone or in different combinations. 15 × 106 BALB/c bone marrow cells were transplanted after varying doses of cGy of total body irradiation.
Results
Pretreatment of recipients with anti-CD154 and rapamycin with or without T-cell lymphodepletion reduced the TBI requirement to 100 cGy for establishing stable mixed chimerism. The mixed chimeras accepted donor islet allografts long term. Lymphocytes from mixed chimeras did not respond to host or donor antigens, yet were reactive to MHC-disparate third-party alloantigens, demonstrating functional donor-specific T-cell tolerance. No antibodies against donor and host were detected in mixed chimeras, suggesting humoral tolerance. Mixed chimeras showed no cytotoxicity to donor cells, but a similar rapid killing rate for MHC-disparate third-party B10.BR cells compared with T-cell-deficient and wildtype controls in in vivo cytotoxicity assays, suggesting donor-specific tolerance in the innate immune cells was achieved in mixed chimeras.
Conclusions
Mixed chimeras prepared with very low intensity nonmyeloablative conditioning exhibit systemic tolerance in innate immunity as well as tolerance in adaptive T- and B-cell immune responses.
Keywords: mixed chimerism, bone marrow transplantation, tolerance, innate immunity
Introduction
Donor-specific transplantation tolerance remains an elusive goal in transplantation. Mixed hematopoietic chimerism generated from bone marrow transplantation (BMT) provides a powerful means to induce donor-specific tolerance to solid organ grafts (1). As a result the toxicities associated with anti-rejection drugs, including opportunistic infection (2), increased frequency of malignancies (3), and end-organ toxicity (4,5) can be avoided.
Mixed chimerism can be achieved in immunologically competent recipients through nonmyeloablative conditioning that does not completely eradicate the host immune system. Mixed chimerism is associated with superior immunocompetence compared to MHC-mismatched fully allogeneic chimerism prepared in mice with ablative conditioning (6-8) due to the presence of recipient-derived antigen presenting cells. Historically, T-cell tolerance in mixed chimeras has been well studied in vivo and in vitro. Although donor antigens are continuously presented in mixed chimeras, the recipients do not generate anti-donor antibody. These data indicate that B-cell tolerance is also achieved (9,10). NK-cell tolerance in vivo to both donor and host antigens has been shown in fully MHC-mismatched, mixed allogeneic chimeras prepared with a nonmyeloablative regimen (11). Once mixed chimerism is achieved in the rat to mouse species combination, both adaptive (T- and B-cell) and NK-cell tolerance is also observed (12-15). However, other than NK-cell tolerance, general innate immune tolerance in mixed chimeras has not been addressed.
In the present studies, pretreatment of the recipient with anti-CD154 (CD40 ligand) and rapamycin with or without anti-αβ-TCR could reduce the minimum total body irradiation (TBI) requirement to 100cGy for establishing mixed chimerism. We further demonstrated the systemic tolerance of adaptive (T- and B-cell) and innate immunity in these mixed chimeras.
Results
Host pretreatment with anti-αβ-TCR, anti-CD154, and rapamycin lowers the minimum TBI required for engraftment
Anti-αβ-TCR mAb (TCR) was used to deplete αβ-TCR+ T-cells in vivo; anti-CD154 mAb (MR1) to block CD40:CD154 co-stimulatory pathway; and rapamycin (Rapa) to block late-stage T-cell activation by inhibiting IL-2 responsiveness. To determine the optimal combinations of these conditioning agents, recipient B6 mice were conditioned with anti-αβ-TCR, anti-CD154, and rapamycin alone, or in different combinations, plus 300cGy TBI (Table 1). With 300cGy TBI, engraftment did not occur with conditioning of anti-αβ-TCR alone, anti-CD154 alone, or rapamycin alone. Only 40% of animals engrafted when conditioned with TCR/Rapa. In contrast, 100% of animals preconditioned with TCR/MR1/Rapa and MR1/Rapa engrafted, respectively.
Table 1.
Percent engrafted in mice pretreated with indicated agents or combinations and irradiated with 300 cGya
| Group | n | % Engraftmentb (1 month) |
|---|---|---|
| Anti-αβ-TCR | 5 | 0 |
| Anti-CD154 | 5 | 0 |
| Rapa | 5 | 0 |
| Anti-αβ-TCR, Rapa | 5 | 40 |
| Anti-αβ-TCR, Anti-CD154, Rapa | 7 | 100 |
| Anti-CD154, Rapa | 7 | 100 |
Recipient B6 mice were pretreated with the indicated agents or combinations of these agents peri-BMT. On day 0, they were transplanted with 15 × 106 untreated BMC from BALB/c donors 4-6 h after conditioning with 300 cGy of TBI. Animals were tested for chimerism by flow cytometric analysis one month after BMT.
Percentage of animals that engrafted with ≥ 1% donor cells in PBL. Results are a summary of 5 experiments.
A dose-titration of TBI was performed to determine the minimal TBI requirement in those 2 groups with 100% engraftment at 300cGy TBI (Fig. 1A and B). With TCR/MR1/Rapa, 100% engraftment was achieved with either 300cGy or 200cGy TBI, with donor chimerism 22.0±7.9% or 17.7±8.1%, respectively. When T-cell lymphodepletion was omitted, conditioning with MR1/Rapa also resulted in 100% engraftment in recipients received 300cGy or 200cGy TBI, with donor chimerism 20.5±5.6% and 7.4±3.2%, respectively. 57.1% or 60.0% of mice engrafted with 100cGy TBI when treated with TCR/MR1/Rapa or MR1/Rapa. No engraftment was observed in the absence of TBI in all groups. The engraftment was durable for up to 6 months (Fig. 1C) and multilineage including T-cells, B-cells, NK-cells, dendritic cells (DC), macrophages, and neutrophils (Table 2).
Figure 1. Immune-based nonmyeloablative conditioning reduces TBI requirement to minimum and induces tolerance to islet and skin graft.
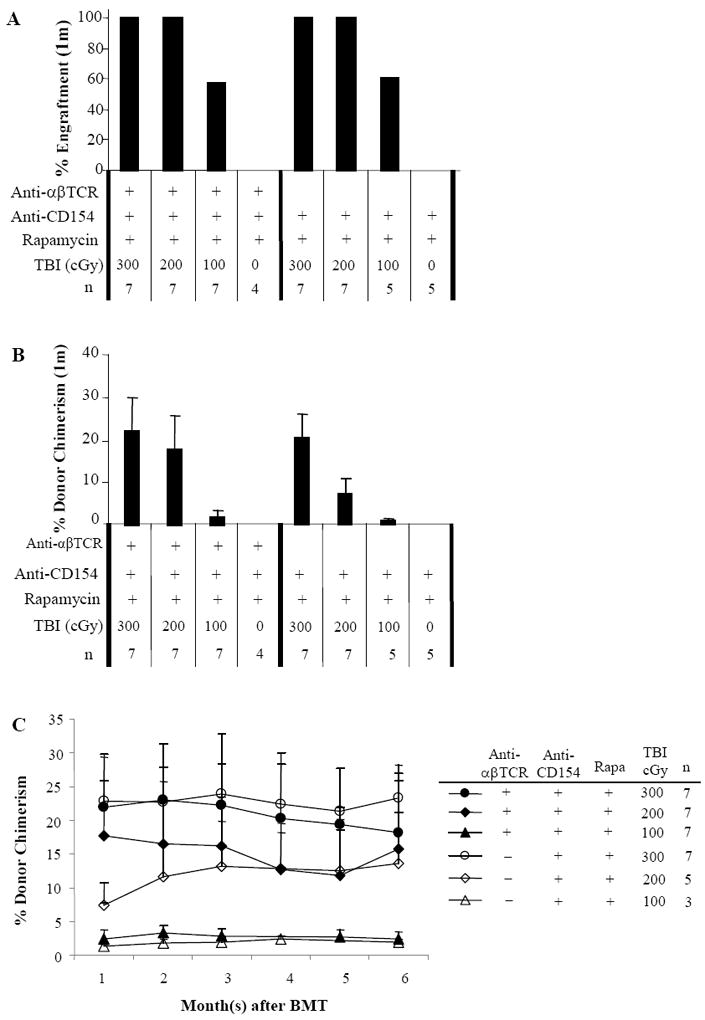
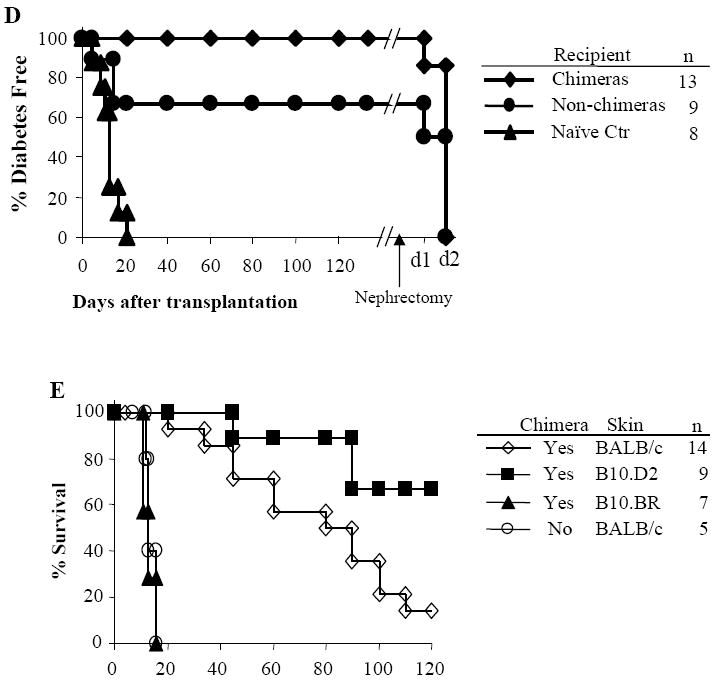
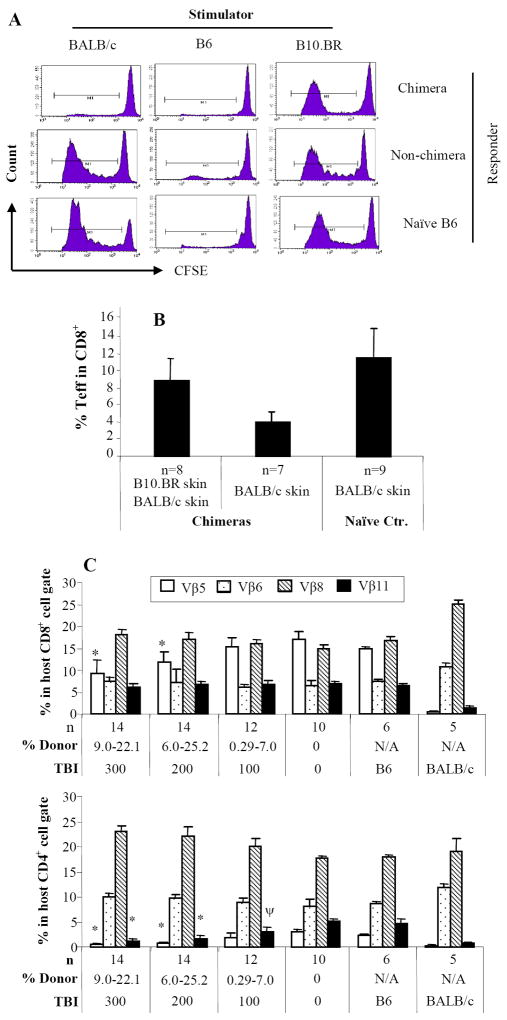
Allogeneic bone marrow transplants were performed between MHC plus minor antigen-disparate C57BL/6 (H2b) and BALB/c (H2d) mice. Recipients were treated with anti-αβ-TCR mAb (30μl/mouse, day -3), anti-CD154 mAb (0.5mg/mouse, day 0 and +3), or rapamycin (3mg/kg, day 0 to +4) alone or in combination. Recipient mice were transplanted with 15 × 106 allogeneic BALB/c donor marrow cells 4-6 hours following TBI. Animals were analyzed for engraftment by flow cytometric analysis monthly after BMT up to 6 months. Peripheral blood was stained with antibodies specific for MHC Class I antigens of donor (anti-H-2Kd-PE) and recipient (anti-H-2Kb FITC) for 30 minutes. The analysis was carried out on a FACSCalibur (Becton Dickinson Mountain View, CA). n = number of animals in each experiment. This figure shows the frequency of engraftment at 1 month after BMT (A), level of chimerism in animals that engrafted (percentage of donor cells in PBL) at 1 month (B), and kinetics of engraftment for up to 6 months after BMT (C) as assessed by PBL typing. The results are the summary of 6 experiments. Transplantation tolerance in mixed chimeras was assessed with islet or skin allograft transplantation 2-5 months after BMT. Non-chimeras and naïve B6 recipients served as controls. In islet transplantation (D), recipients were rendered diabetic through treatment with streptozocin. Each animal received approximately 500-600 mouse islets transplanted beneath the left renal capsule. Rejection was considered to have occurred when the blood glucose levels were > 200 mg/dl for 3 consecutive days. Nephrectomy was performed in long-term acceptors (>120 days) to remove kidney with transplanted islet. In skin transplantation (E), each chimeric animal received skin grafts from donor-specific (BALB/c) and third-party (B10.BR, H-2k) and the grafts were then monitored up to 200 days. One control group of chimeras received B10.D2 (H-2d) skin grafts, which had same MHC background as donor BALB/c.
Table 2.
Multilineage Engraftmenta
| TBI cGy | nb | % donor | % in donor (H2Kd+) lymphoid gate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| αβ-TCR | CD8 | CD4 | B220 | DX5 | CD11c | Mac-1 | |||
| 300 | 7 | 22.5±6.5 | 16.7±7.9c | 2.7±1.3 | 13.6±6.3 | 78.2±8.4 | 10.0±3.8 | 1.4±0.37 | 4.2±0.46 |
| 200 | 7 | 18.4±5.4 | 7.6±3.0 | 1.2±0.62 | 6.2±1.6 | 88.0±2.3 | 7.9±4.7 | 0.77±0.58 | 3.4±0.95 |
| 100 | 5 | 3.1±1.0 | 9.9±6.4 | 1.1±0.28 | 9.1±8.3 | 81.1±9.2 | 4.3±3.4 | 0.91±0.58 | 5.5±3.0 |
Multilineage typing was performed in mice between 2 and 4 mo after BMT. Multi-lineage engraftment was assessed in 50 μl PB by four-color staining for FITC-conjugated anti-donor-specific antibody (H-2Kd) and different fluorochrome (PE, PerCP and APC)-conjugated lineage makers, including T-cells (anti-CD4, anti-CD8α, and anti-TCR-β), B-cells (anti-B220), NK-cells (anti-DX5), DC (anti-CD11c), and myeloid cells (macrophages [anti-Mac-1]).
Mice were conditioned with TCR/MR1/Rapa or MR1/Rapa
Mean ± SD percentage of donor cells in donor lymphoid gate.
Chimeras induced through minimal conditioning demonstrate islet and skin graft tolerance
The chimeras for islet transplantation were from all groups 2-5 months after BMT. Donor chimerism ranged between 28.4% to 1.6% (Fig. 1D). The mixed chimeras accepted donor BALB/c grafts up to 3 months. 66.7% of non-chimeras also accepted donor islet grafts long term. Islet grafts were rapidly rejected by naïve controls (MST: 12.7±4.8 days). After nephrectomy in chimeras that had accepted their islet allografts, mice became diabetic in 2 days, demonstrating that the source of insulin was from the transplanted islets.
Skin grafts were performed on chimeras to assess tolerance in vivo (Fig. 1E). Chimeras received skin grafts from donor-specific (BALB/c) and third-party (B10.BR) strains 3-5 months after BMT. Third-party grafts were promptly rejected. In chimeras, the majority of donor-specific skin grafts were rejected chronically within 100 days as expected due to the skin-specific antigens in BALB/c strain (16,17). To avoid chronic rejection due to skin-specific antigens (8), B10.D2 (H-2d) skin grafts were used. The B10.D2 skin grafts were accepted in 6 of 9 chimeric mice. The non-chimeras rejected donor BALB/c skin grafts (MST=13±5.0 days) with a time course similar to that of third-party grafts in chimeras. The MST for B10.D2 skin grafts in chimeras was significantly prolonged compared to the BALB/c skin grafts (P = 0.019).
T-cells from chimeras are functionally tolerant in in vivo mixed lymphocyte reactions (MLR)
Chimeric T-cells were not reactive to donor BALB/c alloantigens (Fig. 2A). T-cells from non-chimeras exhibited strong reactivity to donor. T-cells from both chimeric and non-chimeric mice showed strong proliferation to B10.BR third-party alloantigens. These data suggest that donor-specific tolerance is achieved in T-cells from chimeras.
Figure 2. Donor-specific T-cell, humoral, and innate immune tolerance in mixed chimeras.
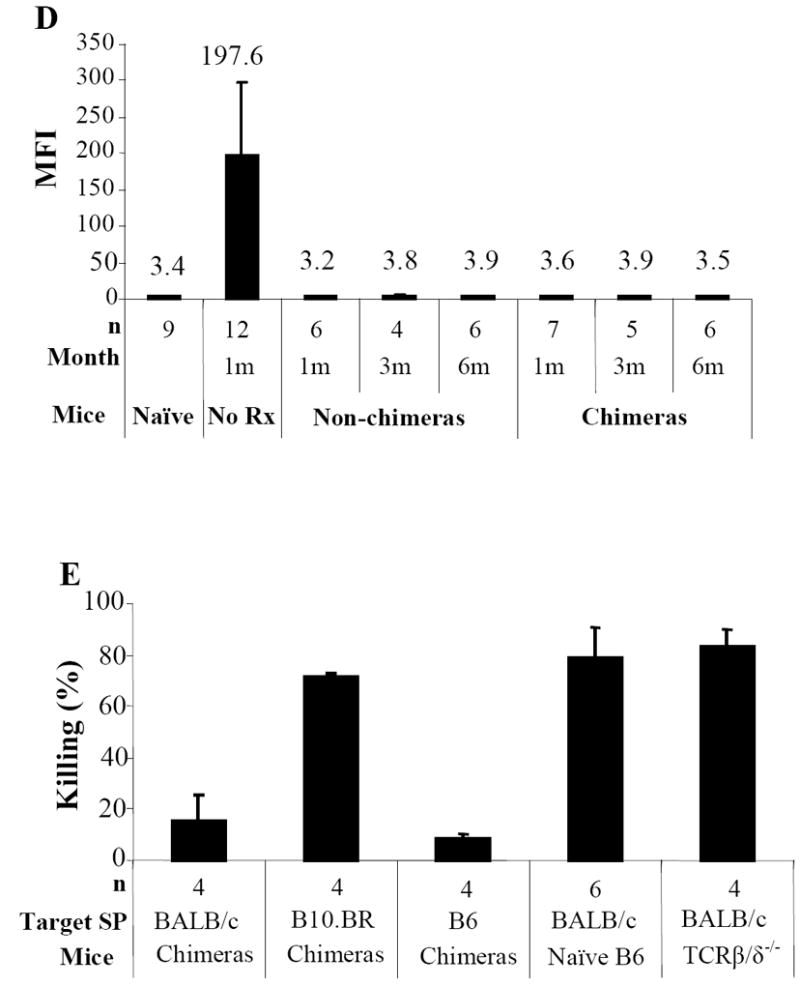
Chimeras (BALB/c → B6) were evaluated for donor-specific T-cell tolerance with in vivo MLR (A). Twenty million carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes from chimeras, non-chimeras or naïve B6 were injected i.v. into irradiated (1000cGy) stimulators of donor BALB/c, recipient B6, or third-party B10.BR mice. At day 3, the spleens were harvested and stained with anti-mouse CD8 PerCP and anti-CD4-APC. CFSE intensities were analyzed in CD4+ or CD8+ cells. The proliferating T-cells lost half CFSE fluorescence intensity sequentially with each division. The proliferation resulted in cell events towards downstream divisions. Data from one representative experiment of 3 are shown. Each chimeric animal received skin grafts from donor-specific (BALB/c) and third-party (B10.BR, H-2k) Donor-specific tolerance was further evaluated with effector/memory T-cell expression in chimeras received donor or donor/third-party skin grafts (B). Mixed chimeras received either donor BALB/c or both BALB/c and third-party skin grafts. Recipient PB was collected at day 14 after skin transplantation and cells were stained with anti-CD44-FITC, anti-CD62L-PE, anti-CD4-PerCP, and anti-CD8-APC. Percentages of CD8+/CD44high/CD62Llow/-effector/memory T-cells were measured in CD8+ T-cell gate. For clonal deletion of activated T-cells, expression of Vβ5.1/2, Vβ6, Vβ8.1/2, and Vβ11 in unmanipulated hosts (B6), unmanipulated donors (BALB/c), experimental animals with different levels of donor chimerism from groups of 0 to 300cGy TBI were measured by FACS analysis (C). Relative expression represents the percentage of Vβ-positive cells within the CD8+ or CD4+ T-cell subsets of the host (H2Kb) lymphogate in peripheral blood. Data from 3 experiments are depicted as mean ± SD. Vβ-TCR expression in either experimental group was compared with that in B6 mice using two tailed t-test. Significant P values are indicated above the respective data bars (* P ≤ 0.0005 and ψ P ≤ 0.05). Humoral tolerance in mixed chimeras was studied with FCXM assay (D). Sera were collected from chimeric or non-chimeric mice 1, 3 and 6 months after BMT. Sera from mice receive BMC infusion only and naïve served as controls. Anti-donor antibodies were detected by FCXM assay. One half-million splenocytes from naive BALB/c mice were incubated with 10 μl of sera for 30 minutes. Cells were washed and incubated with FITC-conjugated polyclonal goat anti-mouse Ig, followed by third incubation with PE-conjugated anti-mouse CD4 plus CD8. Gating was carried out on the CD4+ and CD8+ T-cell fraction, and reported as mean fluorescence intensity (MFI). Innate immune tolerance in mixed chimeras was analyzed using in vivo cytotoxicity assays (E). Single splenocyte suspensions were prepared. Target (BALB/c) and internal control (B6) splenocytes (100 × 106/mL in PBS) were incubated with 4.0 μM or 0.2 μM CFSE, respectively, at room temperature for 10 minutes. Fetal bovine serum was added to quench the reaction. Cells were mixed in a 1:1 ratio and resuspended in PBS, and 20 × 106 cells from each were injected intravenously to chimeras, non-chimeras, T-cell deficient mice (TCRβ/δ-/-), and naïve B6 mice. Chimeras received target and internal control cells both from B6 was served as one of controls. Peripheral blood was collected 18 hours after cell infusion. Peripheral-blood lymphocytes were analyzed for CFSE expression. The percentage of killing was determined by calculating the ratio of target to internal control cells.
The donor-specific tolerance in mixed chimeras was also demonstrated in the generation of CD44high/CD62Llow/- effector CD8+ T-cells in response to skin grafting (Fig. 2B). The CD8+ effector population was significantly increased in chimeras that received third-party skin compared with chimeras that were transplanted with donor-specific skin (8.8±2.6% vs. 4.0±1.2% respectively; P = 0.0009). The CD8+ effector T-cell level in chimeras that rejected third-party skin grafts was comparable to the level of CD8+ effector T-cells in naïve recipients that rejected donor BALB/c skin grafts (P = 0.08). These data suggest that the effector T-cells are third-party antigen-specific and are not generated in response to donor bone marrow cells (BMC) or acute rejection of donor-specific skin grafts.
Partial clonal deletion of donor-reactive TCR-Vβ subsets occurs in chimeras
The superantigen I-E is expressed in BALB/c mice but not in B6 mice, resulting in deletion of Vβ5.1/2+ and Vβ11+ T-cells only in BALB/c mice. Recipient B6 CD8 cells from chimeras with 200 or 300cGy TBI showed significantly more expression of Vβ5.1/2 (P < 0.0001) compared with naïve BALB/c mice and significantly less expression of Vβ5.1/2 (P < 0.0005) compared with naïve B6 mice, indicating partial deletion. No deletion was observed in chimeras prepared with 100cGy TBI. Recipient CD4 T-cells from chimeras conditioned with 300cGy TBI showed the same relative Vβ expression as BALB/c mice, indicating that Vβ5.1/2+ and Vβ11+ subfamilies of CD4+ T-cells had been deleted completely (Fig. 2C). The levels of Vβ5.1/2+ and Vβ11+ CD4 T-cells in chimeras with 200cGy TBI were significantly higher (P < 0.05) than those in chimeras with 300cGy TBI but significantly less than naïve B6 (P < 0.0005). With 100cGy TBI, Vβ11 but not Vβ5 expression in mice was significantly lower (P < 0.05) than in naïve B6 mice. This negative selection was specific, as Vβ6 and Vβ8.1/2 subsets were not deleted.
Peripheral regulation does not appear to be required for the maintenance of chimerism and induction of tolerance in chimeras
Because incomplete Vβ deletion occurred in the current study, the role of regulatory T-cells (Treg) in maintenance of tolerance was studied in our model by depleting CD4+ T-cells to break the tolerance as this should result in the depletion of CD4+/CD25+ Treg at the same time. Anti-mouse CD4 mAb was used to deplete the CD4+ T-cells in chimeras, chimeras with islet tolerance, or non-chimeras with islet tolerance (Table 3). Three days after anti-CD4 mAb injection, >90% CD4+ T-cells were depleted. After anti-CD4 mAb treatment, all the chimeric mice showed durable and stable donor chimerism during the follow-up period. All the mice tolerant to their islets, including chimeras and non-chimeras, maintained normal levels of blood glucose except one non-chimeric mouse with islet acceptance. The blood glucose level in this mouse was >200 mg/dl at 28 days after mAb treatment. These data suggest that regulatory mechanisms of Treg do play a major role in maintenance of tolerance in our current model.
Table 3.
The effect CD4 T-cell depletion on the transplant tolerance
| Group | n | % donor | CD4 depletion | % donor | Blood glucose (mg/dl) at day 28 | Blood glucose (mg/dl) at day 50 |
|---|---|---|---|---|---|---|
| Chimeras | 7 | 11.3 ± 9.4 (2.12 – 30.50) | + | Stable > 60 days | N/Aa | N/Aa |
| Chimeras + Islet tolerance | 8 | 4.8 ± 6.8 (1.3 – 21.12) | + | Stable > 60 days | 101-136 | 102-149 |
| Non-chimeras + Islet tolerance | 8 | 0.0 ± 0.0 | + | N/Ab | 108-153, 223 | 123-150, 328 |
Not applicable as no islet transplantation was performed in this group.
Not applicable as no donor cells were detectable before CD4 depletion.
Evidence for humoral tolerance
Anti-donor antibody was present in mice at 4 weeks after receiving donor BMC without conditioning, with titers of mean fluorescence intensity (MFI) 197.6±111.8. However, anti-donor antibodies were not detected in either chimeric or non-chimeric mice that received conditioning and BMC infusion at 4 weeks as the MFI was 2.8±0.36 and 3.0±0.25 (Fig. 2D), respectively. There was no significant difference (P > 0.05) between these chimeras, non-chimeras, and naïve B6 mice (MFI: 3.2±0.26). Moreover, the humoral tolerance observed in chimeras was robust as no anti-donor antibodies were generated after donor-specific skin grafting, even in those mice that exhibited chronic rejection of donor skin.
Evidence for systemic donor-specific tolerance of innate immunity
In in vivo cytotoxicity assays (Fig. 2E), The elimination rate of donor BALB/c cells tested 1 day after cell infusion in TCR β/δ-/- mice was 83.2±6.3%. This was similar to the cytotoxic rate in wildtype B6 controls (79.8±9.7%; P > 0.05), indicating that acute rejection in wildtype mice was not T-cell-mediated. As T-cell activation requires at least 3 days after exposure to antigen, the effectors mediating donor cell rejection must be innate cells. Mixed chimeras showed 16.4±8.7% cytotoxicity to donor cells, which was not significantly different from the killing rate to syngeneic controls in the chimeras (9.9±0.8%; P >0.05). Cytotoxicity in mixed chimeras to third-party B10.BR cells was similar to that in wildtype B6 (P >0.05). These data suggest that innate immune tolerance is achieved in mixed chimeras.
Discussion
Hematopoietic stem cell (HSC) transplantation leading to allogeneic chimerism has been proposed as one approach for the induction of transplantation tolerance. Implementation of HSC transplantation has been limited by the side effects associated with myelotoxic conditioning required to establish engraftment. As a result, we and others previously found that the host-versus-graft barrier is the dominant one to control by conditioning rather than preparing vacant “niches” (18-23). This critical paradigm shift allowed the development of reduced-intensity immune-based conditioning approaches to establish chimerism. Recently, we reported in a mouse model that targeting of host CD8+ and αβ-TCR+ T-cells significantly reduced the minimum dose of TBI required for engraftment (9,21). However, at this threshold conditioning we observed a dichotomy between donor chimerism and tolerance, only animals with donor T-cell production were durably chimeric and tolerant to donor skins. The addition of cyclophosphamide to T-cell depletion-based conditioning allows donor T-cell engraftment and long-term donor-specific tolerance (9,21). In our current study, we found that pretreatment of recipients with anti-CD154 and rapamycin with or without T-cell depletion can reduce the TBI requirement to 100cGy for establishing mixed chimerism. The synergistic effect of anti-CD154 and Rapamycin observed in our current study to promote allogeneic BMC engraftment may be due to their overlapping inhibitory functions on the adaptive immune response. This conditioning strategy can replace cyclophosphamide in BMT to make the conditioning less toxic and also avoid the dissociation of donor chimerism and tolerance due to the lack of donor T-cell engraftment. These results may have direct clinical application for tolerance protocols.
When bone marrow is transplanted in a nonmyeloablatively conditioned recipient, the pluripotent hematopoietic stem cells engraft and co-exist with recipient stem cells to give rise to all hematopoietic lineages in the recipient. A hybrid immune system is established in mixed chimeras. The mutual tolerance in this hybrid immune system must be systemic as suggested by stable and durable coexistence of genetically different donor and recipient hematopoietic components in mixed chimerism. T- and B-cell tolerance in mixed chimerism has been well studied. The role for innate immune tolerance in mixed chimerism needs to be further examined.
The mechanism of T-cell tolerance has been well established through central deletional mechanisms in mouse studies, in which the allo-activated T-cells are deleted by negative selection in the thymus (21,24,24). Mixed allogeneic chimerism provides the absolute paradigm for robust bidirectional deletional tolerance. The presence of the autologous bone marrow provides the host-type of antigen presenting cells essential for full immunocompetence while the allogeneic marrow provides a persistent source of antigen for induction and maintenance of donor-specific tolerance by deletion (25,26). Functional donor-specific T-cell tolerance has been shown in in vivo MLR as lymphocytes from mixed chimeras specifically did not respond to host and donor antigens. The robustness of T-cell tolerance has been demonstrated in mixed chimeras in response to secondary donor antigen challenge from the skin graft. The chimeras did not generate CD44high/CD62Llow/- Teff cells in response to donor skin but did in response to third-party skin grafts.
Interestingly, we observed a direct correlation between the robustness of deletion of Vβ5 and Vβ11 and degree of conditioning and chimerism in our current study. The Vβ clonal deletion only partially occurred in these chimeric mice, especially in CD8+ T-cell lineage, suggesting other mechanisms in maintaining long-term tolerance. Several other mechanisms by which BMC induce antigen-specific tolerance have been proposed, including anergy (27,28), ignorance, and peripheral regulation via Treg (29,30). CD4+/CD25+ Treg have been shown to be capable of inducing and maintaining transplantation tolerance (31-34). Depletion of CD4+ T-cells resulting in CD4+/CD25+ Treg depletion did not break the tolerance either to islets or donor BMC. Our present data suggest that peripheral regulatory mechanisms of Treg may not play a major role in our current nonmyeloablative conditioning BMT model. Our findings are consistent with reports that peripheral regulatory mechanisms are not sufficient to contribute to induction of tolerance in mixed chimeras (25,26,35,36). However, the majority of non-chimeras also accepted donor islets but the T-cells from these animals exhibited strong alloreactivity to donor antigens. Central deletional mechanisms are not applicable as no alloreactive Vβ deletion is observed. The possible peripheral mechanism for acceptance of less immunogenic islet grafts in non-chimeras may involve immunomodulation by donor bone marrow cell infusion and conditioning regimens used in BMT. Similar graft acceptance was recently reported in human recipients of renal allografts plus BMC. Although chimerism was present at very low levels for ≤ 3 weeks, subjects were successfully weaned from immunosuppression (37). The intrinsic mechanisms for this immunomodulation need further investigation.
In current study, evidence for humoral tolerance in mixed chimeras is supported by the fact that there are no detectable antibodies against donor and host. The mechanism is most likely through T-cell-dependent B-cell-immune response (38-40). As activated T-cells are deleted by negative selection in mixed chimeras, there are no donor-specific antigen activated T-cells in the periphery to interact with B-cells. B-cell activation uniquely requires interaction with activated helper CD4+ T-cells. Therefore, long-term humoral immune tolerance can be achieved in mixed chimeras. Central T-cell tolerance in mixed chimeras and the consequent absence of T-cell help results in B-cell unresponsiveness. The explanation for the absence of anti-donor Ab in non-chimeric mice is due to the conditioning used at BMT, especially the use of anti-CD154 (40,41). CD154 has been demonstrated to be the critical pathway in B-cell activation (42). Alternatively, clonal deletion in vivo of B-cells has been reported as a mechanism for B-cell tolerance (43,44). This mechanism of alloreactive B-cell deletion also applies in solid organ and bone marrow transplantation for humoral tolerance (45,46).
The immune system has evolved to mount immune responses against foreign antigens. The first line of defense is the innate immune response. Until now, most research has focused on T- and B-cell responses and tolerance to transplanted grafts. Little is known about innate immune cell tolerance in mixed chimeras. If innate cell tolerance does not develop in mixed chimeras, this could result in loss of donor chimerism with time. The long-lasting and stable mixed chimerism observed in our study provides strong evidence of mutual tolerance of donor and host immune system. The T-cell-independent mechanism of early allogeneic BMC rejection was clearly demonstrated in the results showing the similar cytotoxicity between T-cell-deficient and wildtype B6 mice. Thus the effectors mediating BMC rejection at this early time would be innate immune cells: macrophages, neutrophils, or NK-cells. Therefore, the similar cytotoxicity to donor cells and to syngeneic cells and significant cytotoxicity to third-party cells in mixed chimeras suggest donor-specific innate immune tolerance is achieved in mixed chimeras. The donor and host-derived NK-cells in mixed allogeneic chimerism have been shown to be tolerant of both the donor and the recipient in allogeneic and xenogeneic settings (11,15,47). To our knowledge, this is the first experimental evidence of general innate immune tolerance other than only NK-cell tolerance induced in mixed chimeras.
In conclusion, mixed chimeras prepared with our current, very reduced intensity nonmyeloablative conditioning exhibit systemic donor-specific immune tolerance for innate immunity as well as adaptive T- and B-cell-immune responses. The achievement of donor-specific immune tolerance in mixed chimerism, a state of specific unresponsiveness to the donor graft, has the potential to overcome the current major limitations to progress in organ transplantation, including late graft loss, organ shortages and the toxicities of chronic nonspecific immunosuppressive therapy.
Materials and Methods
Animals
Male C57BL/6 (B6; H-2b), BALB/c (H-2d), and B10.BR (H-2k), B10.D2 (H-2d), B6.129P2-Tcrbtm1MomTcrdtm1Mom/J (TCRβ/δ-/-, B6 background) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in the barrier facility at the Institute for Cellular Therapeutics and cared for according to National Institutes of Health guidelines.
Chimera preparation
Recipient B6 mice were conditioned with 300-100cGy total body irradiation (TBI) (γ cell 40; Nordion, Ontario, Canada). B6 mice were pre-treated i.p. with anti-αβ-TCR (H57-597, generated in our laboratory), anti-CD154 (BioXcell, Lebanon, NH) and rapamycin (LC Laboratories, Woburn, MA) alone or in combination. 15×106 untreated BALB/c BMC were transplanted 4-6 h after TBI.
Flow cytometric analysis of TCR Vβ families
Peripheral blood (80-100μl) from unmanipulated control mice and mixed chimeras was stained with anti-Vβ 5.1/2-FITC, Vβ 6-FITC, Vβ 8.1/2-FITC or Vβ 11-FITC vs. anti-host H-2Kb-PE, anti-CD8-PerCP, and anti-CD4-APC (all from PharMingen, San Diego, CA) for 30 min at 4°C. The host lymphoid gate was used in the current study to analyze host Vβ clonal deletion.
Islet transplantation
Recipients were rendered diabetic with streptozocin (200mg/kg ip; Upjohn, Kalamazoo, MI). Mice with a positive blood glucose test (>200mg/dl) at least 3 consecutive days were used as recipients. Each animal received approximately 500-600 mouse islets transplanted beneath the left renal capsule. Blood glucose was monitored daily for the first 2 weeks and weekly thereafter. Rejection was considered if positive results were at 3 consecutive days.
Flow cytometric cross-match (FCXM) assay
0.5×106 splenocytes from naive BALB/c mice were incubated with 5μl of sera for 30 min. The cells were washed and incubated with FITC-conjugated polyclonal goat anti-mouse Ig (Immunology Consultants Laboratory, Inc., Newberg, OR), followed by a third incubation with PE-conjugated anti-CD4 plus -CD8 (PharMingen). Levels of circulating alloantibodies were determined by FACSCalibur (BD Biosciences, Mountain View, CA), gating on the CD4+ plus CD8+ T-cell fraction.
Statistical analysis
Data are presented as Average ± Standard Deviation (SD). Two tailed Student’s t-test (two sample assuming unequal variances) was used to evaluate statistical differences. The difference between groups was considered to be significant when P < 0.05.
Acknowledgments
The authors thank Carolyn DeLautre for manuscript preparation; and the staff of the University of Louisville animal facility for outstanding animal care.
Abbreviations
- BMT
bone marrow transplantation
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cells
- FCXM
flow cytometry cross-match
- HSC
hematopoietic stem cell
- MFI
mean fluorescence intensity
- MST
mean survival time
- SD
standard Deviation
- Treg
regulatory T-cells
Footnotes
This work was supported in part by the following: NIH R01 DK069766 and NIH 5RO1 HL063442; This publication was also made possible by Award No.W81XWH-07-1-0185 and W81XWH-09-2-0124 from the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD,21702-5014 (Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Army Research); the Commonwealth of Kentucky Research Challenge Trust Fund; the W. M. Keck Foundation; and The Jewish Hospital Foundation. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statues relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. This work is dedicated to the memory of Michael Tanner.
Reference List
- 1.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 2.Farges O, Saliba F, Farhamant H, et al. Incidence of rejection and infection after liver transplantation as a function of the primary disease: possible influence of alcohol and polyclonal immunoglobulins. Hepatology. 1996;23:240–248. doi: 10.1053/jhep.1996.v23.pm0008591847. [DOI] [PubMed] [Google Scholar]
- 3.Penn I. Malignancy. Surg Clin N Am. 1994;74:1247–1257. [PubMed] [Google Scholar]
- 4.Platz KP, Mueller AR, Blumhardt G, et al. Nephrotoxicity after orthotopic liver transplantation in cyclosporin A and FK 506-treated patients. Transpl Int. 1994;7(Suppl 1):S52–S57. doi: 10.1111/j.1432-2277.1994.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 5.Vercauteren SB, Bosmans JL, Elseviers MM, Verpooten GA, De Broe ME. A meta-analysis and morphological review of cyclosporine-induced nephrotoxicity in auto-immune diseases. Kidney Int. 1998;54:536–545. doi: 10.1046/j.1523-1755.1998.00017.x. [DOI] [PubMed] [Google Scholar]
- 6.Singer A, Hathcock KS, Hodes RJ. Self recognition in allogeneic radiation bone marrow chimeras. A radiation-resistant host element dictates the self specificity and immune response gene phenotype of T-helper cells. J Exp Med. 1981;153:1286–1301. doi: 10.1084/jem.153.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinkernagel RM, Althage A, Callahan G, Welsh RM. On the immunoincompetence of H-2 incompatible irradiated bone marrow chimeras. J Immunol. 1980;124:2356–2365. [PubMed] [Google Scholar]
- 8.Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras: Immunocompetence, in vitro reactivity and genetic specificity of tolerance. J Exp Med. 1985;162:231–244. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Chilton PM, Huang Y, Schanie CL, Yan J, Ildstad ST. Addition of cyclophosphamide to T-cell depletion based nonmyeloablative conditioning allows donor T-cell engraftment and clonal deletion of alloreactive host T-cells after bone marrow transplantation. Transplantation. 2007;83:954–963. doi: 10.1097/01.tp.0000258679.18684.b0. [DOI] [PubMed] [Google Scholar]
- 10.Pilat N, Baranyi U, Klaus C, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 2010;10:751–762. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Ohdan H, Manilay JO, Sykes M. NK cell tolerance in mixed allogeneic chimeras. J Immunol. 2003;170:5398–5405. doi: 10.4049/jimmunol.170.11.5398. [DOI] [PubMed] [Google Scholar]
- 12.Sharabi Y, Aksentijevich I, Sundt TM, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: Production of mixed rat/mouse lymphohematopoietic chimeras using a non-lethal preparative regimen. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohdan H, Yang YG, Swenson KG, Kitamura H, Sykes M. T cell and B cell tolerance to GALalpha1,3GAL-expressing heart xenografts is achieved in alpha1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Transplantation. 2001;71:1532–1542. doi: 10.1097/00007890-200106150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Aksentijevich I, Sachs DH, Sykes M. Humoral tolerance in xenogeneic BMT recipients conditioned by a nonmyeloablative regimen. Transplantation. 1992;53:1108–1114. doi: 10.1097/00007890-199205000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara T, Rodriguez-Barbosa JI, Zhao Y, Zhao G, Sykes M. Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am J Transplant. 2007;7:2090–2097. doi: 10.1111/j.1600-6143.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyse EA, Carswell EA, Scheid MP, Old LJ. Tolerance of Sk-incompatible skin grafts. Nature. 1973;244:441–442. doi: 10.1038/244441a0. [DOI] [PubMed] [Google Scholar]
- 17.Steinmuller D, Lofgen JS. Differential survival of skin and heart allografts in radiation chimeras provides further evidence of skin histocompatibility antigens. Nature (Lond) 1974;248:796–797. doi: 10.1038/248796a0. [DOI] [PubMed] [Google Scholar]
- 18.Colson YL, Wren SM, Schuchert MJ, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 19.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–2829. [PubMed] [Google Scholar]
- 20.Xu H, Exner BG, Cramer DE, Tanner MK, Mueller YM, Ildstad ST. CD8(+), αβ-TCR(+), and γδ-TCR(+) Cells in the Recipient Hematopoietic Environment Mediate Resistance to Engraftment of Allogeneic Donor Bone Marrow. J Immunol. 2002;168:1636–1643. doi: 10.4049/jimmunol.168.4.1636. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Chilton PM, Huang Y, Schanie CL, Ildstad ST. Production of donor T cells is critical for induction of donor-specific tolerance and maintenance of chimerism. J Immunol. 2004;172:1463–1471. doi: 10.4049/jimmunol.172.3.1463. [DOI] [PubMed] [Google Scholar]
- 22.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 24.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 25.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 26.Sykes M, Szot GL, Swenson KA, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nat Med. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Cobbold S, Benjamin R, Waldmann H. Induction of classical transplantation tolerance in the adult. J Exp Med. 1989;169:779–794. doi: 10.1084/jem.169.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayumi H, Himeno K, Tanaka K, Tokuda N, Fan JL, Nomoto K. Drug-induced tolerance to allografts in mice. IX. Establishment of complete chimerism by allogeneic spleen cell transplantation from donors made tolerant to H-2-identical recipients. Transplantation. 1986;42:417–422. [PubMed] [Google Scholar]
- 29.Qin S, Cobbold SP, Pope H, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 30.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607. [PubMed] [Google Scholar]
- 31.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168:2274–2281. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 33.Li XC, Wells AD, Strom TB, Turka LA. The role of T cell apoptosis in transplantation tolerance. Curr Opin Immunol. 2000;12:522–527. doi: 10.1016/s0952-7915(00)00133-3. [DOI] [PubMed] [Google Scholar]
- 34.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 35.Graca L, Le MA, Lin CY, Fairchild PJ, Cobbold SP, Waldmann H. Donor-specific transplantation tolerance: the paradoxical behavior of CD4+CD25+ T cells. Proc Natl Acad Sci U S A. 2004;101:10122–10126. doi: 10.1073/pnas.0400084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvinen LZ, Blazar BR, Adeyi OA, Strom TB, Noelle RJ. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–1379. doi: 10.1097/01.TP.0000093462.16309.73. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard A, Coitot S, Bremont A, Bernard G. T and B cell cooperation: a dance of life and death. Transplantation. 2005;79:S8–S11. doi: 10.1097/01.tp.0000153290.75695.31. [DOI] [PubMed] [Google Scholar]
- 39.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Yan J, Huang Y, et al. Co-stimulatory blockade of CD154:CD40 in combination with T-cell lymphodepletion results in prevention of allogeneic sensitization. Blood. 2008;111:3266–3275. doi: 10.1182/blood-2006-10-053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Huang Y, Chilton PM, et al. Strategic nonmyeloablative conditioning: CD154:CD40 costimulatory blockade at primary bone marrow transplantation promotes engraftment for secondary bone marrow transplantation after engraftment failure. J Immunol. 2008;181:6616–6624. doi: 10.4049/jimmunol.181.9.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop GA, Hostager BS. Molecular mechanisms of CD40 signaling. Arch Immunol Ther Exp (Warsz) 2001;49:129–137. [PubMed] [Google Scholar]
- 43.Nemazee D, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 44.Hartley SB, Crosbie J, Brink R, Kantor AA, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci U S A. 2007;104:12093–12098. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M. Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection. J Clin Invest. 1999;104:281–290. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Rezzoug F, Chilton PM, Grimes HL, Cramer DE, Ildstad ST. Matching at the MHC Class I K locus is essential for long-term engraftment of purified hematopoietic stem cells: a role for host NK cells in regulating HSC engraftment. Blood. 2004;104:873–880. doi: 10.1182/blood-2003-11-3910. [DOI] [PubMed] [Google Scholar]