Abstract
MicroRNAs (miRNAs) are critical regulators of nervous system function, and in vivo knockout studies have demonstrated that miRNAs are necessary for multiple aspects of neuronal development and survival. However, the requirements of miRNA biogenesis in the formation and function of synapses in the cerebral cortex are only minimally understood. Here, we have generated and characterized a mouse line with a conditional neuronal deletion of Dgcr8, a miRNA biogenesis protein predicted to process miRNAs exclusively. Loss of Dgcr8 in pyramidal neurons of the cortex results in a non-cell-autonomous reduction in parvalbumin interneurons in the prefrontal cortex, accompanied by a severe deficit in inhibitory synaptic transmission and a corresponding reduction of inhibitory synapses. Together, these results suggest a vital role for miRNAs in governing essential aspects of inhibitory transmission and interneuron development in the mammalian nervous system. These results may be relevant to human diseases such as schizophrenia, where both altered Dgcr8 levels as well as aberrant inhibitory transmission in the prefrontal cortex have been postulated to contribute to the pathophysiology of the disease.
Keywords: microRNA, Dgcr8, inhibitory synaptic transmission, parvalbumin interneurons, prefrontal cortex
1 Introduction
MicroRNAs (miRNAs) constitute a class of highly-conserved, ~22–24 nucleotide (nt) long non-coding RNAs that bind to the 3′ untranslated region of target mRNA species, leading to translational repression or mRNA degradation (reviewed in Bartel, 2004). The classifications of miRNAs and other small non-coding RNAs have been determined conventionally by the sequential processing reactions that constitute their biogenic pathway (reviewed in Cao et al., 2006; Kim, 2005). Canonical, or “classic,” miRNAs are generated by the double-stranded RNA-binding protein DiGeorge Critical Region Gene 8 (Dgcr8), which complexes with the RNase III enzyme Drosha to cleave initially long primary-miRNA transcripts into ~70 nt stem-loop precursor-miRNAs. These in turn are then cleaved by the RNase III endonuclease Dicer to produce 22–24 nt mature miRNAs.
In mammals, miRNAs are expressed in the brain (Krichevsky et al 2003; Sempere 2003; Miska 2004) where their abundance and powerful regulatory capabilities may have particular importance for controlling both homeostatic and dynamic aspects of neural function. Initial investigations of miRNA-dependent phenotypes in rodent brain have principally focused on characterizations of total miRNA knockdown via genetic ablation of Dicer. These in vivo and in vitro studies have ascribed critical roles for miRNAs in regulating developmental processes including neurogenesis, proliferation, cell fate determination, and survival (Cheng et al 2009; Shibata et al 2008; Davis et al 2008; De Pietri Tonelli 2008). These and other seminal reports have greatly advanced the miRNA field; however, recent studies now indicate that Dicer does not exclusively process miRNAs and in fact has important functions outside of the miRNA pathway. Consequently, some of the phenotypes associated with Dicer deletion could be attributable to its miRNA-independent effects, via pathways involving endogenous small interfering RNAs, non-canonical miRNAs, or Alu RNA elements (Babiarz et al., 2008; Kaneko et al., 2011; Shapiro et al., 2010). Because the RNase III enzyme Drosha has been reported to have a distinct role in pre-rRNA processing (Wu et al., 2000) Dgcr8 may be the only protein within the processing pathway that is specific to miRNAs. Therefore, identifying the neuronal phenotypes associated with Dgcr8 loss might be the only experimental paradigm capable of deconvoluting the specific roles of miRNAs within the brain. Moreover, the development of cortical microcircuitry and synaptic function remain almost entirely unexplored in this context.
In order to investigate these outstanding questions, we generated a novel mouse line with Dgcr8 conditionally deleted from principal neurons of the cortex (Dgcr8fl/fl; Cre) using a Cre line driven by the NEX promoter (Goebbels et al., 2006). These mice presented with a dramatic phenotype, including ataxia, tremors and microencephaly. Analysis of synaptic currents onto Dgcr8fl/fl;Cre pyramidal neurons in the prefrontal cortex (PFC) revealed a strong decrease in the frequency of inhibitory postsynaptic currents (IPSCs). Coinciding with this deficit were selective reductions in parvalbumin interneuron numbers and perisomatic inhibitory synapses. Together, these findings demonstrate for the first time the consequences of conditional, specific miRNA loss in the mammalian cortex as well as reveal an important non-cell-autonomous role for miRNAs on inhibitory synaptic transmission and interneuron development.
2 Materials and Methods
2.1 Generation of conditional knockout mice
Conditional deletion mice were generated using a previously established line in which the third exon of Dgcr8 was floxed (Dgcr8fl/fl) via targeted homologous recombination (Rao et al., 2009; Wang et al., 2007). Mice were maintained in a C57/Bl6 background and backcrossed at least 4generations. Dgcr8fl/fl mice were crossed to a line expressing Cre under the endogenous NEX promoter, which is expressed nearly exclusively in pyramidal cells from the neocortex and hippocampus (Goebbels et al., 2006). A heterozygous mating scheme was used to maintain at least one wildtype copy of the NEX gene in all progeny. A tdTomato strain driven by the ubiquitous Rosa promoter followed by a loxP-flanked STOP cassette (Rosa-LSL-tdTomato, Jackson Labs) was also crossed to the Dgcr8fl/fl;Cre line. In this line, fluorescent tdTomato was expressed in all cells having undergone Cre recombination; tdTomato could therefore be used as a reporter for Cre expression. All animals used in this study were Dgcr8fl/fl;Cre or Dgcr8fl/fl littermate controls. Animal procedures were performed according to protocols approved by the appropriate Institutional Animal Care and Use Committee under the federal and state regulations.
2.2 qRT-PCR
Total RNA was extracted from the PFC of P16-P17 mice using traditional Trizol methods. To evaluate gene expression, 100ng of total RNA was used to generate cDNA using the Taqman Reverse Transcription Kit (Applied Biosystems). qPCR was performed using SYBR GreenER qPCR SuperMix (Invitrogen) on a CFX96 Real-Time System and a C1000 Thermal Cycler (Bio-Rad). GAPDH was used as an internal control. To evaluate miRNA expression, 1μg of total RNA was reverse-transcribed using the Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems). miRNA qPCR was performed using the Taqman Universal PCR Master Mix (Applied Biosystems) and custom-designed Taqman probes (IDT DNA) using techniques previously described (Tang et al., 2006). For sequences, see http://urology.ucsf.edu/blellochlab/protocols.htm. The U6 snRNA (ABI) was used as an internal control. All qPCR reactions were performed in triplicate and relative quantifications were calculated using the Pfaffl method (Pfaffl, 2001).
2.3 Anatomy and cellular analysis
For Nissl staining, brains were extracted from P16-P17 animals and fixed overnight in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. After cryoprotection in 30% sucrose, 25 μm coronal sections were serially collected using a Leica CM1900 cryostat. Sections were stained using an aqueous chrome alum-gallocyanine method (Kiernan, 1990) and images were obtained at 2.5x magnification using an Olympus SZH10 microscope and at 10x using a Zeiss Axio Imager M1fluorescent microscope. Anatomical measurements were evaluated using ImageJ software (Rasband, NIH). Cross sectional cortical thickness was measured across the primary somatosensory area at Bregma -0.08; brain diameter was measured at the widest point across at Bregma -0.08; and the cross-sectional area of the hippocampus was measured at Bregma -2.055. Prefrontal cortical measurements were taken at Bregma 1.545 (Bregma values are given for corresponding coronal sections in the wildtype based on anatomical landmarks). For Golgi staining, the FD Rapid Golgi stain Kit (FD Neurotechnologies) was performed according to the manufacturer’s instructions. Sholl analyses were conducted on Golgi-impregnated sections using NeuronJ, a customized plug-in for ImageJ. Pyramidal neurons from the medial prefrontal cortex region were analyzed, and dendritic intersections were counted with a radius step size of 12 μm. TUNEL labeling was performed in PFC coronal sections using the FragEL DNA Detection Kit (Calbiochem) according to the manufacturer’s instructions.
2.4 Chemoconvulsant-induced seizures
Prolonged seizures were induced in P17–18 Dgcr8fl/fl;Cre and Dgcr8fl/fl animals by intraperitoneal (IP) injection of pilocarpine (320 mg/kg, Sigma). Scopolamine methylnitrate (1 mg/kg, Sigma) was administered via IP injection 30 min prior to the pilocarpine injection to limit peripheral cholinergic effects. Mice were observed for at least 30 minutes after pilocarpine injection, and seizures were quantified by using the classification of Racine (1972). According to this classification, seizures are defined by facial automatisms, head and body tremors, forelimb clonus, rearing, and falling. Stage 3 is defined by the onset of forelimb clonus. The latency to the first stage 3 seizure after pilocarpine injection was recorded.
2.5 Electrophysiology
Prefrontal cortical slices were prepared from Dgcr8fl/fl;Cre and littermate control mice of either sex, age P15–17. Animals were anesthetized with isoflurane and decapitated, and the whole brain was removed and transferred into ice-cold cutting solution containing (in mM): 75 sucrose, 87 NaCl, 25 glucose, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2, equilibrated with 95% O2/5% CO2. Coronal sections 250–275 μm thick were cut on a vibratome and then transferred into a 33°C incubation chamber with artificial cerebral spinal fluid (ACSF) containing (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 1 CaCl2, and 10 glucose, equilibrated with 95% O2/5% CO2 prior to recording. Whole-cell patch-clamp recordings were performed using a MultiClamp 700A amplifier (Molecular Devices) in a submerged bath recording chamber superfused with ACSF at a rate of 2 mL/minute. Recording electrodes were made of borosilicate glass and had a resistance of 2.5–4 MΩ when filled with intracellular solution, which contained (in mM): 135 CsCl, 10 HEPES, 10 EGTA, 5 QX-314 and 2 MgCl2. For recording IPSCs, cells were voltage clamped at −60 mV and the external ACSF contained 20 μM DNQX and 50 μM AP-5. For miniature IPSC (mIPSC) recordings 1 μM tetrodotoxin (TTX, Ascent Scientific) was included in the ACSF. For excitatory postsynaptic current (EPSC) and current-clamp recordings the intracellular solution contained (in mM): 110 K-gluconate, 20 KCl, 10 HEPES, 0.4 EGTA, 2 MgCl2; pH was 7.25 and osmolarity was adjusted to 280–290 mOsm with sucrose. During voltage clamp recordings, cells were clamped at −70 mV and IPSCs were blocked by bath application of the GABA-A receptor antagonist bicuculline methiodide (10 μM, Tocris). The liquid junction potential was 12.3 mV and corrected. Access resistance was monitored and cells were included for analysis only if the series resistance was <20 MΩ and the change of resistance was <25% over the course of the experiment. Data were acquired at 50 kHz using pClamp 10.2 (Molecular Devices) and filtered at 2 kHz.
2.6 Immunohistochemistry
P16-P17 animals were deeply anesthetized and transcardially perfused with saline followed by 4% PFA. Brains were then harvested, post-fixed, and cryoprotected in 30% sucrose. Coronal sections were collected at 20μm using a Leica CM1900 cryostat. Staining was performed in phosphate buffer (PB) containing 0.2% Triton-X and 10% goat serum overnight at 4°C using antibodies against calretinin (1:3000, Millipore), somatostatin (1:250, Millipore), or parvalbumin (1:1000, Swant). AlexaFluor (Invitrogen) secondaries were used at 1:1000 for 1 hour. Sections were mounted using Vectashield with DAPI (Vector Labs) and imaged using a Zeiss Axio Imager M1fluorescent microscope. To quantify cell number, ImageJ was used to count the absolute number of cells across all areas of a coronal slice taken at Bregma 1.545. In order to assess the integrity of cortical lamination, 40μm sections were stained using the upper layer marker Cux1 (1:250 Santa Cruz) and the deeper layer marker Foxp2 (1:1000 AbCam). For staining of inhibitory synapses, a tissue slab containing the PFC was dissected on ice and lightly fixed in 2% PFA in 0.1 M PB for 20 minutes followed by cryoprotection in 30% sucrose. 20 μm coronal sections were stained against VGAT (1:1000, Synaptic Systems) and gephyrin (1:1000, Synaptic Systems) overnight at 4°C, followed by AlexaFluor secondary staining (1:1000). TrkB (1:1000 BD Biosciences) and PV co-staining was performed using the same light fixation technique. Sections were imaged on a LSM 5 Pascal Confocal microscope. Inhibitory synapses were quantified by counting the number of overlapping VGAT and gephyrin puncta and dividing by the total number of VGAT plus gephyrin puncta. To quantify perisomatic synapses, 15 μm-diameter circles were traced around DAPI-labeled nuclei and the number of overlapping puncta within the region was divided by the total number of overlapping puncta in the field of view.
2.7 Western blotting
Total PFC brain lysates were extracted from P16-P17 animals using a lysis buffer containing 150 mM NaCl, 50mM Tris pH = 8.0, 1% Triton-X 100 with complete protease inhibitors (Roche). 15μg of protein per well was loaded into a 10% Bis-Tris SDS-PAGE gel (Invitrogen) under denaturing conditions. Proteins were transferred to PVDF membranes (Millipore), blocked in TBS-T, and blotted against TrkB (1:1000, B.D. Biosciences), BDNF (1:500, courtesy of Xin-Fu Zhou (Zhou and Rush, 1996)), or actin (1:1000, Sigma) overnight at 4°C.
3 Results
3.1 Conditional deletion of Dgcr8 results in microencephaly and decreased neuron size
In order to specifically remove miRNAs from principal neurons of the cortex, we generated a conditional Dgcr8 knockout mouse line (Dgcr8fl/fl;Cre) by crossing a floxed Dgcr8 allele mouse strain (Rao et al., 2009; Wang et al., 2007) to a mouse expressing Cre recombinase driven by the endogenous NEX promoter. In this line, Cre recombinase is expressed in post-mitotic cortical pyramidal neurons and is absent from GABAergic interneurons (Goebbels et al., 2006). To confirm loss of Dgcr8 and miRNAs in the cortex, we performed quantitative real-time PCR on Dgcr8 and on a select panel of miRNAs highly expressed in neurons using total RNA isolated from Dgcr8fl/fl;Cre and Dgcr8fl/fl controls. Dgcr8 levels were reduced compared to controls, as expected (39 ± 5%; Dgcr8fl/fl n = 7; Dgcr8fl/fl;Cre n = 6, p < 0.0005), and we also observed significant reductions in miRNA levels in the Dgcr8fl/fl;Cre compared to controls (miR-23a: 43 ± 9%; miR-124: 56 ± 8%, miR-132: 87 ± 2%; miR-134: 41 ± 9%; let-7c: 24 ± 12%; Dgcr8fl/fl n = 7; Dgcr8fl/fl;Cre n = 6, p < 0.05; Supplemental Figure 1).
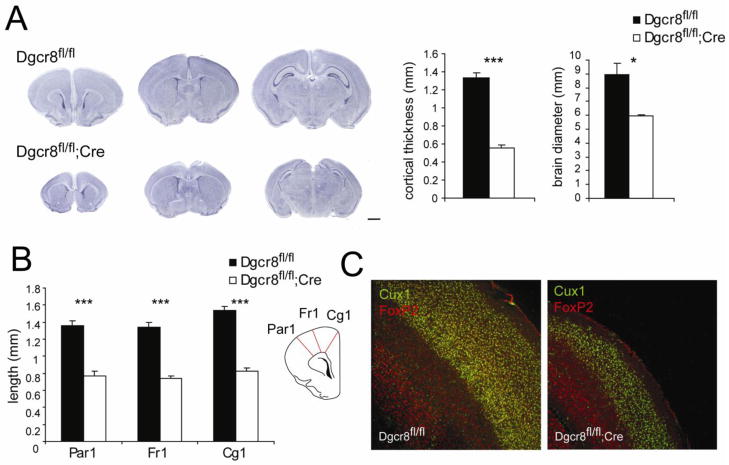
We initially evaluated the impact of Dgcr8 loss on gross neuroanatomy by performing Nissl staining on control and Dgcr8fl/fl;Cre brains. Similar to previous findings in a conditional Dicer knockout (Davis et al., 2008), we found a dramatic reduction in brain diameter (Dgcr8fl/fl = 8.67 ± 0.79 mm; Dgcr8fl/fl;Cre = 5.83 ± 0.08 mm; p = 0.02; Figure 1A), cortical thickness (Dgcr8fl/fl = 1.33 ± 0.06 mm; Dgcr8fl/fl;Cre = 0.56 ± 0.04 mm; p = 0.0003; Figure 1A), and hippocampal size (Dgcr8fl/fl = 2.36 ± 0.34 mm2; Dgcr8fl/fl;Cre = 0.70 ± 0.03 mm2; p = 0.009; n = 6 per genotype; example shown in Fig 1A). Frontal cortex measurements were also greatly reduced (parietal cortex: Dgcr8fl/fl = 1.36 ± 0.06 mm; Dgcr8fl/fl;Cre = 0.77 ± 0.06 mm; p < 0.0001; frontal cortex: Dgcr8fl/fl = 1.34 ± 0.06 mm; Dgcr8fl/fl;Cre = 0.74 ± 0.03 mm; p < 0.0001; cingulate cortex: Dgcr8fl/fl = 1.54 ± 0.05 mm; Dgcr8fl/fl;Cre = 0.83 ± 0.04 mm; p < 0.0001; n = 6 per genotype; Figure 1B). To investigate whether the severe microcephaly was due to a failure of newly-born neurons to migrate to their proper cortical positions, we examined gross cortical layer organization using immunocytochemistry. Staining for upper (Cux1) and deeper (Foxp2) layer cortical markers showed that these layer positional identities were retained, suggesting overall intact lamination (n = 2 per genotype; Figure 1C).
Figure 1. Gross anatomical phenotypes of Dgcr8fl/fl vs Dgcr8fl/fl;Cre mice.
(A) Nissl staining showing microencephaly of the Dgcr8fl/fl;Crebrain. Scale bar = 1 mm. Quantification reflects reduced cortical thickness and brain diameter in the conditional Dgcr8fl/fl;Cre. (B) Nissl staining quantification shows reduced PFC measurements in the Dgcr8fl/fl;Cre. (C) Immunostaining for the upper layer marker Cux1 (green) and the deeper layer marker FoxP2 (red) demonstrated that overall cortical positional identities for these markers were retained in Dgcr8fl/fl;Cre animals. Error bars represent SEM.
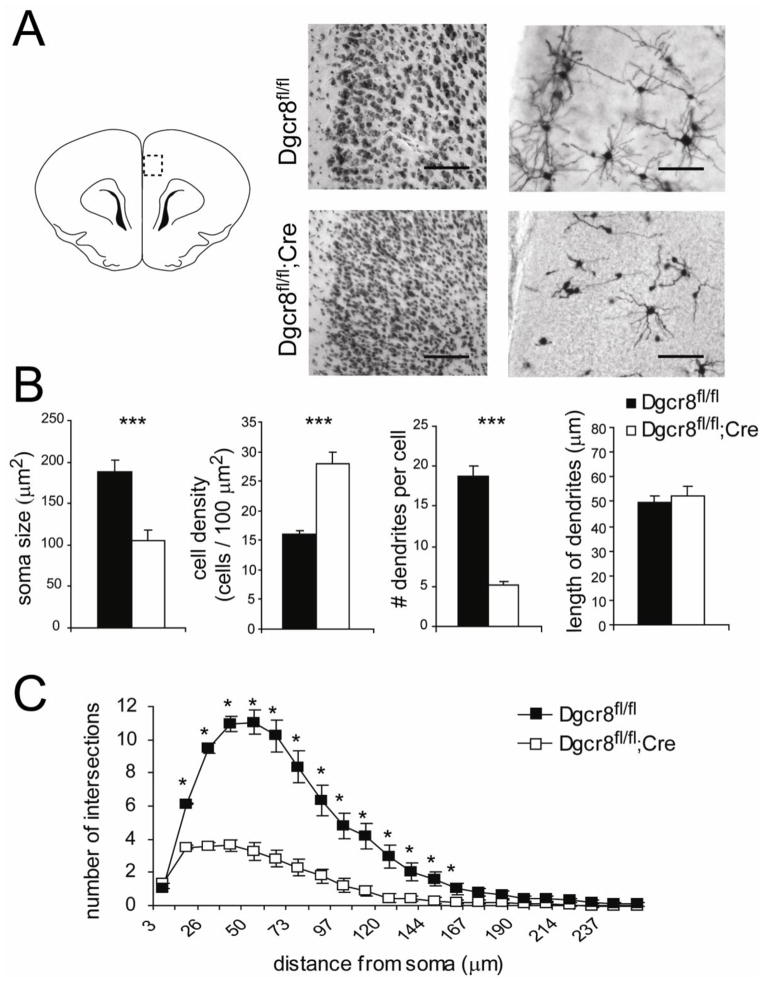
We next sought to determine whether the cortical microencephaly was due to a reduction in cell number, a reduction of cell size, or both. First, cell density of the PFC region was evaluated by counting cells per μm2. An increase in cell density was observed in the Dgcr8fl/fl;Cre animals (Dgcr8fl/fl = 16.1 ± 0.6 cells/100 μm2; Dgcr8fl/fl;Cre = 27.9 ± 2.1 cells/100 μm2; p < 0.0001; n = 6 per genotype). This was accompanied by an increase in cell death as revealed by TUNEL staining (n = 2 per genotype, Supplemental Figure 2) as well as a significant shrinkage in the average soma size (Dgcr8fl/fl = 188.5 ± 13.8 μm2; Dgcr8fl/fl;Cre = 105.1 ± 13.4 μm2; p < 0.001; Figure 2A, B), demonstrating that both cell loss and a reduction in cell body size contribute to the loss in cortical thickness. Golgi staining of PFC neurons supported the finding of cell shrinkage, as neurons in the Dgcr8fl/fl;Cre displayed reduced dendritic arborization, with only 5.17 ± 0.48 basal dendrites per neuron compared to 18.70 ± 1.26 in the controls (n = 30 neurons from 4 animals per genotype, p < 0.001). No differences were observed in the average length of dendrites (Dgcr8fl/fl = 49.9 ± 2.6 μm; Dgcr8fl/fl;Cre = 52.6 ± 3.8 μm; Figure 2B). Quantification by Sholl analysis confirmed a reduction in dendritic complexity in Dgcr8fl/fl;Cre neurons (Figure 2C). Together, these anatomical findings indicate that Dgcr8-dependent miRNA biogenesis is required for the proper morphogenesis of cerebral cortical neurons.
Figure 2. Reduced soma size and dendritic elaboration in the cortex of the Dgcr8fl/fl;Cre.
(A) Cellular quantification in the medial PFC region of Dgcr8fl/fl under 10x Nissl (left) and 10x Golgi staining (right). Scale bars = 0.1 mm. (B) Soma size is reduced, cell density is increased, and the number of dendrites per cell is significantly reduced in the Dgcr8fl/fl;Cre. However, the average dendritic length is unchanged. (C) Sholl analysis of mPFC pyramidal neurons reflects reduced dendritic elaboration in the Dgcr8fl/fl;Cre. Error bars represent SEM.
3.2 Conditional deletion of Dgcr8 results in increased susceptibility to spontaneous and chemoconvulsant-induced seizures
Dgcr8fl/fl;Cre mice display a severe behavioral phenotype, including clasping, ataxia and tremors (Supplemental Figure 3A), and they succumb to early postnatal lethality. To determine whether these phenotypes were accompanied by other neurological symptoms such as seizures, Dgcr8fl/fl and Dgcr8fl/fl;Cre mice were monitored for susceptibility to spontaneous and chemoconvulsant-induced seizures. Behavioral observation of Dgcr8fl/fl;Cre mice indicated a predisposition to spontaneous seizure activity induced by cage-handling stress; precautions were therefore taken to ensure minimal stress of the mice prior to seizure testing. Chemoconvulsant-induced seizures were provoked by IP injection of 320 mg/kg pilocarpine preceded 30 min by 1 mg/kg scopolamine methylnitrate. Seizures were provoked in both genotypes, but Dgcr8fl/fl;Cre mice exhibited a significantly shortened latency to stage 3 seizures as compared to their control littermates. Dgcr8fl/fl mice developed stage 3 seizures in 14.15 ± 3.23 min (n = 5), whereas Dgcr8fl/fl;Cr mice progressed to stage 3 seizures in 5.31 ± 0.67 min (n = 6; p = 0.016; Supplemental Figure 3B).
3.3 Reduced inhibitory postsynaptic currents at Dgcr8fl/fl;Cre pyramidal neurons
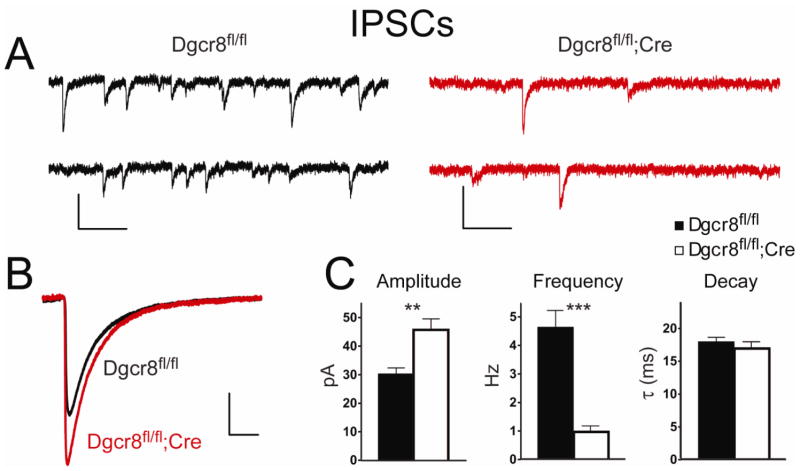
Common forms of ataxia, seizures, and tremors are predicted to be primarily GABAergic in origin (Chiu et al., 2005). Accordingly, we hypothesized that there might be perturbations to inhibitory circuitry in the cortex of the conditional Dgcr8 knockout mice. To assess this, we performed whole-cell patch-clamp electrophysiological recordings on layer II/III pyramidal neurons in the PFC of Dgcr8fl/fl (n = 13) and Dgcr8fl/fl;Cre mice (n = 13; Figure 3) and examined pharmacologically isolated spontaneous IPSC events. Here, we found a profound reduction in the frequency of IPSCs in Dgcr8fl/fl;Cre mice (Dgcr8fl/fl = 4.6 ± 0.6 Hz; Dgcr8fl/fl;Cre = 1.0 ± 0.2 Hz; p < 0.0001 Figure 3A, C). Coinciding with this deficit in frequency, the remaining events in Dgcr8fl/fl;Cre mice displayed a significant increase in IPSC amplitude (Dgcr8fl/fl = 30 ± 2 pA; Dgcr8fl/fl;Cre = 46 ± 4 pA; p = 0.002; Figure 3A, C). The kinetics profiles of IPSC events were similar between genotypes (Figure 3B, C). Because alterations in IPSC frequency and amplitude can be attributable to changes in the action potential firing capabilities of presynaptic interneurons, we examined the mIPSC population by recording events in the presence of 1 μM tetrodotoxin. Similar to the spontaneous data, we found a striking reduction in mIPSC event frequency (Dgcr8fl/fl = 3.9 ± 0.5 Hz; Dgcr8fl/fl;Cre = 0.6 ± 0.1 Hz; p < 0.0001) and gain in mIPSC amplitude (Dgcr8fl/fl = 24 ± 1 pA; Dgcr8fl/fl;Cre = 30 ± 2 pA; p = 0.02). Together, these data show that Dgcr8-dependent miRNA biogenesis is required for the normal development of inhibitory synaptic transmission, and pyramidal neurons deficient for Dgcr8 display a decrease in IPSC frequency that is accompanied by an increase in IPSC amplitude.
Figure 3. Altered inhibitory synaptic transmission in pyramidal neurons in the Dgcr8 conditional knockout cortex.
Representative IPSC recordings from layer II/III pyramidal neurons from Dgcr8fl/fl and Dgcr8fl/fl;Cre mice. Scale bar = 50 pA, 200 ms. (B) Mean IPSC responses (average of >50 isolated events) from individual Dgcr8fl/fl (black) and Dgcr8fl/fl;Cre (red) neurons superimposed on the same scale illustrate the gain in amplitude with no changes to IPSC kinetics. (C) Summary of IPSC parameters from Dgcr8fl/fl (n = 13) and Dgcr8fl/fl;Cre (n = 13) neurons demonstrate a statically significant decrease in frequency and increase in amplitude. The biexponential decay time (τ) was unaltered. Error bars represent SEM.
3.4 Perisomatic inhibitory synapses are reduced onDgcr8 knockout pyramidal neurons
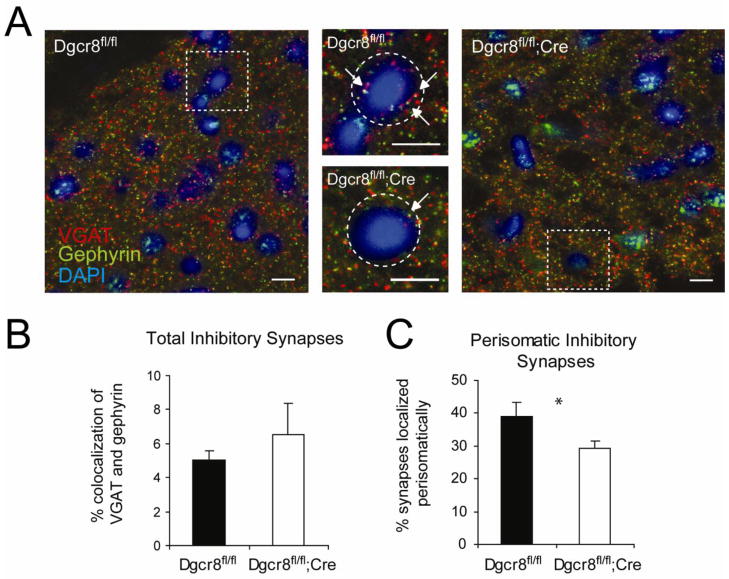
In order to determine whether changes in inhibitory synapse number could account for the changes in IPSC event frequency, we used immunohistochemical colocalization of the presynaptic vesicular GABA transporter (VGAT) and postsynaptic gephyrin to quantify the number of inhibitory synapses. Quantification of the percentage of colocalized pre- and post-synaptic puncta in the PFC revealed no significant differences between Dgcr8 knockouts and wildtypes, suggesting no change in the total number of inhibitory synapses in this region (Dgcr8fl/fl = 11.8 ± 1.2%, n = 3 animals; Dgcr8fl/fl;Cre = 14.2 ± 3.6%, n = 3 animals; Figure 4A, B). However, certain classes of inhibitory neurons form a small percentage of the total number of synapses yet exert a powerful influence on inhibitory drive due to the localization and properties of these synapses (Markram et al., 2004; Miles et al., 1996). We therefore extended our examination to specifically quantify the inhibitory synapses surrounding the cell bodies. This analysis revealed a 25% decrease in the number of inhibitory synapses localized perisomatically (Dgcr8fl/fl = 39.2 ± 4.1%, n = 3 animals; Dgcr8fl/fl;Cre = 29.4 ± 2.1%, n = 3 animals; p = 0.02; Figure 4C). We therefore predict that this reduction in perisomatic inhibitory synapses can at least in part account for the reduction in IPSC frequency.
Figure 4. Immunostaining of inhibitory synapses in the PFC.
(A) VGAT (red), gephyrin (green), and DAPI (blue) staining taken at 63x. Scale bars on all images = 10 μm. Arrows indicate colocalization of VGAT and gephyrin signal within the perisomatic space. (B) Quantification of VGAT and gephyrin colocalization as a measure of total inhibitory synapses in the PFC. (C) Percentage of colocalized signal that was located perisomatically, reflecting a reduction in perisomatic inhibitory synapses in the Dgcr8fl/fl;CrePFC. Error bars represent SEM.
3.5 Selective loss of parvalbumin interneurons in the PFC of Dgcr8 conditional knockouts
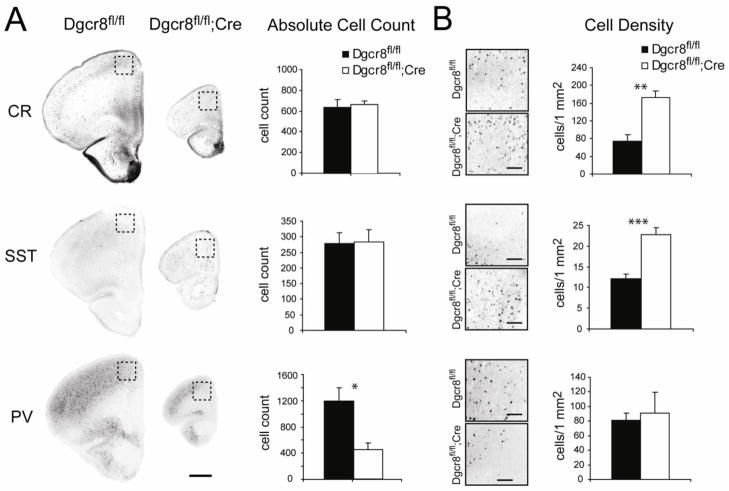
In order to determine whether the changes in inhibitory transmission and the selective reduction of perisomatic inhibitory synapses could be attributed to a specific class of interneuron, the total population of calretinin (CR), somatostatin (SST), and parvalbumin (PV) subtypes was measured using immunohistochemistry. ~95% of all cortical interneurons can be accounted for using these three markers (Wonders and Anderson, 2006). CR immunostaining in the PFC revealed no significant changes in the absolute number of CR-positive cells (Dgcr8fl/fl = 640 ± 79 cells; Dgcr8fl/fl;Cre = 667 ± 33 cells; Figure 5A). SST staining similarly showed no differences in absolute cell number (Dgcr8fl/fl = 278 ± 35 cells; Dgcr8fl/fl;Cre = 284 ± 39 cells; Figure 5A). In contrast, when we examined the third class of interneurons, we found a dramatic reduction in the number of PV-positive cells in the Dgcr8fl/fl;Cre cortex (Dgcr8fl/fl = 1182 ± 204 cells; Dgcr8fl/fl;Cre = 441 ± 105 cells; n = 3–4 per genotype for all stainings; p = 0.01; Figure 5A). Whereas the absolute number of CR and SST cells remained unchanged, the density of these cells increased, due to the smaller overall brain size (CR: Dgcr8fl/fl = 57.1 ± 9.3 cells/mm2; Dgcr8fl/fl;Cre = 187.4 ± 9.2 cells/mm2; p <0.001; SST: Dgcr8fl/fl = 12.1 ± 1.1 cells/mm2; Dgcr8fl/fl;Cre= 22.8 ± 1.6 cells/mm2; p < 0.001; Figure 5B). The density of PV cells, however, remained unchanged in spite of the smaller brain size (81.0 ± 9.6 cells/mm2; Dgcr8fl/fl;Cre = 91.0 ± 28.3 cells/mm2; n = 3–4 per genotype for all stainings; Figure 5B). To establish that the reduction of PV interneurons was not simply due to misexpression of the Cre recombinase outside of the NEX lineage, we crossed a Rosa-LSL-tdTomato reporter line to our recombinant strains. Staining for CR, SST, and PV showed no overlap with Cre reporter expression, which confirms the published expression of the NEX-Cre line (Goebbels et al., 2006) and indicates that the phenotypes observed were not due to Cre expression within these inhibitory cell types (Supplemental Figure 4). Together, these results demonstrate a selective loss of PV interneurons in the Dgcr8fl/fl;Cre PFC.
Figure 5. Dgcr8fl/fl;Cre animals show a selective reduction of parvalbumin inhibitory interneurons in the PFC.
(A) Representative images showing calretinin (CR), somatostatin (SST), and parvalbumin (PV) immunostaining in the PFC. Scale bar = 1 mm. Quantification of the absolute number of labeled cells in a coronal plane at Bregma 1.545 illustrates a significant reduction in PV cells in the conditional Dgcr8fl/fl;Cre. (B) Cell density of CR, SST, and PV neurons in the same coronal plane. Scale bar = 0.25 mm. Error bars represent SEM.
3.6 BDNF and TrkB expression are reduced in the Dgcr8fl/fl;Cre PFC
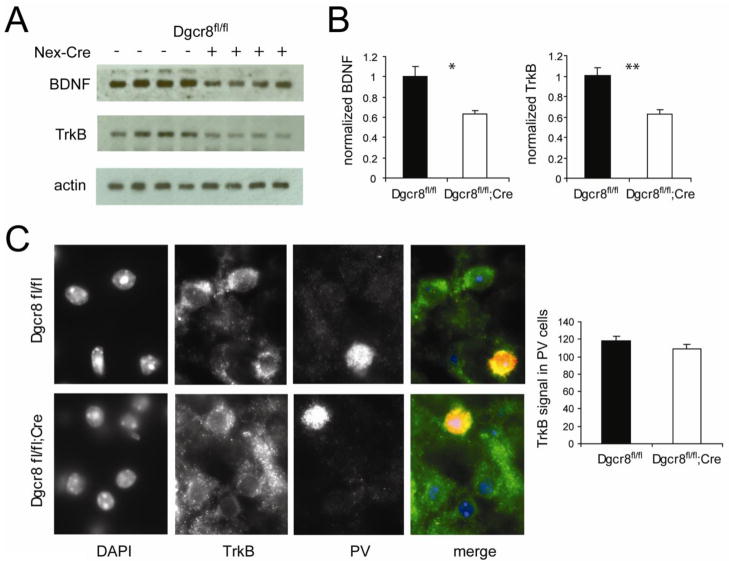
Maturation of interneuron subclasses is regulated by a combination of intrinsic molecular programs and extrinsic signaling cues (Wonders and Anderson, 2006). For example, the development of PV interneurons is mediated by extrinsic signaling through the brain-derived neurotrophic factor (BDNF) and tropomyosin-related receptor tyrosine kinase B (TrkB) pathway (Eto et al., 2010; Patz et al., 2004; Wahle et al., 2000). To evaluate whether this pathway is altered in the Dgcr8fl/fl;Cre mice, Western blots were used to measure the levels of BDNF and TrkB expression in the PFC of Dgcr8fl/fl and Dgcr8fl/fl;Cre animals. Significant reductions in both BDNF (37 ± 3%, p = 0.01) and TrkB protein (38 ± 5%, p = 0.006) were observed in the Dgcr8fl/fl;Cre when normalized to control values (n = 4 per genotype; Figure 6A, B). Since TrkB is expressed in both pyramidal neurons and PV-positive interneurons, we evaluated the TrkB expression at the cellular level to determine what cells are responsible for the decrease in expression. Co-staining of TrkB and PV revealed TrkB expression in the PV-positive interneurons in the Dgcr8fl/fl;Cre PFC (Dgcr8fl/fl = 30 cells; Dgcr8fl/fl;Cre = 33 cells; 2 animals per genotype; Figure 6C). The presence of TrkB in the PV cells that remain in the deletion animal is consistent with the fact that TrkB expression is required for normal PV interneuron development (Patz et al., 2004) and suggests that the cells which have reduced or lost TrkB expression fail to mature into PV interneurons. Additional studies examining the developmental time course of the TrkB loss will be required to resolve this point.
Figure 6. Reduced BDNF and TrkB protein levels in the Dgcr8fl/fl;Cre.
(A) Western blots from total PFC lysates from Dgcr8fl/fl and Dgcr8fl/fl;Cre animals. Each lane represents one animal. (B) Quantification of BDNF and TrkB band intensities normalized to actin. (C) Co-staining of TrkB and PV, showing similar levels of TrkB expression in PV-positive cells. Error bars represent SEM.
3.7 Additional electrophysiological characterization of PFC pyramidal neurons from control and Dgcr8 knockout mice
One alternative explanation for the phenotypes described by our data set is widespread cell death throughout the cortex accompanied by dysfunctional neurons. In order to rule this out as a major determining factor, we performed additional physiological analysis of pyramidal neurons from control and Dgcr8 conditional knockout mice. Pyramidal neurons in the cortex receive extensive excitatory connections from intralaminar, interlaminar and subcortical afferents. Accordingly, if massive cellular necrosis were occurring, a marked reduction in the frequency of excitatory synaptic events would be predicted. However, our analysis of spontaneous excitatory postsynaptic current (EPSC) recordings from control (Dgcr8fl/fl n = 11) and Dgcr8 conditional knockout (Dgcr8fl/fl;Cre n = 16) pyramidal neurons showed similar frequencies of events between genotypes (Dgcr8fl/fl = 1.7 ± 0.1 Hz; Dgcr8fl/fl;Cre = 2.0 ± 0.5 Hz; p = 0.57; Supplemental Figure 5) indicating that Dgcr8 deletion did not result in a reduced capacity for pyramidal neurons to functionally integrate into excitatory circuitry within the cortex. Interestingly, when we analyzed the shape of isolated EPSC events, we found notable differences between genotypes. EPSC events from Dgcr8 knockouts were larger in amplitude than control (Dgcr8fl/fl = 10 ± 1 pA; Dgcr8fl/fl;Cre = 12 ± 1 pA; p = 0.03). Also, the kinetics were markedly faster in the knockouts, as both the activation times (event 10–90% rise time: Dgcr8fl/fl = 1.2 ± 0.1 ms; Dgcr8fl/fl;Cre = 0.8 ± 0.1 ms; p < 0.0001) and decay times (event half-width: Dgcr8fl/fl = 8.4 ± 0.5 ms; Dgcr8fl/fl;Cre = 3.8 ± 0.5 ms; p < 0.0001) were faster in the Dgcr8 knockout than in the control.
Lastly, we examined the passive electrical properties of control and Dgcr8 conditional knockout pyramidal neurons. As expected for neurons of smaller size, input resistance (Rin) measured through the I-V plot of whole-cell current responses to a series of 5 mV voltage steps was significantly increased in Dgcr8fl/fl;Cre neurons compared to control (Dgcr8fl/flRin = 440 ± 32 MΩ, n = 15 cells; Dgcr8fl/fl;Cre Rin = 701 ± 97 MΩ, n = 17 cells, p = 0.02; Supplemental Figure 5). Conversely, measurement of the whole-cell capacitance (Cc) showed that this value was significantly decreased in Dgcr8fl/fl;Cre pyramidal neurons (Dgcr8fl/fl Cc = 69 ± 5 pF, n = 17 cells; Dgcr8fl/fl;Cre Cc = 36 ± 2 pF, n = 17 cells; p < 0.0001). Next, we examined the membrane time constants (τm) and resting membrane potential of pyramidal neurons. τm values were determined by the single exponential fit of the time course of the membrane voltage response to a −25 pA current step, and these values were similar between genotypes (Dgcr8fl/flτm = 48 ± 4 ms, n = 16 cells; Dgcr8fl/fl;Creτm = 39 ± 5 ms, n = 17 cells, p = 0.22). The resting membrane potential was also not significantly different in the conditional knockout (Dgcr8fl/fl Vm = 68 ± 2 mV, n = 10 cells; Dgcr8fl/fl;Cre Vm = 60 ± 5 mV, n = 15 cells, p = 0.16). Together, these data show that Dgcr8fl/fl;Cre neurons display altered whole-cell electrical properties consistent with morphological changes, but with no observable changes to specific membrane properties or leak conductances that may be associated with neuronal death.
4 Discussion
In this study, we demonstrate a critical role for Dgcr8-dependent miRNA biogenesis in the development of the cytoarchitecture of the cerebral cortex. We find that conditional ablation of Dgcr8 in newly-born pyramidal neurons results in profound morphological defects, including microencephaly due to cortical thinning, decreased soma size, and loss of dendritic complexity. These results are largely in agreement with the anatomical findings from postmitotic deletions of Dicer (Davis et al., 2008; De Pietri Tonelli et al., 2008; Kawase-Koga et al., 2009); however, we did not observe the grossly disrupted lamination which has consistently been reported across multiple conditional Dicer strains (De Pietri Tonelli et al., 2008; Kawase-Koga et al., 2009), suggesting that miRNA-independent pathways may underlie this component of the Dicer phenotype. In addition, we performed immunohistological, electrophysiological, and behavioral analyses that have not previously been conducted in Dicer models, leading to the identification of a novel effect of miRNAs on inhibitory transmission.
This work is notable as the first molecular- to systems-level characterization of a conditional ablation of Dgcr8 in the mammalian CNS. As such, this study is the first to explicitly address the impact of miRNA loss in the developing cortex. Loss of other classes of small RNAs may have confounded the results of previous studies in which Dicer deletion was used as a measure of miRNA function. Side-by-side deep sequencing of conditional Dicer versus Dgcr8 neuronal knockouts has identified a pool of highly-expressed non-canonical miRNAs that are processed by Dicer but not by Dgcr8. In addition, the Dicer knockout animals exhibited earlier lethality and significant structural abnormalities compared to the Dgcr8 knockout animals (Babiarz et al., 2011). This is an important issue given that the identity and mechanism of action of other Dicer-dependent RNAs is largely unknown, and evidence suggests that their function may be distinct from that of canonical miRNAs. For instance, endo-siRNAs processed by Dicer typically result in target degradation, whereas Dgcr8-derived miRNAs more commonly act via translational repression (Ambros, 2004; Bartel, 2004). Most recently, Dicer has been shown to have a novel miRNA-independent function in processing Alu RNAs, and conditional loss of Dicer induces Alu RNA toxicity leading to a macular degeneration phenotype, an effect not observed following loss of Dgcr8 (Kaneko et al., 2011). Previous studies examining Dicer-dependent effects did not analyze Alu RNAs, and it is possible that toxicity induced by Alu RNAs may be a component of these phenotypes (Meister, 2011). To isolate the miRNA pathway, it is therefore critical to characterize a system via depletion of Dgcr8 rather than Dicer.
Our finding that PV interneurons are selectively reduced in the PFC of Dgcr8fl/fl;Cre mice was unexpected because the Cre recombinase expression is limited to post-mitotic principle neurons of the cortex. This would suggest that elements extrinsic to interneurons, such as trophic factors expressed by principle neurons, might be dysregulated in Dgcr8fl/fl;Cre mice. BDNF and TrkB are required for the proper maturation of PV interneurons in the cortex (Eto et al., 2010; Patz et al., 2004; Wahle et al., 2000), and loss of activity-dependent BDNF results in reductions in PV expression as well as changes in IPSCs in the mPFC (Sakata et al., 2009). The reductions of both BDNF and TrkB in the PFC of Dgcr8fl/fl;Cre mice suggests that disrupted signaling through the BDNF/TrkB pathway may be in part responsible for the observed changes in PV expression and inhibitory transmission. miRNAs have been predicted to target BDNF itself; however, brain-specific miRNAs have also been shown to target molecules which regulate BDNF expression, including RE-1 silencing transcription factor (REST) (Conaco et al., 2006; Packer et al., 2008; Wu and Xie, 2006) and methyl CpG-binding protein 2 (MeCP2) (Im et al., 2010; Klein et al., 2007), through homeostatic and double-negative feedback interactions. Furthermore, miRNAs are thought to fine-tune gene expression and are commonly observed in feedforward and feedback loops which serve to maintain neuronal homeostasis (Tsang et al., 2007). Given the pleiotropic effects of BDNF signaling as well as the multiple levels of regulation acting on BDNF, it is likely that the interaction between miRNAs and BDNF expression are complex and interconnected. Decreases in BDNF and TrkB are likely to have many downstream effects, and alterations in PV interneurons is only one of many possible consequences. Additional studies will be necessary to determine whether the loss of PV expression is directly due to the downregulation of BDNF or to other indirect effects of miRNA loss, as well as to determine the precise mechanism underlying the loss of PV neurons.
The selective reduction of PV interneurons is particularly interesting given that PV is expressed by basket cell interneurons which are known to primarily synapse perisomatically (DeFelipe, 1997; Freund and Katona, 2007; Williams et al., 1992). Accordingly, reductions in this powerful source of inhibition would likely result in hyperexcitability and aberrant network activity, which may underlie some of the severe behavioral deficits observed in Dgcr8fl/fl;Cre mice. While it is possible that the reduction in perisomatic inhibitory synapses may be in part due to the smaller cell bodies in the Dgcr8fl/fl;Cre, the change in inhibition is supported by electrophysiological recordings. Specifically, although IPSC frequency was dramatically reduced in pyramidal neurons, we found that the remaining IPSC events were significantly larger in amplitude, a result that was confirmed through mIPSC recordings as attributable to a postsynaptic origin. These results would indicate that despite the loss of miRNA expression, pyramidal neurons are still able to form functional postsynaptic GABA-A receptor complexes. The larger IPSC amplitudes could reflect a compensatory mechanism, such as synaptic scaling, in response to the deficit in perisomatic inhibition.
Our electrophysiological analysis of Dgcr8fl/fl;Cre pyramidal neurons also indicates that despite their morphogenic defects, these neurons sit hyperpolarized (Vm = ~−60 mV), display a normal membrane time constant, fire action potentials, and receive excitatory as well as inhibitory synaptic transmission (Supplemental Figure 5). While the elevated levels of cell death we observe could account for the microencephaly and for some of the changes in gross morphology, these physiological properties are not hallmarks of dying neurons, suggesting that the pyramidal neurons that survive are alive, functional and integrated into cortical circuitry. We argue that increased cell death is unlikely to result in a consistent phenotype including decreased IPSC frequency, a specific loss of perisomatic inhibitory synapses, decreases in PV but not CR or SST interneurons, and seizure activity, which together suggest that mechanisms specific to inhibitory transmission are at work. Thus, the loss of a single class of inhibitory neurons (that have normal miRNA expression), while other types of interneurons develop normally, is both unexpected and novel. Together, our data suggest a model in which loss of Dgcr8 in pyramidal neurons leads to a BDNF-dependent, non-cell-autonomous effect on PV interneuron development. This selective reduction of PV interneurons accounts for the decrease in perisomatic synapses, which in turn, corresponds to the changes in inhibitory transmission.
Finally, our findings have potential implications for understanding the molecular mechanisms underlying human neuropsychiatric disease and illness. Abnormalities in interneuron development and function have been implicated in seizure disorders as well as in autism, anxiety disorders, and schizophrenia (Cobos et al., 2005; Powell et al., 2003; Wonders and Anderson, 2006). Decreases in PV interneurons in the PFC specifically have been described in schizophrenia (Blum and Mann, 2002; Mellios et al., 2009; Zhang and Reynolds, 2002), while changes in miRNA expression patterns have been reported in schizophrenia postmortem studies (Beveridge et al., 2008; Perkins et al., 2007). In addition, Dgcr8 haploinsufficiency contributes to neurological, behavioral, and anatomical phenotypes of the 22q11 Deletion Syndrome (Schofield et al., 2011; Stark et al., 2008), which has been proposed as a genetic subtype of schizophrenia (Liu et al., 2002). Our findings delineate a novel connection between Dgcr8-dependent miRNAs in the cortex and PV neuron maturation, and as such, provide a potential inroad toward a mechanistic understanding of the cellular and molecular changes observed in schizophrenia. However, as human schizophrenia lacks the severe gross cortical malformations observed in Dgcr8fl/fl;Cre mice, a more likely scenario is that small-scale changes of specific miRNA levels during postnatal development similar to those observed in the heterozygous Dgcr8 mice (Fenelon et al., 2011; Schofield et al., 2011), are involved in the emergent phenotypes of schizophrenia, including the changes in PV expression. Identification of individual miRNAs in the cortex mediating such effects will be the basis of future studies.
Supplementary Material
To confirm loss of Dgcr8 as well as miRNAs in the conditional Dgcr8 mouse, qRT-PCR was performed on PFC tissue. Results showed significantly reduced (A) Dgcr8 (B) and miRNA levels. Data are normalized to internal controls and to the Dgcr8fl/flcontrol. Error bars represent SEM.
Cell death via apoptotic pathways was observed in the Dgcr8fl/fl;CrePFC.
(A) Front and hind limb clasping observed in Dgcr8fl/fl;Cre mice. (B) Seizures were induced in mice by intraperitoneal (IP) injection of pilocarpine, and the latency to the first stage 3 seizure was measured. The Dgcr8fl/fl;Cre mice had a decreased latency compared with Dgcr8fl/fl control littermates. Error bars represent SEM.
NEX-Cre animals were crossed to the Rosa-LSL-tdTomato reporter strain in order to label all cells expressing Cre recombinase. Brains were immunostained for CR, SST, or PV. Representative images from the PFC show the absence of Cre reporter expression (red) in the respective interneuron populations (green). Scale bar = 100 μm.
Representative EPSC recording traces from layer II/III pyramidal neurons from (A) Dgcr8fl/fl and (B) Dgcr8fl/fl;Cre mice; scale bar = 20 pA, 200 ms. (C) Mean EPSC responses (average of >50 isolated events) from individual Dgcr8fl/fl (black) and Dgcr8fl/fl;Cre (red) neurons superimposed on the same scale to illustrate changes to EPSC kinetics; scale bar = 2 pA, 10 ms. (D) Whole-cell current-clamp recordings from layer II/III pyramidal neurons demonstrate membrane voltage responses and action potential firing capabilities elicited by a series of current injections (waveform diagrams = −100, −75, −50, −25, 0, +175 pA) to Dgcr8fl/fl (black) and Dgcr8fl/fl;Cre (red) neurons; scale bar = 75 mV, 0.5 s.
Acknowledgments
Funding for this project was supported in part by the NIH T32EY07120 (RH); NIH R21-MH083090 and Autism Speaks (EMU); and K08 NS48118, R01 NS057221, and California Institute of Regenerative Medicine New Faculty Award RN2-00906 (RB). This work was made possible in part by the NIH-NEI EY002162 Core Grant for Vision Research. The authors are grateful to Tigwa Davis, Selina Koch, Gemma Rooney, Caitlyn Gertz, and Robert Krencik for helpful discussion, critical readings of the manuscript, and technical assistance. We thank Lawrence Sincich and the laboratory of Jonathan Horton for aiding in image acquisition and cell density analyses. We also thank Ryan Sze and Tony Tran for assistance with genotyping and Beth Grande for administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–85. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiarz JE, Hsu R, Melton C, Thomas M, Ullian EM, Blelloch R. A role for non-canonical microRNAs in the mammalian brain revealed by phenotypic differences in Dgcr8 versus Dicer1 knockouts and small RNA sequencing. RNA. 2011 doi: 10.1261/rna.2442211. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I, Cairns MJ. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–68. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–79. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–45. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–30. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–21. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Eto R, Abe M, Kimoto H, Imaoka E, Kato H, Kasahara J, Araki T. Alterations of interneurons in the striatum and frontal cortex of mice during postnatal development. Int J Dev Neurosci. 2010;28:359–70. doi: 10.1016/j.ijdevneu.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Fenelon K, Mukai J, Xu B, Hsu PK, Drew LJ, Karayiorgou M, Fischbach GD, Macdermott AB, Gogos JA. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:4447–52. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–21. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee DK, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, Zhang Q, Grossniklaus HE, Provis JM, Madigan MC, Milam AH, Justice NL, Albuquerque RJ, Blandford AD, Bogdanovich S, Hirano Y, Witta J, Fuchs E, Littman DR, Ambati BK, Rudin CM, Chong MM, Provost P, Kugel JF, Goodrich JA, Dunaief JL, Baffi JZ, Ambati J. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–30. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase-Koga Y, Otaegi G, Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev Dyn. 2009;238:2800–12. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan JA. Histological and histochemical methods: theory and practice. 2. Pergamon; 1990. [Google Scholar]
- Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19:1–15. [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–4. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002;99:16859–64. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Meister G. Vision: Dicer leaps into view. Nature. 2011;471:308–9. doi: 10.1038/471308a. [DOI] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–14. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28:14341–6. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–51. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–31. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, Reinhardt F, Liao R, Krieger M, Jaenisch R, Lodish HF, Blelloch R. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105:585–94. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–7. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CM, Hsu R, Barker AJ, Gertz CC, Blelloch R, Ullian EM. Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex. Neural Dev. 2011;6:11. doi: 10.1186/1749-8104-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JS, Varble A, Pham AM, Tenoever BR. Noncanonical cytoplasmic processing of viral microRNAs. RNA. 2010;16:2068–74. doi: 10.1261/rna.2303610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Tang F, Hajkova P, Barton SC, Lao K, Surani MA. MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res. 2006;34:e9. doi: 10.1093/nar/gnj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J, Zhu J, van Oudenaarden A. MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol Cell. 2007;26:753–67. doi: 10.1016/j.molcel.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle P, Gorba T, Wirth MJ, Obst-Pernberg K. Specification of neuropeptide Y phenotype in visual cortical neurons by leukemia inhibitory factor. Development. 2000;127:1943–51. doi: 10.1242/dev.127.9.1943. [DOI] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–5. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol. 1992;320:353–69. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wu H, Xu H, Miraglia LJ, Crooke ST. Human RNase III is a 160-kDa protein involved in preribosomal RNA processing. J Biol Chem. 2000;275:36957–65. doi: 10.1074/jbc.M005494200. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–53. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To confirm loss of Dgcr8 as well as miRNAs in the conditional Dgcr8 mouse, qRT-PCR was performed on PFC tissue. Results showed significantly reduced (A) Dgcr8 (B) and miRNA levels. Data are normalized to internal controls and to the Dgcr8fl/flcontrol. Error bars represent SEM.
Cell death via apoptotic pathways was observed in the Dgcr8fl/fl;CrePFC.
(A) Front and hind limb clasping observed in Dgcr8fl/fl;Cre mice. (B) Seizures were induced in mice by intraperitoneal (IP) injection of pilocarpine, and the latency to the first stage 3 seizure was measured. The Dgcr8fl/fl;Cre mice had a decreased latency compared with Dgcr8fl/fl control littermates. Error bars represent SEM.
NEX-Cre animals were crossed to the Rosa-LSL-tdTomato reporter strain in order to label all cells expressing Cre recombinase. Brains were immunostained for CR, SST, or PV. Representative images from the PFC show the absence of Cre reporter expression (red) in the respective interneuron populations (green). Scale bar = 100 μm.
Representative EPSC recording traces from layer II/III pyramidal neurons from (A) Dgcr8fl/fl and (B) Dgcr8fl/fl;Cre mice; scale bar = 20 pA, 200 ms. (C) Mean EPSC responses (average of >50 isolated events) from individual Dgcr8fl/fl (black) and Dgcr8fl/fl;Cre (red) neurons superimposed on the same scale to illustrate changes to EPSC kinetics; scale bar = 2 pA, 10 ms. (D) Whole-cell current-clamp recordings from layer II/III pyramidal neurons demonstrate membrane voltage responses and action potential firing capabilities elicited by a series of current injections (waveform diagrams = −100, −75, −50, −25, 0, +175 pA) to Dgcr8fl/fl (black) and Dgcr8fl/fl;Cre (red) neurons; scale bar = 75 mV, 0.5 s.