Abstract
The vitamin D binding protein (DBP) is the major plasma carrier for vitamin D and its metabolites, but it is also an actin scavenger, and is the precursor to the immunomodulatory protein, Gc-MAF. Two missense variants of the DBP gene – rs7041 encoding Asp432Glu and rs4588 encoding Thr436Lys – change the amino acid sequence and alter the protein function. They are common enough to generate population-wide constitutive differences in vitamin D status, based on assay of the serum metabolite, 25-hydroxyvitamin D (25OHD). Whether these variants also influence the role of vitamin D in an immunologic milieu is not known. However, the issue is relevant, given the immunomodulatory effects of DBP and the role of protracted innate immune-related inflammation in response to tissue injury or repeated infection. Indeed, DBP and vitamin D may jointly or independently contribute to a variety of adverse health outcomes unrelated to classical notions of their function in bone and mineral metabolism. This review summarizes the reports to date of associations between DBP variants, and various chronic and infectious diseases. The available information leads us to conclude that DBP variants are a significant and common genetic factor in some common disorders, and therefore, are worthy of closer attention. In view of the heightened interest in vitamin D as a public health target, well-designed studies that look simultaneously at vitamin D and its carrier in relation to genotypes and adverse health outcome should be encouraged.
Keywords: Association studies; cancer; chronic and infectious diseases, common polymorphisms; inflammation; GC gene; vitamin D binding protein/group-specific component/Gc-globulin; vitamin D binding protein-macrophage activating factor
Introduction
DBP structure and function
The vitamin D binding protein (DBP), originally known as the Group-specific component (Gc-globulin), is a 51–58 kDa multifunctional serum glycoprotein synthesized in large quantities by hepatic parenchymal cells and secreted into the circulation as a monomeric mature peptide of 458 residues and three structural domains1–3. Two binding regions are well characterized – a vitamin D/fatty acid binding domain located between residues 35–49, and an actin binding domain positioned between residues 350–403. Recently, DBP cell surface binding sites have been localized to N-terminal and C-terminal domains (domain I and III), which may be necessary to mediate DBP cellular functions4.
The functions of DBP are still being defined, but they include the transport of vitamin D and its metabolites in the circulation5 , 6, as well as actin scavenging and fatty acid binding7–12. Proteomic analysis shows that DBP is a hepatic acute phase reactant and is upregulated in patients with diabetes mellitus13, early-stage breast cancer14, oral squamous cell carcinoma15, aggressive periodontitis16, idiopathic temporal lobe epilepsy17, Alzheimer and Parkinson disease18 and myopia19. It is downregulated in patients with sepsis20, neuromyelitis optica21, cutaneous malignant melanoma22, hepatocellular carcinoma23 , 24, primary non-metastatic breast cancer25, Klinefelter syndrome26 and type I diabetes27. These observations provide an attractive prospect for clinically relevant biomarker status, but further characterization is needed to establish its usefulness in patient care.
The GC gene encodes DBP
DBP is encoded by the single copy GC gene (NCBI GENE ID2638) located on chromosome 4q12-q13. The genomic sequence is 35 kb in length with a single start site, 13 exons with flanking untranslated regions at both ends and a key-enhancing motif (DNase Hypersensitivity Site IV) in intron 1 (Figure 1). The DBP gene is a member of a multigene family that includes albumin (ALB), α-fetoprotein (AFP), and α-albumin/afamin (AFM), tandemly linked in the following order: centromere–DBP–ALB–AFP–AFM-telomere28 . Despite tight linkage, DBP, in an opposite direction of gene transcription, is separated from ALB by a non-transcribed sequence 1.5 Mb in length and is under autonomous regulatory control29.
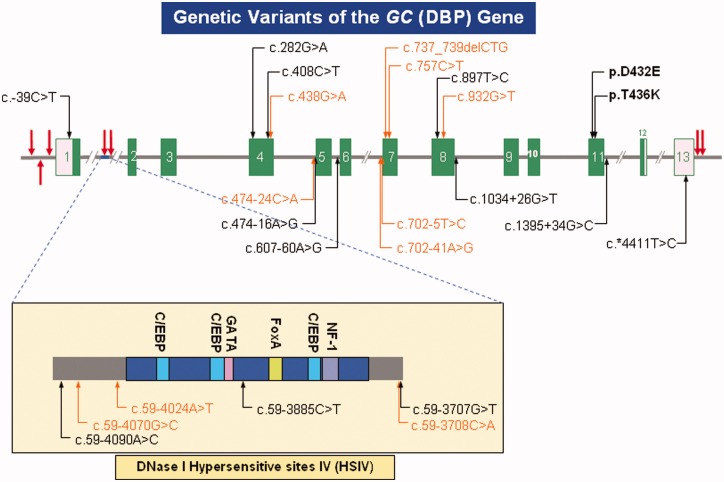
Figure 1.
Genetic variants of the vitamin D-binding protein gene (GC). Shown in this schematic are the 13 exons (coding regions as green bars and untranslated sequences as pink boxes), separated by variable length introns (horizontal grey line, interrupted). Also shown are the DNase I hypersensitive sites (vertical red arrows). Extensively involved in control of gene expression, Site IV (HSIV), located in Intron I, is depicted in greater detail. Binding elements specific for Ccaat-enhancer-binding proteins (C/EBP, blue), GATA transcription factors (GATA, pink), hepatocyte nuclear factor 3-alpha (FoxA, lime) and nuclear factor-1 (NF-1, purple) are indicated. Besides the common missense SNPs – c.1296T > G specifying p.D432E, and c.1307C > A specifying p.T436K – there are a number of other well documented (black) and novel (orange) single-nucleotide variants scattered throughout the gene of relevance to future genetic association studies.
A number of genetic variants are known (Figure 1). The two most common polymorphisms [c.1296T > G encoding D432E (SNPdb rs7041) and c.1307C > A encoding T436K (SNPdb rs4588)] are found in exon 11 in complete linkage disequilibrium, so only six haplotypes are observed in any significant frequency.
Genotype-phenotype associations and vitamin D metabolism
Mature serum DBP concentration is a quantitative trait with a significant hereditable component (66%), as established by twin studies published at least a decade ago30 , 31. Functional studies of the protein polymorphisms were initiated prior to molecular identification since the three protein variants, Gc1S, Gc1F and Gc2, can be distinguished by electrophoretic migration differences32 , 33. This is related to the differences in glycosylation pattern, resulting from replacement of the O-glycosylation moiety by a positively charged lysine residue at the T436K position as well as small differences in amino acid charge in the D432E variant (Figure 2). The full functional consequences of these variants (which probably include protein half-life and cell transit times) have not yet been delineated, although their impact on serum DBP and 25(OH)D concentrations has been demonstrated. Mean serum DBP concentrations stratified by DBP electrophorectic phenotypes were examined in 586 women34. Serum DBP levels did not differ between Gc1F and Gc1S alleles (D432E); however, Gc2 (T436K) showed a significantly lower level compared with Gc1 (Gc1F and Gc1S combined) (p = 0.001). The highest DBP serum concentration was in Gc1-1 subjects (272 ± 2 mg/L), the lowest in Gc2-2 (226 ± 2 mg/L) and intermediate levels were found in heterozygous subjects (249 ± 3 mg/L)34. Other investigators have confirmed these findings30 , 35. Similarly, our study also confirmed that the lowest serum DBP concentration was in the subjects with 436KK genotype (encoding the Gc2-2 protein variant)36.
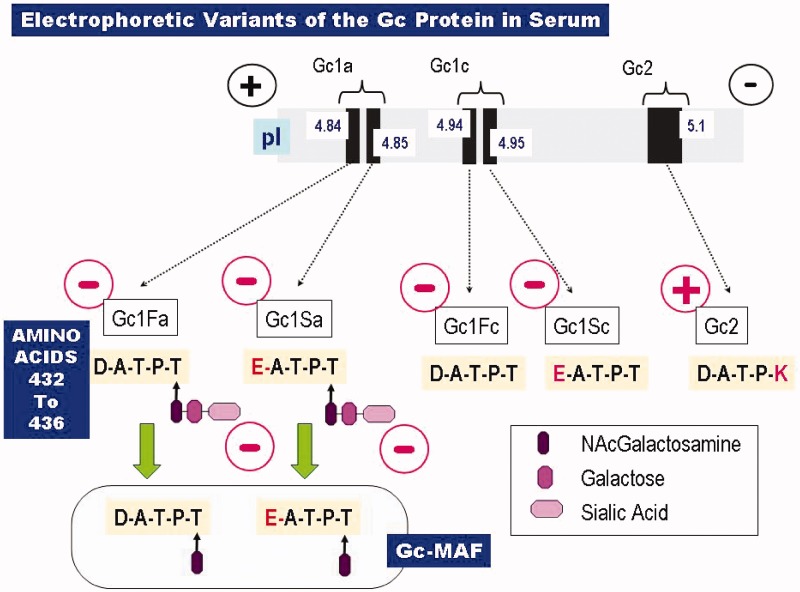
Figure 2.
Common electrophoretic variants of the Gc protein (DBP). Shown at the top is the relative electrophoretic separation of the various Gc species, based on their isoelectric points (pI). The corresponding molecular structures are given below that. Only five residues of primary amino acid sequence (residues 432–436 = Asp–Ala–Thr–Pro–Thr = D–A–T–P–T) are depicted, along with their O-linked saccharides. Note that the D432E mutation results in a very small separation (ΔpI = 0.01), since the carboxyl side chains of the wild-type aspartate residue (in the Gc1F species) and the mutant glutamate residue (in Gc1S) have such similar dissociation constants. There is larger separation of the two Gc1 species if the negatively charged sialic acid residue (generating the anodal form, Gc1a) is removed, generating Gc1c, the cathodal form. Gc-MAF, shown at the bottom, arises as a result of sequential deglycosylation removing first the sialic acid and then the galactose residues. At the T436 position, the genetic variant Gc2 shows a more marked cathodal shift in electrophoretic migration due to replacement of the O-glycosylation site by a positively charged lysine residue (436 K).
Conclusions based on this type of candidate gene approach have been confirmed and strengthened by the results of genome-wide association studies (GWAS). In populations of European ancestry, two independent studies found that an intronic SNP rs2282679 which is in tight linkage disequilibrium with D432E had the strongest genome-wide significant associations with 25(OH)D concentrations37 , 38.
As noted before, the physiological relationship between the genetically determined differences in circulating DBP molecules and overall DBP concentrations is uncertain, but it seems likely that they specify not only the D432E and T436K substitutions in the peptide, but also affect glycosylation status39 , 40, and consequently, macrophage stimulating activity. While these common polymorphisms are associated with DBP status, others have demonstrated association between genotype and serum vitamin D metabolite levels.
A recent study examining a cohort of 595 Danish Caucasian menopausal women showed that after adjusting for confounders, DBP phenotype is an independent predictor of 25(OH)D (p = 0.016)41. Similar analysis of a large Han Chinese cohort (n = 3210) found four SNPs at the GC locus (D432E, T436K, rs2282679 and rs1155563) significantly associated with lower plasma 25(OH)D42. Of note, is the finding that T436K and rs2282679 remained significant after adjusting for the other two SNPs. A cross-sectional study examining 741 premenopausal white women of French-Canadian descent demonstrated that each additional copy of the 432D or 436 K alleles reduced 25(OH)D concentrations significantly (p < 0.0003 and p < 0.0001, respectively)43. Similarly, in a study population of 98 healthy adult women, we found that the 436KK homozygotes exhibited reduced baseline vitamin D serum levels44.
Of more immediate clinical interest, perhaps, was our observation that this allele was also correlated with the response of serum 25(OH)D levels to long-term vitamin D supplementation. The subjects with 436KK genotype showed the largest proportional increase in response to a vitamin D supplement. The population-wide implications of these findings for vitamin D in public health are substantial, given that the 436 K allele is less prevalent in Africans (15%) than in Caucasians (23%), Hispanics (20%) or Asians (29%)45. Indeed, we have recently shown that this is mirrored in immigrant sub-populations found in large North American cities46.
Vitamin D, DBP and innate immunity – an emerging theme
The role of DBP in the biology of vitamin D as it relates to the endocrine status of bone and mineral metabolism is well established and the reader is referred to reviews47 , 48, monographs49 and authoritative web sites50. DBP is thought to regulate the bioavailability of 25-hydroxycholecalciferol [25(OH)D3], acting as the main transporter from liver to kidney for the synthesis of the principal active metabolite, 1,25-dihydroxycalciferol [1,25(OH)2D]. Alternatively, 25(OH)D delivered to the kidney may undergo catabolic hydroxylation there to the considerably less active 24,25-dihydroxycalciferol [24,25(OH)2D] metabolite, which is then subject to further degradation and renal excretion.
In contrast, the role of vitamin D and DBP in innate immunity has been appreciated only more recently, either through its involvement in the vitamin D transport pathway or by independent mechanisms51. The vitamin D3 form, 1,25-dihydroxycholecalciferol [1,25(OH)2D3], and the vitamin D2 form, 1,25-dihydroxyergocalciferol [1,25(OH)2D2], exhibit similar properties not only for skeletal effects, but also for their anti-inflammatory actions52. Moreover, epidemiologic evidence for mitigating chronic diseases such as cancer53, and for upregulation of antimicrobial peptide synthesis54 , 55 applies to both vitamers.
Interestingly, DBP has also been shown to play a more direct role in the inflammatory cascade by enhancing the leukocyte chemo-attractant effect of compliment activation peptide C5a, one of the most potent of the early-acting chemo-attractants56–60. The role of DBP as a C5a adjuvant is apparently not strictly vitamin D independent as 1,25(OH)2D3 can mitigate the synergistic effect of DBP and C5a60. The stepwise glycohydrolysis promoted by T and B cells leads to loss of the O-glycosylated oligosaccharide moiety in the DBP peptide, transforming it into a potent macrophage activating factor (Gc-MAF)61 , 62.
Finally, while DBP plays a proximate role in innate immune regulation, it may also act more distantly through actin scavenging to protect the host from the effects of cell damage due to tissue ischemia, inflammation or mechanical injury. Whereas excessive globular actin released from damaged cells can cause intravascular coagulation resulting in multi-organ dysfunction and cardiac arrest, DBP functions to bind and sequester actin in circulation released from damaged cells, thus potentially mitigating adverse outcomes of tissue injury7 , 11 , 63 , 64.
The various roles that DBP plays in different inflammatory processes suggests that functional polymorphisms in the DBP gene, GC, may therefore predict adverse outcomes in a wide range of chronic and infectious diseases associated with these processes. Below we summarize the evidence supporting this contention, and examine the strength of the findings to date (Table 1).
Table 1.
Association between common genetic variants of the vitamin D binding protein gene (GC) and various diseases.
|
Sample size (N) |
|||||||
|---|---|---|---|---|---|---|---|
| Disease | Reference | Cases | Controls | Country | Ethnicity | GC polymorphisms studied | Findings |
| Cancer | |||||||
| Breast Cancer | Abbas et al.160 | 1402 | 2608 | Germany | Caucasiana | Gc1F, Gc1S, Gc2 | Genotype Gc2-2 was associated with decreased risk of postmenopausal breast cancer, [OR = 0.72 (CI = 0.54–0.96, p = 0.04)], when compared to the most frequently observed genotype, Gc1S-1S. Gc2 carriers had an OR of 0.88 (CI = 0.77–1.01, p = 0.02) compared to non-carriers. |
| Anderson et al.161 | 1560 | 1633 | Canada | Caucasian | rs7041, rs4588 | TT genotype of rs7041 was associated with breast cancer, OR = 1.23 (CI = 1.01–1.51, p < 0.05). Resulting genotype Gc2-2 had OR = 1.22 (CI = 0.93–1.59), when compared to genotype Gc1-1. | |
| McCullough et al.159 | 500 | 500 | United States | 99% Caucasianb | rs7041, rs4588 | NS | |
| Gastrointestinal Cancer (includes Colorectral Cancer) | Hibler et al.169 | 1439 | N/A | United Status | Caucasian | rs7041 and six other SNPs in GC gene | NS |
| Poynter et al.168 | 1806 | 2879 (siblings) | United States, Canada, Australia | 87% non-Hispanic White; remainder Black, Asian and Other | rs7041, rs4588 and other SNPs in the Gc gene | NS | |
| Zhou et al.170 | 964 | 1187 | China | Han Chinese | D432E, T436K | Homozygous KK genotype of T436K had elevated risk for colorectal cancer in comparison to 436 T/T genotype, OR = 3.41 (CI = 1.85–6.57, p < 0.001). When all gastrointestinal cancer types were combined, KK genotype had 1.15 fold increased risk over T/T (CI = 1.02–1.30, p = 0.020) after adjustment for age, sex and smoking status. Carriers of D432- 436 K haplotype (associated with Gc2 allele) also had an increased risk for developing gastrointestinal cancer, OR = 1.22 (CI = 104–1.39, p = 0.015) | |
| Prostate Cancer | Ahn et al.166 | 749 | 781 | United States | Non-Hispanic White | rs7041, rs4588 and other SNPs in the Gc gene | NS |
| Corder et al.167 | 181 | 181 | United States | 182 White, 180 Black | Gc1F, Gc1S, Gc2 | NS | |
| Dimopoulos et al.165 | 115 | 155 | Greece | Caucasiana | Gc1F, Gc1S, Gc2 | Increased disease risk for carriers of Gc2 allele; RR = 1.81 (p < 0.01) | |
| Skin Cancer, Basal Cell Carcinoma (BCC) | Flohil et al.171 | 7983 | N/A | Netherlands | Caucasian | rs7041, rs4588 | Heterozygous Gc1F carriers were significantly more likely to develop a first BCC compared to non-carriers, HR = 1.40 (CI = 1.11–1.78). Homozygote Gc1S carriers aged less than 65 years old has significantly lower risk of BCC compared to non-carriers, HR = 0.53 (CI = 0.31–0.91). |
| Chronic Diseases | |||||||
| Asthma | Wjst et al.117 | 947 (201 families) | 191 | Germany and Sweden | Caucasiana | rs7041, rs4588 and other SNPs in the GC gene | NS. Weak association with D432E (rs7041) and total serum IgE (p = 0.0249) |
| Li et al.116 | 467 | 288 | China | Chinese Han | rs7041, rs4588 | Gc2 allele was associated with asthma susceptibility, OR = 1.35 (CI = 1.01–1.78, p = 0.006); Gc2-2 genotype was strongly associated with risk of asthma compared to Gc1-1, OR = 13.13 (CI = 2.42–7.13, p = 0.001) | |
| Chronic Obstructive Pulmonary Disease (COPD) | Horne et al.90 | 104 | 413 | Canada | Caucasian | Gc1F, Gc1S, Gc2 | Gc2 allele may confer disease protection; Highest disease risk in Gc1F-1F genotype (RR = 4.8), and lowest risk in Gc2-1F genotype (RR = 0.5) |
| Ito et al.93 | 103 | 88 | Japan | Japanese | T436K, D432E | Increased risk of COPD for Gc1F-1F genotype; significantly larger proportion of Gc1F homozygotes in patients (32%) compared to healthy smokers (17%) p = 0.014; OR for Gc2-2 is 2.3 (CI = 1.2–4.6). | |
| Janssens et al.97 | 262 | 152 | Belgium | Caucasian a | rs7041, rs4588 | Individuals with homozygous TT at rs7041 have increased risk for COPD; OR = 2.11 (CI = 1.2–3.71, p = 0.009) for COPD. No association with heterozygous T carriers. | |
| Kasuga et al.98 | 532 (low lung function) | 537 (high lung function) | Canada | Caucasian | Gc1F, Gc1S, Gc2 | NS | |
| Kueppers et al.91 | 114 | 114 | United Sates | Caucasiana | Gc1F, Gc1S, Gc2 | Gc2-2 may offer a protective advantage; Gc2-2 genotype was underrepresented in the COPD group (1%) versus the control group (5%), p = 0.049. | |
| Schellenberg et al.89 | 75 | 64 | Canada | Caucasiana | Gc1F, Gc1S, Gc2 | Homozygous Gc2 appears protective against disease. OR for Gc2-1 = 1.01 (CI = 0.49–2.10) OR for Gc2-2 = 0.17 (CI = 0.03–0.83) | |
| Shen et al.95 | 100 | 100 | China | Chinese Han | Gc1F, Gc1S, Gc2 | Decreased COPD disease risk for Gc2. OR for Gc1F-1F = 3.08(CI = 1.498–6.347, p = 0.003) OR for Gc2-2 = 0.215 (CI = 0.06–0.772, p = 0.0017) | |
| Wood et al.92 | 611 (471 with AATD and 140 with COPD | 480 | United Kingdom | Caucasian | rs7041, rs4588 and other SNPs in Gc gene | Gc2 decreased risk of COPD, OR = 0.79 (CI = 0.65–0.99, p = 0.048). Gc2 had increased risk of bronchiectasis, OR = 1.51 (CI = 1.02–2.22, p = 0.034). rs7041 was associated with bronchiectasis in α1-antitrypsin deficiency (AATD), OR = 0.52 (0.29–0.93, p = 0.027). No association with rs4588. | |
| Diabetes (Type I) | Cooper et al.224 | 8517 | 104 381 and 1933 families | United Kingdomc | Caucasian | rs7041, rs4588 | Association between rs4588 and type 1 diabetes was observed in the case/control dataset, OR = 0.95 (CI = 0.91–1.00, p = 0.05). No associations were observed in the family collection data (p = 0.071). No association with D432E. |
| Hodge et al.225 | 103 | N/A | United Sates | Caucasian | Gc1F, Gc1S, Gc2 | Excess of Gc2-1 and Gc1-1 phenotypes (0.05 > p > 0.02); however, association not significant when corrected for multiple testing. | |
| Pani et al.66 | 527 (152 families with at least one affected offspring) | N/A | Germany | Caucasiana | Gc1F, Gc1S, Gc2; number of repeats for intron 8 [(TAAA)n] | NS | |
| Diabetes (Type II) (including insulin resistance and altered glucose tolerance) | Baier et al.70 | 578 | 595 | United States | Pima Indians | D432E, T436K | Gc genotypes differed in plasma glucose concentrations in response to oral glucose tolerance test, highest concentrations observed in Gc1F and lowest in Gc2 (p < 0.028, repeated measures ANOVA). |
| Hirai et al.72 | 208 | 209 | Japan | Japanese | Gc1F, Gc1S, Gc2 | Reduced risk for individuals with Gc1F allele. Gc1F-1F genotype was less frequent in cases versus controls (11% versus 19%, p < 0.001). Gc1S-2 genotype was more common in cases than controls (48% versus 28%, p < 0.02). | |
| Hirai et al.68 | N/A | 82 | Japan | Japanese | Gc1F, Gc1S, Gc2 | Fasting insulin concentrations differed in Gc alleles (p < 0.05); individuals with Gc1S allele had highest concentrations, followed by Gc2 allele carriers, the lowest fasting insulin was observed in Gc1F carriers. HOMA was highest in Gc1S carriers (p < 0.03). | |
| Iyengar et al.71 | N/A | 468 | United States | Anglos (N = 289); Hispanic Americans (N = 179) | Gc1F, Gc1S, Gc2 | Gc1F allele associated with highest levels of plasma glucose (p = 0.033). | |
| Klupa et al.74 | 396 (Type I, N = 181; Type II, N = 215) | 163 | United States | Caucasian | Gc1F, Gc1S, Gc2 | NS | |
| Malecki et al.76 | 231 | 162 | Poland | Caucasian | Gc1F, Gc1S, Gc2 | NS | |
| Szathmary 69 | 144 | N/A | Canada | Dogrib Indians | Gc1F, Gc1S, Gc2 | Gc1F-1F had the lowest fasting glucose level. After adjustment for BMI, Gc genotype was the only variable that had a significant effect on insulin concentrations (p = 0.040). | |
| Ye et al.75 | 237 | 143 | France | Caucasian | Gc1F, Gc1S, Gc2 | NS | |
| Endometriosis | Faserl et al.124 | 57 | 24 | United States | Caucasiana | Gc1F, Gc1S, Gc2 | Gc alleles differed between cases and controls (p = 0.006). 18% of cases expressed Gc2 allele alone, but none of the control cases did. |
| Thyroid Autoimmune Disease | Kurylowicz et al.196 | 332 | 185 | Poland | Caucasians | D432Eand T436E; Variable tandem (TAAA)n-Alu repeat in Intron8 | Increased risk for carriers of K allele at T436K, OR = 1.5 (CI = 1.13–1.99, p = 0.005). OR = 1.63 for homozygous 436KK genotype (CI = 0.80–3.32, p = 0.01). |
| Pani et al.195 | 187 nuclear families with an offspring affected by Graves (N = 95) or Hashimoto thyroiditis (N = 92) | N/A | Germany and Italy | Caucasian | Gc1F, Gc1S, Gc2, Variable tandem (TAAA)n-Alu repeat in Intron8 | Association with intron 8(*8) polymorphisms in Graves Disease patients (p < 0.03). No association with Hashimoto thyroiditis. | |
| Ischemic Stroke | Wang et al.119 | 3550 | 6560 | United States, Europe, China | Varied by study; primarily Caucasians and Chinese | rs7041, rs4588 | NS |
| Inflammatory Bowel Diseases (IBD) | Eloranta et al.125 | 636 (232 with ulcerative colitis; 404 with crohn disease) | 248 | Switzerland | Caucasiana | D432E, T436K | Homozygous 436KK genotype was more common in control group than in IBD patients (p = 0.006). Association was significant in separate comparisons of healthy controls with ulcerative colitis patients (p = 0.022) and Crohn disease patients (p = 0.016). DBP_2 TA haplotype (D432-436 K, Gc2 allele) was found at higher frequencies in healthy controls than in IBD cases [OR = 2.53, (CI = 1.48–4.34, p = 0.0005)], particularly when comparing healthy controls to the ulcerative colitis cases [OR = 4.39, (CI = 1.87–10.31, p = 0.0003)] |
| Liver Disease | Constans et al.35 | 17 (15 cirrhosis; 2 hepatitis) | 100 | France | Caucasiana | Gc1F, Gc1S, Gc2 | Gc1 was overrepresented in the cases versus healthy controls. An unusual electrophoretic form of Gc1 was found in many of the cases (10 out of 17 cases), perhaps differentiated by the presence of two sialic acid residues. |
| Osteoporosis | Al-oanzi et al.87 | 56 | 114 | United Kingdom | Caucasian | Variable tandem (TAAA)n-Alu repeat in Intron8 | Allele *10 was associated with lower risk of osteoporosis, OR = 0.39 (CI = 0.25–0.64, p < 0.0005). Allele 11 was also associated with reduced risk, OR = 0.09 (CI = 0.01–0.67, p < 0.007). Allele *8 and *9 both showed increased susceptibility. |
| Eichner et al.226 | 258 | N/A | United States | Caucasiana (non-Black) | Gc1F, Gc1S, Gc2 | No association between Gc alleles and BMD. Homozygous Gc1F phenotype was associated with higher bone mass density, but only 7 individuals with this phenotype. | |
| Ezura et al.85 | 384 | N/A | Japan | Japanese | rs7041, rs4588 and 12 other SNPs in the Gc gene | Several SNPs were associated with bone mass density (BMD); D432E (rs7041) in conjunction with IVS1 + 827C > T showed the strongest relationship with BMD (r2 = 0.029, p = 0.005). | |
| Fang et al.88 | 6181 | N/A | Netherlands | Caucasians | rs7041, rs4588 | Gc variants were not associated with fracture risk in entire study. In a subgroup of individuals with calcium intake <1.09 g/day, the hazard ratio for fracture risk for the Gc1S homozygote was 1.47 (CI = 1.06–2.05), compared to non-carriers. | |
| Lauridsen et al.77 | 595 | N/A | Denmark | Caucasian | Gc1F, Gc1S, Gc2 | Lowest fracture risk in Gc2-2 group (14%) compared to Gc1-2 (27%) and Gc1-1 (34%) (p = 0.017). Fracture risk OR for Gc2-2 = 0.32 (CI = 0.13–0.80) Compared to the other genotypes, none of the Gc2-2 individuals had any low energy fractures (p = 0.021). | |
| Papiha et al.86 | 26 | 21 | United Kingdom | Caucasian | Gc1F, Gc1S, Gc2; Variable tandem (TAAA)n-Alu repeat in Intron8 | Intron 8*Alu repeat had significant association with lumbar spine and femoral neck BMD. BMD was observed to be lower in men with 10/8 genotype than 10/10 genotype (p < 0.05). | |
| Sarcoidosis | Milman et al.192 | 44 | 44 | Denmark | Caucasiana | Gc1F, Gc1S, Gc2 | NS |
| Infections Diseases | |||||||
| Human Immunodeficiency Virus (HIV) | Alonso et al.216 | 318 (at risk or infected) | 187 | Spain | Caucasian | Gc1F, Gc1S, Gc2 | NS |
| Cleve et al.217 | 97 | 1523 | United Kingdom | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Eales et al.219 | 203 (at risk males) | 177 (50 homo-sexual and 122 hetero-sexual males) | United Kingdom | Caucasiana | Gc1F, Gc1S, Gc2 | 30% of AIDS patients were homozygous for Gc1F compared to 0.8% of controls (p < 0.0001). Gc2-2 individuals were overrepresented in sero-negative contacts of AIDS patients (p < 0.05). | |
| Pronk et al.212 | 447 (96 AIDS patients and 351 homosexual men | 86 | Netherlands | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Putkonen n et al.218 | 125 (85 AIDS patients and 40 couples with 1 infected partner) | 3394 | Sweden | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Rosberger et al.227 | 121 | 1011 | United States | Caucasians and African Americans | Gc1F, Gc1S, Gc2 | NS | |
| Rheumatoid Fever | Bahr et al.208 | 39 | 90 | Kuwait | Arabic | Gc1F, Gc1S, Gc2 | An association was observed between Gc2 allele with rheumatic fever (p = 0.0024); 56% of all rheumatic fever patients were carriers of Gc2 allele. |
| Tuberculosis (TB) | Bahr et al.208 | 41 | 90 | Kuwait | Arabic | Gc1F, Gc1S, Gc2 | No association with GC alleles and TB, although an association was observed between Gc2 allele with rheumatic fever (p = 0.0024); 56% of all rheumatic fever patients were carriers of Gc2 allele. |
| Martineau et al.206 | 123 (United Kingdom); 130 (Brazil); 281 (South Africa) | 140 (United Kingdom); 78 (Brazil); 182 (South Africa) | United Kingdom | Varied based on study locationd | Gc1F, Gc1S, Gc2 | Associations between GC polymorphism and TB only observed in Gujarati Asians from United Kingdom. Gc2-2 associated with susceptibility to active TB compared to wild type (Gc1-1): OR = 2.81 for Gc2-2 versus Gc1-1 (CI = 1.19–6.66, p = 0.009) No associations were observed in the populations from Brazil and or South Africa. | |
| Neurodegeneration | |||||||
| Amyotrophic Lateral Sclerosis (ALS) | Palma et al.182 | 11 (7 ALS, 4 other muscular disorders) | 4 | Portugal | Caucasiana | Gc1F, Gc1S, Gc2 | Gc2 may represent a disease risk, Gc2 was present in all familial ALS patients and 2 patients with other muscular diseases but in none of the healthy controls. |
| Multiple Sclerosis (MS) | Hollsberg et al.176 | 95 | 227 | Denmark | Caucasiana | Gc1F, Gc1S, Gc2 | NS |
| Lindblom et al.177 | 88 | 3394 | Sweden | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Niino et al.173 | 107 | 109 | Japan | Japanese | D432E, T436K | NS | |
| Orton et al.179 | 1364 | 1661 (first degree relatives) | Canada | Caucasiana | rs7041, rs4588 and 2 other SNPs in the Gc gene | NS | |
| Simon et al.178 | 214 | 428 | United States | Various (Nurses Health Study) | rs7041, rs4588 | NS | |
| Steckley et al.174 | 236 sib pairs (1 affected, 1 not) | N/A | Canada | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Parkinson’s Disease | Suzuki et al.191 | 137 | N/A | Japan | Japanesea | Gc1F, Gc1S, Gc2 | No association with GC and severity of Parkinson disease as evaluated by Hoehn & Yahr (HY) stages and Unified Parkinson’s Disease Rating Stage (UPDRS). |
| Schizophrenia | Beckman et al.187 | 47 | 2361 | Sweden | Caucasiana | Gc1F, Gc1S, Gc2 | NS |
| Fananas et al.189 | 162 | 365 | Spain | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Lange, 184 | 222 | 176 | Germany | Caucasiana | Gc1F, Gc1S, Gc2 | Excess of Gc1-1 and a deficit of Gc2-1 and Gc2-2 (p < 0.01) in cases versus controls | |
| Papiha et al.185 | 215 | 402 (203 family &199 unrelated) | United Kingdom | Caucasiana | Gc1F, Gc1S, Gc2 | NS with Gc2 allele. However, lower frequency of Gc1S in female cases than controls (50% versus 63%, p < 0.025) | |
| Rudduck et al.188 | 152 | 3384 | Sweden | Caucasiana | Gc1F, Gc1S, Gc2 | NS | |
| Saha and Tsoi, 186 | 423 males | 595 | Singapore | Asiana | Gc1F, Gc1S, Gc2 | Gc1S appears to offer protection against schizophrenia. Excess of Gc2 allele over Gc1 (p < 0.005) and lack of Gc1S in cases (p < 0.001) was observed. RR for Gc1F, Gc1S, and Gc2 estimated as 1.12, 0.76, and 1.15, respectively. | |
Acronyms used in table: CI = Confidence Interval (95%); OR = Odds Ratio; RR = Relative Risk. Abbreviations used in table: NS = no significant association between polymorphisms studied and disease risk; AATD = α1-antitrypsin deficiency; HOMA (Homeostatis model assessment) is a measure used to quantify insulin resistance and beta cell function. In the cited study, HOMA(R) was calculated as [the fasting plasma glucose concentration (mg/dL)] × [fasting plasma insulin concentration (μU/mL)]/405 68.
aIn these studies, ethnicity was not clearly provided so they are assumed to be representative of the ethnic majority of the country where the study originates.
bSample was 99% Caucasian; the remainder of the sample was comprised of African-American, Asian, Hispanic and other ethnicities.
cFamily collections included samples from Ireland, Romania, Norway and families from the Human Biological Data Interchange.
dEthnicity included Gujarati Asians (United Kingdom), White, Black, Mixed Ethnicity (Brazil), Xhosa and Cape Colored (South Africa).
DBP genotypes and chronic disease
Diabetes
Type I diabetes mellitus results from the autoimmune destruction of insulin producing pancreatic β-cells while type II diabetes is associated with insulin resistance and elevated levels of inflammatory mediators65 , 66. Vitamin D may play a role in the prevention and treatment of diabetes through its anti-inflammatory actions or by modifying calcium homeostasis to improve insulin secretion or enhance insulin sensitivity67. As such, polymorphisms in the DBP gene might provide insight into the role of vitamin D and diabetes.
Several studies have examined whether pre-diabetic phenotypes are associated with DBP variants. In a Japanese study group, composed of 47 males and 35 females with normal glucose tolerance, carriers of Gc1S-2 and Gc1S-1S had significantly higher fasting plasma insulin levels than homozygotes for Gc1F (p < 0.01 and p < 0.03, respectively)68. These findings were supported in an ethnically distinct population made up of 144 Dogrib Indians of the Northwest Territories in Canada69.
On the other hand, Baier et al. found no association with insulin or fasting plasma glucose concentrations in a Pima cohort, although they did report that exon 11 polymorphisms were associated with blood glucose responses to an oral glucose challenge in non-diabetics70. Specifically, individuals homozygous for Gc1F had the highest increment in glucose concentration at 30 and 60 min and the lowest glucose concentration at 180 min. Moreover, carriers homozygous for Gc2 had significantly lower mean incremental glucose concentrations at 60 and 120 min70.
Similarly, in a population-based case-control study composed of Hispanic and Anglo participants from the San Luis Valley Diabetes Study, DBP variants were not associated with insulin levels71. Also, and in contrast with Baier et al., DBP variants were not associated with postprandial glucose, but were associated with fasting glucose levels (F = 2.46; p = 0.033), the Gc1F genotype being more commonly observed in individuals with the highest levels of plasma glucose71.
Other studies have examined the association between genetic variants of DBP and clinical diabetes. Results from a Japanese study, composed of 208 non-insulin-dependent diabetes mellitus patients and 209 control subjects with normal glucose tolerance, showed that Gc1F-1F homozygosity was less frequently and Gc1S/2 heterozygosity more frequently observed in patients compared to controls (p < 0.001 and p < 0.02, respectively)72. Sequence analysis identified D432E and T436K genotypes in a cohort of 912 Pima Indians (578 diabetic; 334 nondiabetic) from Gila River Indian Community in Arizona, USA70, but no association was reported between either variant and the prevalence of type II diabetes.
In a family-based study, the tetranucleotide repeat in intron 873 and D432E and T436K polymorphisms were studied in 152 Caucasian families of German origin with at least one child developing type I diabetes66. Transmission disequilibrium testing (TDT) showed neither allelic distortion of intron 8 or exon 11 alleles between cases and controls, nor of their combined haplotypes66. Similarly, allele, genotype and haplotype frequencies of SNPs in exon 11 did not differ between non-diabetic controls (n = 163) and type I (n = 181) or type II (n = 215) diabetics in a Caucasian American population74. These findings were substantiated in French and Polish Caucasian case-control studies that additionally reported that phenotypes, defined by haplotype combinations, were equally distributed between Type II diabetics and non-diabetics75 , 76.
In aggregate, these studies fail to support a strong, consistent role for DBP in the pathobiology of diabetes. It is likely that ethnicity and lifestyle factors contribute to the lack of concordance among these studies.
Osteoporosis
Osteoporosis is a common disorder of aging that disproportionately affects post-menopausal women. It is characterized by low bone mass and micro-architectural changes in bone which contribute to increased bone fragility and risk of fracture77. The primary clinical marker of osteoporosis, lower bone mineral density (BMD), is associated with vitamin D insufficiency78–80. However, the links between DBP and bone biology are not well understood. Whereas Gc-MAF is shown to increase phagocytosis and free radical (superoxide) production in macrophages61 , 81, further work has implicated it as a potent activator of osteoclasts82. Indeed, osteopetrotic patients (characterized by excessively dense bone and high BMD) appear to lack the ability to generate Gc-MAF83. Osteopetrotic rats treated with Gc-MAF showed a reduction in BMD and associated skeletal defects84.
In the ongoing 20-year Danish Osteoporosis Prevention Study comprised of 595 white postmenopausal women, Scandinavian investigators found a three-fold lower fracture risk associated with Gc2-2 compared with Gc1-1 genotype (p = 0.014, OR = 0.32, 95% CI = 0.13–0.80)77. A more comprehensive set of SNPs distributed across promoter, intronic and exonic regions of DBP was analyzed in 384 Japanese in a search for association with BMD85. Six SNPs (−39C > T, IVS1 + 827C > T, IVS1 + 1916C > T, IVS1-1154A > G, D432E and IVS11 + 1097G > C) were either significantly associated or suggestively associated with BMD, the IVS11 + 1097G > C SNP in intron 11 showing the strongest correlation (p = 0.006). Furthermore, a common haplotype (T-C-C-G-T-C) derived from the six SNPs showed significant association with adjusted radial BMD (r = 0.15, p = 0.008). The functional consequence(s) of these SNPs/haplotypes are not known.
Another polymorphism in the form of an (TAAA)n-Alu repeat or repetitive element downstream of intron 8 was examined in a cohort of Caucasian men from northeast England for association with DBP serum levels, BMD and osteoporosis86. The (TAAA)8 (GC-I8*8) allele is the most prevalent one in this study group. Upon analyzing 26 men with vertebral fractures and 21 controls, the GC-I8*10/*8 genotype was found to be associated with decreased BMD lumbar spine and femoral neck BMD and increased vertebral fractures compared with GC-I8*10/*10 (p < 0.05, OR = 56; 95% CI = 7–445)86. Interestingly, GC-I8*10/*8 was also associated with elevated serum DBP (p = 0.049) which, in turn, may provide greater potential for Gc-MAF activation of osteoclasts in Gc1 carriers. Results of this study remained consistent in another male cohort made up of 56 individuals with idiopathic osteoporosis and 114 controls, but the odds ratio was much more modest87. Carriers of the GC-I8*10/*8 genotype and the GC-I8*9 allele were at increased risk of osteoporosis (OR = 2.88, CI = 1.31–6.32, p = 0.013 and OR = 1.86, CI = 1.07–3.24, p = 0.038, respectively). Moreover, combining all genotypes that include GC-I8*8 is associated with a two-fold risk of osteoporosis (OR = 2.38, CI = 1.24–4.58, p = 0.014). However, GC-I8*10 allele carriers and GC-I8*10/*10 homozygotes showed increasingly substantial protection from osteoporosis (OR = 0.40, CI = 0.25–0.64, p = 0.0005 and OR = 0.13, CI = 0.05–0.36, p = 0.0005, respectively). Whether this effect is related in part to linkage disequilibrium with the nearby functional polymorphisms (D432E and T436K) has not been examined.
In a common aging disorder like osteoporosis, many genes are involved and interaction with non-genetic factors (e.g. calcium and vitamin D intake) is common. A recent prospective study examining more than 6100 elderly Caucasians found no association between DBP phenotype and osteoporosis; however, a 33% increased risk of fracture was detected in Gc1S carriers homozygous for polymorphisms in the 3′ untranslated region (UTR) of the vitamin D receptor (VDR) in haploblock 5 compared with non-carriers (p = 0.005)88. Moreover, a hazard ratio of 1.47 (95% CI = 1.06–2.05) was observed for homozygous Gc1S carriers (compared with non-carriers) with low dietary calcium intake (<1.09 g/day).
Collectively, these studies emphasize the importance of gene-gene and gene-environment interactions in complex trait analysis and underscore the need to characterize the role of non-coding genetic variants associated with osteoporosis and related phenotypes.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is characterized by decreased pulmonary elastic recoil, expiratory obstruction and hyperinflation secondary to inflammation of peripheral airways89. The role of DBP as a modulator of inflammation suggests that it plays a role in the chronic pulmonary inflammation characteristic of COPD. Consistent with this hypothesis, an association study in a Caucasian population of 104 COPD patients and 413 controls indicated that Gc1F-1F and Gc1S carriers (at least one copy) were seven-fold and two-fold more likely, respectively, to have COPD than individuals with at least one Gc-2 allele90.
In another study of 75 Caucasian COPD patients, Gc-2 homozygosity was found to be underrepresented in COPD patients compared to 64 controls (OR = 0.17, CI = 0.03–0.83)89. This is in agreement with an earlier report examining 114 COPD cases in a match pair design study showing an excess of Gc2 homozygotes in the control group (p = 0.049)91.
Similarly, a recent study of 471 unrelated Caucasian subjects confirmed that the Gc2 variant was associated with a decreased risk of COPD (OR = 0.79, CI = 0.65–0.99; p = 0.048), though it was associated with an increased risk of bronchiectasis in patients with α1-antitrypsin deficiency (OR = 1.51, CI = 1.02–2.22). The authors suggested this may be due to reduced capacity to produce Gc-MAF, thereby stimulating macrophage-induced pathogen clearing from the airway92.
A Japanese case-control study (103 cases, 88 controls) found that Gc1F homozygotes were more susceptible to chronic bronchitis and emphysema due to COPD than were healthy smokers (p = 0.01, OR = 2.3, 95% CI = 1.2–4.6)93. In a second Japanese cohort, Gc1F homozygotes were overrepresented among COPD patients (36.5%) compared to controls (20.7%; OR = 2.2; 95% CI = 1.1–4.6). However, no association was found with diffuse panbronchitis, also characterized by chronic airflow limitation94. In a case-control study of a Han Chinese cohort (100 cases, 100 controls), the COPD patient group was found to be enriched for Gc1F homozygous individuals while the frequency of Gc2 was significantly lower95.
Together, these findings are consistent with evidence suggesting that the presence of the 436 K minor variant (Gc2) precludes the conversion of DBP to Gc-MAF, potentially reducing macrophage-related inflammation96. Another recent report indicates that, 25(OH)D deficiency is more common in COPD patients from Belgium and correlates with disease severity97. In the same study, homozygous T436 subjects were found to have a 25% reduction in vitamin D serum levels (p < 0.0001) and were enriched amongst the COPD patient population (OR = 2.11; CI = 1.20–3.71). It is conceivable, therefore, that anti-inflammatory actions exerted downstream of 25(OH)D52 , 97 are reduced in patients. The nature by which DBP influences COPD pathogenesis deserves further investigation, however unlikely it is to affect the rate or onset of deteriorating lung function98 , 99.
Asthma
Asthma is a chronic inflammatory condition characterized by airway obstruction and elevated serum IgE levels100, most often found to be a consequence of enhanced acquired reactions to external allergens. It is associated with various reactive pro-inflammatory states including allergic rhinitis and eczema. Ecological studies indicate that vitamin D deficiency is associated with asthma and asthma related phenotypes101–105, but some studies contradict these findings reporting a positive association106 , 107, whereas others show no association108. Further evidence suggesting an inverse correlation between vitamin D and asthma is provided by genetic109 , 110 and epidemiologic (geospatial and solar radiation) studies111. However, in another study, Hughes et al. reported no association between ultraviolet radiation (UVR) or vitamin D serum levels; yet, cod liver oil supplementation earlier in life was associated with an increased risk of asthma112.
Overall evidence suggests that vitamin D protects against asthma, but the precise nature of this relationship is ambiguous and inconsistent findings are likely a function of epidemiological context. A molecular mechanism by which vitamin D may affect asthma is unknown and its relationship to atopy is unclear. However, emerging evidence suggests that 1,25(OH)2D3 inhibits Th2-related asthmatic inflammation either directly or via immunosuppressive T-regulatory cell induction113–115. However, these findings were not substantiated in a recent case-control study examining 467 asthmatic patients and 288 unrelated healthy controls in a Chinese Han population, which showed an enrichment of Gc2-2 in asthma cases (OR = 1.35: CI = 1.01–1.78; p = 0.006)116. A TDT of more than 200 families from the German Asthma Family Study found no significant association with asthma, but rs222040 and rs7041 (D432E) were both weakly associated with total serum IgE (p < 0.02 and p < 0.03, respectively)117.
Ischemic stroke
Risk of ischemic stroke is associated with elevated levels of vascular inflammation118 , 119 and lower 25(OH)D120, both of which are thereby linked to DBP. In six study populations from the United States, Europe and China (3550 cases, 6560 controls), a recent study evaluated 105 variants in 64 inflammation- and cardiovascular-related genes for association with ischemic stroke. Among the SNPs evaluated were DBP D432E and T436K, but neither was significantly associated119.
Endometriosis
Endometriosis is a uterine disorder in which large overgrowths of endometrium accumulate inside or adjacent to the uterus121. The precise etiology remains unclear, but one theory holds that the peritoneal epithelia can differentiate into endometrial tissue as a result of chronic inflammation122. Furthermore, macrophage activation has been associated with disease progression123, thereby highlighting a potential role for DBP. In a recent cross-sectional study of premenopausal women undergoing laparoscopy, analysis of specific Gc allele products using nano-scale liquid chromatography-electrospray ionization-mass spectrometry indicated that the Gc-2 allele product was enriched in serum samples in women with endometriosis (p = 0.006)124. The authors speculate that the lack of activated macrophages’ phagocytic function in those Gc2 carriers may increase the risk for implantation of endometriosis tissue in the peritoneal cavity. Further investigation of this hypothesis is warranted.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) includes Crohn’s disease and ulcerative colitis, both of which are characterized by chronic inflammation of the gastrointestinal tract125. Data collected as part of the Swiss Inflammatory Bowel Disease Cohort Study was used to test for associations between common DBP polymorphisms and risk of IBD125. Homozygotes for the K allele of T436K SNP were more common in non-IBD controls than in IBD patients (p = 0.006). Significant associations were observed between SNP variants and disease risk in separate comparisons of healthy controls with ulcerative colitis (p = 0.022) and Crohn’s disease patients (p = 0.016)125. The DBP-2 haplotype (consisting of D432-436 K) was found at higher frequencies in healthy controls than in IBD cases (OR = 2.53, CI = 1.48–4.34, p = 0.0005), especially when comparing healthy controls to the ulcerative colitis cases (OR = 4.39, CI = 1.87–10.31, p = 0.0003).
Cancer
Several observational studies have demonstrated an inverse relationship between UVB irradiance (required for the cutaneous synthesis of vitamin D) or vitamin D status [serum 25(OH)D concentrations] and the incidence of mortality from endometrial126, lung127 , 128, breast127 , 129–133, prostate134–137, ovarian138 , 139 and colorectal cancer140–149. The underlying mechanism by which vitamin D status affects cancer risk is unknown, however, 1,25(OH)2D3 has been shown to modulate cell proliferation and differentiation of both normal and malignant cells. Alternatively, inflammatory modulation effects of 1,25(OH)2D3 or Gc-MAF may play important roles in cancer pathogenesis.
Breast cancer
Ecological case-control studies indicate an inverse correlation between vitamin D status and breast cancer risk, however, the relationship is less clear in longitudinal studies150 , 151. Much work has been done to elucidate a vitamin D-related mechanistic role in breast cancer. Gc-MAF exhibits anti-breast tumor activities in mice152 and shows immunotherapeutic properties in metastatic breast cancer patients153. Exposure to Gc-MAF significantly reduces vimentin expression in human breast cancer cells, suggesting a reversal of breast cancer progression154. Alternatively, the active metabolite of vitamin D, calcitriol, has been shown to inhibit cell proliferation and differentiation and promote apoptosis of breast tumor tissue155–158.
Genetic studies examining the relationship between functional DBP variants and breast cancer (in situ and invasive) have yielded inconsistent findings. In a nested case-control study derived from the Cancer Prevention Study II Nutrition Cohort (500 cases, 500 controls), D432E and T436K were not associated with postmenopausal breast cancer risk159. Interestingly, a German population-based case-control study comprised of 1,402 post-menopausal women with in situ or invasive breast cancer and 2608 population controls reported a protective effect of Gc2-2 carriers (OR = 0.72, 95 % CI = 0.54–0.96)160. Both these studies argue against a role for Gc-MAF or vitamin D in breast cancer pathogenesis. In contrast, subjects with the rs7041 TT genotype were at increased risk of breast cancer (OR = 1.23; 95% CI = 1.01–1.51) based on a study of 1560 invasive breast cancer patients and 1633 controls from the Ontario Women’s Diet and Health Study161. Altogether, these findings underscore the complex pathogenesis of breast cancer and highlight the need to consider gene-environment interactions.
Prostate cancer
Studies have shown that vitamin D can inhibit proliferation and differentiation of human prostatic cells in vitro 162 and 1,25(OH)2D3 has marked anti-tumor effects in animal models163. Furthermore, recent work has demonstrated that Gc-MAF directly inhibits proliferation and migration of prostate cancer cells as well as expression of a tumor metastasis-associated gene, urokinase plasminogen activator receptor164 consistent with an early study demonstrating the elevated risk of Gc2 genotype and carcinoma of the prostate165. However, in a comprehensive study of 749 cases and 781 controls from the PLCO Cancer Screening Trial, Ahn et al. found no association between risk of prostate cancer and 15 SNPs in GC, which included D432E and T436K166. An earlier study examining D432E and T436K in 181 prostate cancer patients (90 black and 91 white) and sex-, age- and race-matched controls also found no differences in allelic/genotypic frequencies between patients and controls; however, frequencies were strikingly different between blacks and whites167.
Gastrointestinal and colorectal cancer
In a well established colorectal cancer cohort (Colon Cancer Family Registry), Poynter et al. explored previous epidemiological evidence suggesting a reduced risk associated with vitamin D. No evidence for association between DBP genotype and risk of colorectal cancer was found, although associations between DBP SNPs and microsatellite-unstable colorectal cancer were reported168. A more recent study examining associations between polymorphisms in the GC and CASR genes similarly found no associations in any of the tested GC SNPs169.
Zhou et al.170 examined associations between variants of D432E (rs7041) and T436K (rs4588) and risk of four types of gastrointestinal cancers (hepatocellular, esophageal, gastric and colorectal) in a Han Chinese population. An elevated risk for colorectal cancer was observed in individuals homozygous for the 436 K allele, in comparison to individuals homozygous for the wildtype (OR = 3.41; CI = 1.85–6.57, p < 0.001). When all four types of cancer were combined in the analysis, the 436 K homozygotes had 1.15–fold increased risk over wildtype (CI = 1.02–1.30, p = 0.020) after adjustment for age, sex and smoking status. Gc2 allele carriers also had an increased risk for developing gastrointestinal cancer (OR = 1.22; CI =104–1.39, p = 0.015)
Skin cancer
Associations between GC SNPs and basal cell carcinoma (BCC) were examined as part of the prospective cohort Rotterdam Study (n = 7983). The study found no significant association between the GC genotypes and haplotypes and risk of developing at least one BCC. However, Gc1F carriers were more likely to develop a first BCC compared to non-carriers (HR = 1.40; 95% CI = 1.11–1.78). When stratified by age, individuals younger than 65 years of age and homozygous for Gc1S had about half the risk of developing a first BCC, compared to non-carriers (HR = 0.53; CI = 0.31–0.91)171.
Neurodegenerative disorders
Multiple sclerosis
Multiple sclerosis (MS) is a form of disseminated encephalomyelitis that appears to be partly autoimmune in origin though its etiology is not known. The rates of MS exhibit a pattern of increasing prevalence with increasing latitude, indicating a protective role of ultraviolet radiation in the development of MS172. Moreover, several studies have shown an inverse correlation between serum levels of vitamin D and MS173 , 174, suggesting that polymorphisms influencing vitamin D status might contribute to the initiation or progression of disease.
Serum DBP was found to be upregulated along with other acute phase reactive proteins in a small sample (n = 9) of pediatric MS patients175, however, the clinical relevance is not known. Early reports examining the distribution of DBP phenotypes among European adults did not find significant correlations with presence of disease, disease natural history or age of onset176 , 177. Similarly, in 107 Japanese MS patients and 109 controls, neither DBP phenotypes nor D432E/T436K genotypes (examined independently) were associated173, a finding that was recently confirmed178 , 179. To control for population admixture/stratification, both SNPs were evaluated in a family-based design study of 187 Canadian families; however, the lack of association between genotype and disease was unchanged174. Ongoing interventional trials with vitamin D and association studies with larger cohorts may offer further opportunity to explore an interactive role of DBP SNPs with vitamin D in MS.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is another neuropathy of unknown etiology that is characterized by the progressive loss of motor neurons in the brain and spinal cord, leading to debilitating, usually fatal, muscle atrophy180. While the vast majority of ALS patients are sporadic, 10–15% of cases show familial clustering. Mutations in the cytosolic superoxide dismutase (SOD1) gene have been associated with ALS, but these account for less than 20% of all familial ALS (FALS)181. In Portuguese patients with FALS, proteomic analysis showed that the Gc-2 phenotype (436 K) was overrepresented in comparison to healthy controls182. The role of DBP in ALS is not known, but it is tempting to speculate that motor neuron damage resulting in the systemic release of actin and ensuing intravascular coagulation and local hypoxia-induced oxidative damage may be mitigated by the actin-scavenging properties of DBP. Whether the apparently reduced capacity of Gc-2 for actin-scavenging, compared with Gc1 30 , 34 , 35, might increase the likelihood of disease progression is not known.
Schizophrenia
A number of hypotheses have been put forth to explain the etiology of schizophrenia, but several lines of observational evidence suggest that vitamin D may be a risk factor183. The underlying molecular mechanism governing this association is not known, but prenatal vitamin D deficiency is associated with structural and functional deficits common to schizophrenic patients. Several early reports examined DBP phenotypes in relation to schizophrenia. An excess of Gc1-1 was observed in cases over controls in a study carried out in a German study (p < 0.01)184. This association was even more compelling for the hebephrenic subgroup (p < 0.01). On the other hand, analysis of a north east England cohort comparing 215 schizophrenic patients with healthy first-degree relatives or non-familial controls reported a significant reduction in Gc1S alleles (p = 0.025) largely within the female subjects185. Findings from a Chinese male cohort of 423 schizophrenic patients also suggested a relative paucity of Gc1S (p < 0.001) and an excess of Gc2 186. However, there are several other studies reporting lack of association between DBP and schizophrenia187–189, and firm conclusions would be premature.
Parkinson disease
Parkinson disease is a progressive movement disorder, the severity of which has been found to be inversely associated with serum levels of 25(OH)D190. However, a study examining GC polymorphisms and severity of Parkinson disease did not identify any significant associations191.
Other chronic diseases
Liver disease
In liver disease, secretion of proteins belonging to the albumin family is usually reduced, and it is therefore not surprising that there are numerous studies reporting lower serum DBP concentrations in this group of disorders. This may also be associated with inability to sufficiently sequester cellular actin released by the dying hepatic cells themselves, thereby contributing to an increased risk of intravascular coagulation. Variants associated with DBP status might therefore be useful as biomarkers of worse outcomes in liver disease. Looking at 17 alcoholic liver cirrhosis patients and 100 healthy controls, French investigators observed an unusual electrophoretic form of non-ligand bound DBP that was characterized by the presence of an additional sialic acid moiety. This form of DBP was found only in Gc-1 carriers and was associated with worse clinical outcome35. To date, this novel phenotype has not been reported in other studies and, therefore, the larger relevance in non-hepatic disorders is unknown.
Sarcoidosis
Sarcoidosis, a granulomatous inflammatory disease, typically affects young adults and is associated with altered immunoglobulin and increased 1,25(OH)2D3 production. DBP phenotypes were examined in a small case-control study of 88 patients, but found no relationship between disease, course of disease, or presentation192. Whether 1,25(OH)2D3 levels might be associated with the D432E or T436K genotypes, as others have observed, was not studied.
Thyroid autoimmune disease
Graves’ disease and Hashimoto thyroidosis are the two common forms of autoimmune thyroid disease. In such disorders, 1,25(OH)2D3 downregulates expression of thyrocyte-derived human leukocyte antigen class II molecules193 and inhibits secretion of proinflammatory cytokines and lymphocyte proliferation194. In 95 and 92 Western European pedigrees positive for Graves’ disease and Hashimoto’s thyroiditis, respectively, Pani et al. looked at TDT of the DBP gene195. Only the Alu repeat in intron 8 (I8*8) was found to be associated with Graves’ disease (p < 0.03), and none were associated with Hashimoto’s thyroiditis. Neither D432E nor T436K was associated with disease outcome. In contrast, association of GC SNPs with Graves’ disease was found in a case-control study evaluating 332 cases and 185 healthy controls of Polish origin. In that study, cases were significantly enriched for the T436K heterozygotes (OR = 1.50; CI = 1.13–1.99; p = 0.005)196.
Infectious diseases
Tuberculosis
Mycobacterium tuberculosis, the causative agent of tuberculosis, infects a third of the world’s population and results in 3 million deaths per year197. Low serum levels of 25(OH)D have been correlated with extant tuberculosis198–204 and incident infection204. Oral supplementation of vitamin D given to tuberculosis contacts enhanced immunity towards the mycobacterium205. A significant advance in our understanding of innate immunity against tuberculosis came with a seminal report showing convergence of the vitamin D metabolic pathway with the Toll-like receptor (TLR2/1) signaling cascade resulting in the induction of cathelicidin, a potent antimicrobial peptide in the innate immunity pathway54 , 55. Subsequently, GC genotypes were examined in several ethnically diverse tuberculosis study populations206. In Gujarati Asians, the homozygous variant Gc2-2 phenotype was strongly associated with susceptibility to active tuberculosis, compared with Gc1-1 using logistic regression to adjust for age and sex (OR = 2.81, CI = 1.19–6.66, p = 0.009). Furthermore, this association was observed only under conditions of low 25(OH)D, suggesting an important gene–environment interaction in this condition.
Rheumatic fever
Rheumatic fever is a systemic inflammatory illness characterized by excessive B cell activity leading to an over-production of antibody to Group A streptococcus. DBP is found on the surface of B-cells, often in a molecular complex with actin and surface immunoglobulin, and may play a role in B cell activation by facilitating signal transduction activity207. Alternatively, inflammatory mediation of DBP may affect the course of disease. In a study of 39 cases and 90 controls of Arab ancestry, a positive association between the Gc-2 allele and rheumatic fever was observed (p = 0.0024)208. The implications of this finding are not altogether clear, but suggest that the anti-inflammatory properties of Gc-2 may not be important in this disorder.
Human immunodeficiency virus
Untreated, human immunodeficiency virus (HIV) infection often progresses to acquired immunodeficiency syndrome (AIDS) with its subsequent complications of opportunistic infection and neoplasia. Macrophages are among the first cell types to be infected by HIV-1 and serve as reservoirs for the virus in affected persons209 , 210. Macrophage activation via Gc-MAF may therefore be an important factor in the acquisition of HIV-1 infection and progression to AIDS. Indeed, immunotherapy of HIV-infected individuals with GcMAF appeared to be significantly curative211. Several studies have examined the association between DBP variants and HIV/AIDS.
DBP phenotypes were examined in a cohort consisting of 86 HIV negative hospital workers, 351 homosexual men that were either HIV negative (as determined by the absence of HIV antibodies) or HIV positive (as determined by the presence of HIV antibodies with or without HIV antigen), and 96 AIDS cases212. It is thought that the presence of HIV antigen is a strong predictive marker of disease progression213–215. During the course of this study, a subset of 62 HIV negative homosexual men seroconverted. No significant difference in DBP distribution was observed between HIV negative heterosexuals, HIV negative homosexuals and patients with AIDS. Moreover, there was no difference in DBP phenotype distribution between homosexual men who remained HIV negative and those who sero-converted during follow-up, arguing against the involvement of DBP in susceptibility to HIV infection. Finally, DBP phenotypes were equally distributed among (i) HIV-positive homosexuals without the presence of HIV-antigen, (ii) HIV positivity with the presence of HIV antigen and (iii) AIDS – effectively excluding a role for DBP in either susceptibility or progression. Other investigators examining Spanish, German and Swedish populations also found no evidence of association between DBP variants and susceptibility to HIV/AIDS216–218. However, an excess of Gc2 was observed in persistently seronegative sexual contacts (engaging in unprotected sex) of AIDS patients, although the sample size was small219.
In summary, there is little evidence to support a role for DBP in susceptibility to HIV/AIDS or progression to AIDS, based on these studies. The interplay between HIV and DBP, however, is complex211 and likely confounds any true associations between genetic variation and infection/disease outcome. Investigators may choose to focus on identifying new genetic variants in or near the GC gene that may provide further insight into a molecular mechanism bridging DBP and HIV/AIDS.
Discussion
We present an overview of studies examining the association of genetic/phenotypic variants of GC and adverse health outcomes, several of which constitute global health problems of major public health importance. It is becoming increasingly evident that the DBP plays an important role in inflammation and immunity, and that genetic variants encoding this protein may, in part, modulate these pathophysiologic pathways. Despite recent progress, the nature of this regulation remains unclear. In comparison to Gc-2 (436 K) types, non-Gc-2 polymorphisms are associated with increased macrophage activity and elevated vitamin D serum levels required for downstream upregulation of antimicrobial peptides220. While it is tempting to speculate that Gc1-related macrophage activity results in prolonged inflammation associated with chronic diseases61, binding of active 1,25(OH)2D3 metabolite to its intracellular receptor (VDR) is anti-inflammatory52 , 221. Included amongst those actions is the negative regulation of nuclear factor kappa B (NFκB), an essential component of the inflammatory response by recruitment of histone deacetylase 352 , 222.
It should be noted that there are inherent limitations in some association studies that are related not to sample size but to intrinsic heterogeneity of disease phenotype. A case in point is schizophrenia, a disorder that is notoriously difficult to classify and has proven quite resistant to confirmation of initially promising results223. Candidate gene studies looking at DBP in this area are no exception.
Further work is needed to characterize more precisely the role of DBP in chronic and infectious disease, however, current evidence from genotype-phenotype association studies does indicate an important role in innate-immune-related inflammation.
Conclusion
There is growing evidence that DBP plays a role in immune modulation, either directly through Gc-MAF, or indirectly by influencing serum levels of vitamin D. It is tempting to speculate that DBP operates as a molecular switch between pro- and anti-inflammation and intervention strategies designed to modify this switch may be important in the control and prevention of chronic and infectious diseases. Nevertheless a precise understanding of the role of DBP in chronic and infectious diseases is still lacking. Correlations between functional DBP variants and adverse health outcomes have been difficult to interpret, in part because there is still much about the biology of DBP that is not well understood. Furthermore, many of the association studies were insufficiently powered and failed to account for multiple comparisons. A greater effort is therefore needed to clarify the relevant gene-gene and gene-environment interactions. Given that GC gene is so polymorphic, it is reasonable to consider that other SNPs in this gene may be more relevant to the disease outcomes being studied, but they have yet to be formally examined. It is becoming clear that DBP is indeed a multifunctional protein with an important auxiliary role in the relationship between vitamin D and innate immunity. The various discordant findings underscore the need for more robust genetic studies and better characterization of just how this protein functions.
Acknowledgements
We appreciate the editorial work of B. Lee and helpful discussions with E. Parra and R. Vieth.
Glossary
Abbreviations
- AFM:
α–albumin/afamin
- AFP:
α-fetoprotein
- AIDS:
acquired immunodeficiency syndrome
- ALB:
albumin
- ALS:
amyotrophic lateral sclerosis
- BCC:
basal cell carcinoma
- BMD:
bone mineral density
- COPD:
chronic obstructive pulmonary disease
- DBP:
vitamin D binding protein/group-specific component/Gc-globulin
- FALS:
familial ALS
- GC:
vitamin D binding protein gene
- Gc-MAF:
vitamin D binding protein-macrophage activating factor
- HIV:
human immunodeficiency virus
- IBD:
inflammatory bowel disease
- MS:
multiple sclerosis
- SNPdb:
single nucleotide polymorphism (SNP) database
- TDT:
transmission disequilibrium test
- UTR:
untranslated region
- VDR:
vitamin D receptor
- 25(OH)D:
25-hydroxycalciferol
- 1,25(OH)2D3:
1,25-dihydroxycholecalciferol
- 1,25(OH)2D2:
1,25-dihydroxyergocalciferol
- 24,25(OH)2D:
24,25-dihydroxycalciferol
Declaration of interest
Contributions of D.E.C.C. and L.F. to this work were supported in part by a grant from the Dairy Farmers of Canada. A.G. has no declaration of interest.
References
- 1.Haddad JG, Hu YZ, Kowalski MA, et al. Identification of the sterol- and actin-binding domains of plasma vitamin D binding protein (Gc-globulin) Biochemistry. 1992;31:7174–81. doi: 10.1021/bi00146a021. [DOI] [PubMed] [Google Scholar]
- 2.Otterbein LR, Cosio C, Graceffa P, Dominguez R. Crystal structures of the vitamin D-binding protein and its complex with actin: structural basis of the actin-scavenger system. Proc Natl Acad Sci USA. 2002;99:8003–8. doi: 10.1073/pnas.122126299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verboven C, Rabijns A, De MM, et al. A structural basis for the unique binding features of the human vitamin D-binding protein. Nat Struct Biol. 2002;9:131–6. doi: 10.1038/nsb754. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Habiel DM, Ramadass M, Kew RR. Identification of two distinct cell binding sequences in the vitamin D binding protein. Biochim Biophys Acta. 2010;1803:623–9. doi: 10.1016/j.bbamcr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillon R, Van BH, Rombauts W, De MP. The purification and characterisation of the human-serum binding protein for the 25-hydroxycholecalciferol (transcalciferin). Identity with group-specific component. Eur J Biochem. 1976;66:285–91. doi: 10.1111/j.1432-1033.1976.tb10518.x. [DOI] [PubMed] [Google Scholar]
- 6.Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci USA. 1975;72:2076–80. doi: 10.1073/pnas.72.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulin: roles in response to injury. Clin Chem. 2006;52:1247–53. doi: 10.1373/clinchem.2005.065680. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R, Xiang DZ, Convents R, Van BH. Polyunsaturated fatty acids decrease the apparent affinity of vitamin D metabolites for human vitamin D-binding protein. J Steroid Biochem Mol Biol. 1992;42:855–61. doi: 10.1016/0960-0760(92)90094-y. [DOI] [PubMed] [Google Scholar]
- 9.Calvo M, Ena JM. Relations between vitamin D and fatty acid binding properties of vitamin D-binding protein. Biochem Biophys Res Commun. 1989;163:14–7. doi: 10.1016/0006-291x(89)92091-3. [DOI] [PubMed] [Google Scholar]
- 10.Ena JM, Esteban C, Perez MD, et al. Fatty acids bound to vitamin D-binding protein (DBP) from human and bovine sera. Biochem Int. 1989;19:1–7. [PubMed] [Google Scholar]
- 11.Van BH, Bouillon R, De MP. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980;255:2270–2. [PubMed] [Google Scholar]
- 12.Williams MH, Van Alstyne EL, Galbraith RM. Evidence of a novel association of unsaturated fatty acids with Gc (vitamin D-binding protein) Biochem Biophys Res Commun. 1988;153:1019–24. doi: 10.1016/s0006-291x(88)81330-5. [DOI] [PubMed] [Google Scholar]
- 13.Cho EH, Kim MR, Kim HJ, et al. The discovery of biomarkers for type 2 diabetic nephropathy by serum proteome analysis. Proteomics Clin Appl. 2007;1:352–61. doi: 10.1002/prca.200600608. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Hawke DH, Liu Y, et al. Proteomic analysis of nipple aspirate fluid from women with early-stage breast cancer using isotope-coded affinity tags and tandem mass spectrometry reveals differential expression of vitamin D binding protein. BMC Cancer. 2006;6:68. doi: 10.1186/1471-2407-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bijian K, Mlynarek AM, Balys RL, et al. Serum proteomic approach for the identification of serum biomarkers contributed by oral squamous cell carcinoma and host tissue microenvironment. J Proteome Res. 2009;8:2173–85. doi: 10.1021/pr800979e. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Shu R, Luo LJ, et al. Initial comparison of proteomic profiles of whole unstimulated saliva obtained from generalized aggressive periodontitis patients and healthy control subjects. J Periodontal Res. 2009;44:636–44. doi: 10.1111/j.1600-0765.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- 17.Xiao F, Chen D, Lu Y, et al. Proteomic analysis of cerebrospinal fluid from patients with idiopathic temporal lobe epilepsy. Brain Res. 2009;255:180–9. doi: 10.1016/j.brainres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Sokal I, Peskind ER, et al. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J Clin Pathol. 2008;129:526–9. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan X, Lu Q, Xue P, et al. Proteomic analysis of aqueous humor from patients with myopia. Mol Vis. 2008;14:370–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori N, Oda S, Sadahiro T, et al. YKL-40 identified by proteomic analysis as a biomarker of sepsis. Shock. 2009;32:393–400. doi: 10.1097/SHK.0b013e31819e2c0c. [DOI] [PubMed] [Google Scholar]
- 21.Bai S, Liu S, Guo X, et al. Proteome analysis of biomarkers in the cerebrospinal fluid of neuromyelitis optica patients. Mol Vis. 2009;15:1638–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Greco M, Mitri MD, Chiriaco F, et al. Serum proteomic profile of cutaneous malignant melanoma and relation to cancer progression: association to tumor derived alpha-N-acetylgalactosaminidase activity. Cancer Lett. 2009;283:222–9. doi: 10.1016/j.canlet.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Chan KY, Lai PB, Squire JA, et al. Positional expression profiling indicates candidate genes in deletion hotspots of hepatocellular carcinoma. Mod Pathol. 2006;19:1546–54. doi: 10.1038/modpathol.3800674. [DOI] [PubMed] [Google Scholar]
- 24.Gressner OA, Gao C, Siluschek M, et al. Inverse association between serum concentrations of actin-free vitamin D-binding protein and the histopathological extent of fibrogenic liver disease or hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2009;21:990–5. doi: 10.1097/MEG.0b013e3283293769. [DOI] [PubMed] [Google Scholar]
- 25.Kim BK, Lee JW, Park PJ, et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009;11:R22. doi: 10.1186/bcr2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anagnostopoulos AK, Kolialexi A, Mavrou A, et al. Proteomic analysis of amniotic fluid in pregnancies with Klinefelter syndrome foetuses. J Proteomics. 2010;73:943–50. doi: 10.1016/j.jprot.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Blanton D, Han Z, Bierschenk L, et al. Reduced serum vitamin d-binding protein levels are associated with type 1 diabetes. Diabetes. 2011;60:2566–70. doi: 10.2337/db11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song YH, Naumova AK, Liebhaber SA, Cooke NE. Physical and meiotic mapping of the region of human chromosome 4q11-q13 encompassing the vitamin D binding protein DBP/Gc-globulin and albumin multigene cluster. Genome Res. 1999;9:581–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Hiroki T, Song YH, Liebhaber SA, Cooke NE. The human vitamin D-binding protein gene contains locus control determinants sufficient for autonomous activation in hepatic chromatin. Nucleic Acids Res. 2006;34:2154–65. doi: 10.1093/nar/gkl174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daiger SP, Miller M, Chakraborty R. Heritability of quantitative variation at the group-specific component (Gc) locus. Am J Hum Genet. 1984;36:663–76. [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter D, De LM, Snieder H, et al. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16:371–8. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 32.Constans J, Viau M. Group-specific component: evidence for two subtypes of the Gc1 gene. Science. 1977;198:1070–1. doi: 10.1126/science.73222. [DOI] [PubMed] [Google Scholar]
- 33.Van BH, Bouillon R, De MP. The heterogeneity of human Gc-globulin. J Biol Chem. 1978;253:6344–5. [PubMed] [Google Scholar]
- 34.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47:753–6. [PubMed] [Google Scholar]
- 35.Constans J, Arlet P, Viau M, Bouissou C. Unusual sialilation of the serum DBP associated with the Gc 1 allele in alcoholic cirrhosis of the liver. Clin Chim Acta. 1983;130:219–30. doi: 10.1016/0009-8981(83)90119-5. [DOI] [PubMed] [Google Scholar]
- 36.Fu L, Cade C, Holm SS, et al. Relationship of functional T436K polymorphism in vitamin D binding protein (DBP) with serum 25-hydroxyvitamin D [25(OH)D], DBP concentration and binding capacity: a pilot study. Clin Chem. 2010;56:A154. [Google Scholar]
- 37.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–45. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borges CR, Jarvis JW, Oran PE, Nelson RW. Population studies of Vitamin D binding protein microheterogeneity by mass spectrometry lead to characterization of its genotype-dependent O-glycosylation patterns. J Proteome Res. 2008;7:4143–53. doi: 10.1021/pr8002936. [DOI] [PubMed] [Google Scholar]
- 40.Borges CR, Jarvis JW, Oran PE, et al. Population studies of intact vitamin D binding protein by affinity capture ESI-TOF-MS. J Biomol Tech. 2008;19:167–76. [PMC free article] [PubMed] [Google Scholar]
- 41.Lauridsen AL, Vestergaard P, Hermann AP, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, Sheng H, Li H, et al. Associations between common variants in GC and DHCR7/NADSYN1 and vitamin D concentration in Chinese Hans. Hum Genet. 2012;131:505–12. doi: 10.1007/s00439-011-1099-1. [DOI] [PubMed] [Google Scholar]
- 43.Sinotte M, Diorio C, Berube S, et al. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–40. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 44.Fu L, Yun F, Oczak M, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–7. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Institute SNP500 cancer database. National Cancer Institute. 2010. Available from: http://variantgps.nci.nih.gov/cgfseq/pages/snp500.do. [last accessed 22 Jan 2013]
- 46.Gozdzik A, Zhu J, Wong BY, et al. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127:405–12. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–57. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 48.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 49.Feldman D, Pike JW, Adams JS. Vitamin D. 3rd. Amsterdam: Academic Press; 2011. [Google Scholar]
- 50.Bikle D. “Vitamin D system in mineral homeostasis”. In: Singer F, editor. Disease of bone and mineral metabolism. Endotext.com; 2009. Available from: http://www.endotext.org/parathyroid/index.htm . [Google Scholar]
- 51.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 52.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 53.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 54.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 55.Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binder R, Kress A, Kan G, et al. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–92. doi: 10.1016/s0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 57.Kew RR, Webster RO. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J Clin Invest. 1988;82:364–9. doi: 10.1172/JCI113596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kew RR, Fisher JA, Webster RO. Co-chemotactic effect of Gc-globulin (vitamin D binding protein) for C5a. Transient conversion into an active co-chemotaxin by neutrophils. J Immunol. 1995;155:5369–74. [PubMed] [Google Scholar]
- 59.Piquette CA, Robinson-Hill R, Webster RO. Human monocyte chemotaxis to complement-derived chemotaxins is enhanced by Gc-globulin. J Leukoc Biol. 1994;55:349–54. doi: 10.1002/jlb.55.3.349. [DOI] [PubMed] [Google Scholar]
- 60.Shah AB, DiMartino SJ, Trujillo G, Kew RR. Selective inhibition of the C5a chemotactic cofactor function of the vitamin D binding protein by 1,25(OH)2 vitamin D3. Mol Immunol. 2006;43:1109–15. doi: 10.1016/j.molimm.2005.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto N, Homma S, Millman I. Identification of the serum factor required for in vitro activation of macrophages. Role of vitamin D3-binding protein (group specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J Immunol. 1991;147:273–80. [PubMed] [Google Scholar]
- 62.Yamamoto N, Kumashiro R, Yamamoto M, et al. Regulation of inflammation-primed activation of macrophages by two serum factors, vitamin D3-binding protein and albumin. Infect Immun. 1993;61:5388–91. doi: 10.1128/iai.61.12.5388-5391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahl B, Schiodt FV, Ott P, et al. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med. 2003;31:152–6. doi: 10.1097/00003246-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 64.Haddad JG, Harper KD, Guoth M, et al. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci USA. 1990;87:1381–5. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badawi A, Klip A, Haddad P, et al. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–86. doi: 10.2147/dmsott.s9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pani MA, Donner H, Herwig J, et al. Vitamin D binding protein alleles and susceptibility for type 1 diabetes in Germans. Autoimmunity. 1999;31:67–72. doi: 10.3109/08916939908993861. [DOI] [PubMed] [Google Scholar]
- 67.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–7. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 68.Hirai M, Suzuki S, Hinokio Y, et al. Variations in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance. J Clin Endocrinol Metab. 2000;85:1951–53. doi: 10.1210/jcem.85.5.6569. [DOI] [PubMed] [Google Scholar]
- 69.Szathmary EJ. The effect of Gc genotype on fasting insulin level in Dogrib Indians. Hum Genet. 1987;75:368–72. doi: 10.1007/BF00284110. [DOI] [PubMed] [Google Scholar]
- 70.Baier LJ, Dobberfuhl AM, Pratley RE, et al. Variations in the vitamin D-binding protein (Gc locus) are associated with oral glucose tolerance in nondiabetic Pima Indians. J Clin Endocrinol Metab. 1998;83:2993–96. doi: 10.1210/jcem.83.8.5043. [DOI] [PubMed] [Google Scholar]
- 71.Iyengar S, Hamman RF, Marshall JA, et al. On the role of vitamin D binding globulin in glucose homeostasis: results from the San Luis Valley Diabetes Study. Genet Epidemiol. 1989;6:691–8. doi: 10.1002/gepi.1370060606. [DOI] [PubMed] [Google Scholar]
- 72.Hirai M, Suzuki S, Hinokio Y, et al. Group specific component protein genotype is associated with NIDDM in Japan. Diabetologia. 1998;41:742–3. doi: 10.1007/s001250050980. [DOI] [PubMed] [Google Scholar]
- 73.Braun A, Bichlmaier R, Muller B, Cleve H. Molecular evaluation of an Alu repeat including a polymorphic variable poly(dA) (AluVpA) in the vitamin D binding protein (DBP) gene. Hum Genet. 1993;90:526–32. doi: 10.1007/BF00217453. [DOI] [PubMed] [Google Scholar]
- 74.Klupa T, Malecki M, Hanna L, et al. Amino acid variants of the vitamin D-binding protein and risk of diabetes in white Americans of European origin. Eur J Endocrinol. 1999;141:490–93. doi: 10.1530/eje.0.1410490. [DOI] [PubMed] [Google Scholar]
- 75.Ye WZ, Dubois-Laforgue D, Bellanne-Chantelot C, et al. Variations in the vitamin D-binding protein (Gc locus) and risk of type 2 diabetes mellitus in French Caucasians. Metabolism. 2001;50:366–69. doi: 10.1053/meta.2001.20172. [DOI] [PubMed] [Google Scholar]
- 76.Malecki MT, Klupa T, Wanic K, et al. Vitamin D binding protein gene and genetic susceptibility to type 2 diabetes mellitus in a Polish population. Diabetes Res Clin Pract. 2002;57:99–104. doi: 10.1016/s0168-8227(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 77.Lauridsen AL, Vestergaard P, Hermann AP, et al. Female premenopausal fracture risk is associated with gc phenotype. J Bone Miner Res. 2004;19:875–81. doi: 10.1359/JBMR.040133. [DOI] [PubMed] [Google Scholar]
- 78.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 79.Kuchuk NO, van Schoor NM, Pluijm SM, et al. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24:693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 80.Tang BM, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539–43. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swamy N, Ghosh S, Schneider GB, Ray R. Baculovirus-expressed vitamin D-binding protein-macrophage activating factor (DBP-maf) activates osteoclasts and binding of 25-hydroxyvitamin D(3) does not influence this activity. J Cell Biochem. 2001;81:535–46. doi: 10.1002/1097-4644(20010601)81:3<535::aid-jcb1067>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto N, Naraparaju VR, Orchard PJ. Defective lymphocyte glycosidases in the macrophage activation cascade of juvenile osteopetrosis. Blood. 1996;88:1473–8. [PubMed] [Google Scholar]
- 84.Schneider GB, Benis KA, Flay NW, et al. Effects of vitamin D binding protein-macrophage activating factor (DBP-MAF) infusion on bone resorption in two osteopetrotic mutations. Bone. 1995;16:657–62. doi: 10.1016/8756-3282(95)00118-w. [DOI] [PubMed] [Google Scholar]
- 85.Ezura Y, Nakajima T, Kajita M, et al. Association of molecular variants, haplotypes, and linkage disequilibrium within the human vitamin D-binding protein (DBP) gene with postmenopausal bone mineral density. J Bone Miner Res. 2003;18:1642–49. doi: 10.1359/jbmr.2003.18.9.1642. [DOI] [PubMed] [Google Scholar]
- 86.Papiha SS, Allcroft LC, Kanan RM, et al. Vitamin D binding protein gene in male osteoporosis: association of plasma DBP and bone mineral density with (TAAA)(n)-Alu polymorphism in DBP. Calcif Tissue Int. 1999;65:262–6. doi: 10.1007/s002239900695. [DOI] [PubMed] [Google Scholar]
- 87.Al oanzi ZH, Tuck SP, Mastana SS, et al. Vitamin D-binding protein gene microsatellite polymorphism influences BMD and risk of fractures in men. Osteoporos Int. 2008;19:951–60. doi: 10.1007/s00198-007-0516-8. [DOI] [PubMed] [Google Scholar]
- 88.Fang Y, van Meurs JB, Arp P, et al. Vitamin D binding protein genotype and osteoporosis. Calcif Tissue Int. 2009;85:85–93. doi: 10.1007/s00223-009-9251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schellenberg D, Pare PD, Weir TD, et al. Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med. 1998;157:957–61. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 90.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40:173–6. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 91.Kueppers F, Miller RD, Gordon H, et al. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977;63:336–42. doi: 10.1016/0002-9343(77)90270-4. [DOI] [PubMed] [Google Scholar]
- 92.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–10. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 93.Ito I, Nagai S, Hoshino Y, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest. 2004;125:63–70. doi: 10.1378/chest.125.1.63. [DOI] [PubMed] [Google Scholar]
- 94.Ishii T, Keicho N, Teramoto S, et al. Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J. 2001;18:753–7. doi: 10.1183/09031936.01.00094401. [DOI] [PubMed] [Google Scholar]
- 95.Shen LH, Zhang XM, Su DJ, et al. Association of vitamin D binding protein variants with susceptibility to chronic obstructive pulmonary disease. J Int Med Res. 2010;38:1093–8. doi: 10.1177/147323001003800337. [DOI] [PubMed] [Google Scholar]
- 96.Nagasawa H, Uto Y, Sasaki H, et al. Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activity. Anticancer Res. 2005;25:3689–95. [PubMed] [Google Scholar]
- 97.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 98.Kasuga I, Pare PD, Ruan J, et al. Lack of association of group specific component haplotypes with lung function in smokers. Thorax. 2003;58:790–3. doi: 10.1136/thorax.58.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandford AJ, Chagani T, Weir TD, et al. Susceptibility genes for rapid decline of lung function in the lung health study. Am J Respir Crit Care Med. 2001;163:469–73. doi: 10.1164/ajrccm.163.2.2006158. [DOI] [PubMed] [Google Scholar]
- 100.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 101.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 102.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 103.Hollams EM, Hart PH, Holt BJ, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011;38:1320–7. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
- 104.Pinto JM, Schneider J, Perez R, et al. Serum 25-hydroxyvitamin D levels are lower in urban African American subjects with chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122:415–7. doi: 10.1016/j.jaci.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 105.Freishtat RJ, Iqbal SF, Pillai DK, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156:948–52. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hypponen E, Sovio U, Wjst M, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann NY Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 107.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 108.Camargo CA, Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 109.Pillai DK, Iqbal SF, Benton AS, et al. Associations between genetic variants in vitamin D metabolism and asthma characteristics in Young African Americans: a pilot study. J Investig Med. 2011;59:938–46. doi: 10.231/JIM.0b013e318220df41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bosse Y, Lemire M, Poon AH, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krstic G. Asthma prevalence associated with geographical latitude and regional insolation in the United States of America and Australia. PLoS One. 2011;6:e18492. doi: 10.1371/journal.pone.0018492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hughes AM, Lucas RM, Ponsonby AL, et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter study. Pediatr Allergy Immunol. 2011;22:327–33. doi: 10.1111/j.1399-3038.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- 113.Annesi-Maesano I. Perinatal events, vitamin D, and the development of allergy. Pediatr Res. 2002;52:3–5. doi: 10.1203/00006450-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 114.Gorman S, Judge MA, Burchell JT, et al. 1,25-Dihydroxyvitamin D3 enhances the ability of transferred CD4+ CD25+ cells to modulate T helper type 2-driven asthmatic responses. Immunology. 2010;130:181–92. doi: 10.1111/j.1365-2567.2009.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pichler J, Gerstmayr M, Szepfalusi Z, et al. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 116.Li F, Jiang L, Willis-Owen SA, et al. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC Med Genet. 2011;12:103. doi: 10.1186/1471-2350-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wjst M, Altmuller J, Faus-Kessler T, et al. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pola R. Inflammatory markers for ischaemic stroke. Thromb Haemost. 2009;101:800–1. [PubMed] [Google Scholar]
- 119.Wang X, Cheng S, Brophy VH, et al. A meta-analysis of candidate gene polymorphisms and ischemic stroke in 6 study populations: association of lymphotoxin-alpha in nonhypertensive patients. Stroke. 2009;40:683–95. doi: 10.1161/STROKEAHA.108.524587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pilz S, Dobnig H, Fischer JE, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–3. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 121.Lobo R. Endometriosis: etiology, pathology, diagnosis, management. In: Katz Vea , editor. Comprehensive gynecology. 5th. Philadelphia: Mosby Elsevier; 2011. pp. 473–500. [Google Scholar]
- 122.Wellbery C. Diagnosis and treatment of endometriosis. Am Fam Physician. 1999;60:1753–8. [PubMed] [Google Scholar]
- 123.Akoum A, Kong J, Metz C, Beaumont MC. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. 2002;77:989–94. doi: 10.1016/s0015-0282(02)03082-0. [DOI] [PubMed] [Google Scholar]
- 124.Faserl K, Golderer G, Kremser L, et al. Polymorphism in vitamin D-binding protein as a genetic risk factor in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2011;96:E233–41. doi: 10.1210/jc.2010-1532. [DOI] [PubMed] [Google Scholar]
- 125.Eloranta JJ, Wenger C, Mwinyi J, et al. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics. 2011;21:559–64. doi: 10.1097/FPC.0b013e328348f70c. [DOI] [PubMed] [Google Scholar]
- 126.Mohr SB, Garland CF, Gorham ED, et al. Is ultraviolet B irradiance inversely associated with incidence rates of endometrial cancer: an ecological study of 107 countries. Prev Med. 2007;45:327–31. doi: 10.1016/j.ypmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 127.Mohr SB, Garland CF, Gorham ED, et al. Could ultraviolet B irradiance and vitamin D be associated with lower incidence rates of lung cancer? J Epidemiol Community Health. 2008;62:69–74. doi: 10.1136/jech.2006.052571. [DOI] [PubMed] [Google Scholar]
- 128.Porojnicu AC, Robsahm TE, Dahlback A, et al. Seasonal and geographical variations in lung cancer prognosis in Norway. Does vitamin D from the sun play a role? Lung Cancer. 2007;55:263–70. doi: 10.1016/j.lungcan.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Gorham ED, Garland FC, Garland CF. Sunlight and breast cancer incidence in the USSR. Int J Epidemiol. 1990;19:820–4. doi: 10.1093/ije/19.4.820. [DOI] [PubMed] [Google Scholar]
- 130.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 131.Lowe LC, Guy M, Mansi JL, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 132.Reinhold U, Schmitz B, Kurbacher C, et al. Circulating 25-hydroxyvitamin D concentration in German cancer patients. Oncol Rep. 2008;20:1539–43. [PubMed] [Google Scholar]
- 133.Agborsangaya CB, Surcel HM, Toriola AT, et al. Serum 25-hydroxyvitamin D at pregnancy and risk of breast cancer in a prospective study. Eur J Cancer. 2010;46:467–70. doi: 10.1016/j.ejca.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 134.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 135.Colli JL, Colli A. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urol Oncol. 2006;24:184–94. doi: 10.1016/j.urolonc.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 136.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colli JL, Grant WB. Solar ultraviolet B radiation compared with prostate cancer incidence and mortality rates in United States. Urology. 2008;71:531–5. doi: 10.1016/j.urology.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 138.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23:1133–6. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 139.Garland CF, Mohr SB, Gorham ED, et al. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med. 2006;31:512–4. doi: 10.1016/j.amepre.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 140.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and serum vitamin D metabolite levels 10–17 years prior to diagnosis. Am J Epidemiol. 1995;142:608–11. doi: 10.1093/oxfordjournals.aje.a117682. [DOI] [PubMed] [Google Scholar]
- 141.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–8. [PubMed] [Google Scholar]
- 142.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 143.Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 144.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 145.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 146.Otani T, Iwasaki M, Sasazuki S, et al. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97:446–51. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–9. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 148.Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–25. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 149.Tangrea J, Helzlsouer K, Pietinen P, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8:615–25. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]
- 150.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–24. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 151.Yin L, Grandi N, Raum E, et al. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 152.Kisker O, Onizuka S, Becker CM, et al. Vitamin D binding protein-macrophage activating factor (DBP-maf) inhibits angiogenesis and tumor growth in mice. Neoplasia. 2003;5:32–40. doi: 10.1016/s1476-5586(03)80015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamamoto N, Suyama H, Yamamoto N, Ushijima N. Immunotherapy of metastatic breast cancer patients with vitamin D-binding protein-derived macrophage activating factor (GcMAF) Int J Cancer. 2008;122:461–7. doi: 10.1002/ijc.23107. [DOI] [PubMed] [Google Scholar]
- 154.Pacini S, Punzi T, Morucci G, et al. Effects of vitamin D-binding protein-derived macrophage-activating factor on human breast cancer cells. Anticancer Res. 2012;32:45–52. [PubMed] [Google Scholar]
- 155.Welsh J, Wietzke JA, Zinser GM, et al. Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr. 2003;133:2425S–33S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 156.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80:1721S–4S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 157.Lowe L, Hansen CM, Senaratne S, Colston KW. Mechanisms implicated in the growth regulatory effects of vitamin D compounds in breast cancer cells. Recent Results Cancer Res. 2003;164:99–110. doi: 10.1007/978-3-642-55580-0_6. [DOI] [PubMed] [Google Scholar]
- 158.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2002;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 159.McCullough ML, Stevens VL, Diver WR, et al. Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2007;9:R9. doi: 10.1186/bcr1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abbas S, Linseisen J, Slanger T, et al. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomarkers Prev. 2008;17:1339–43. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 161.Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20:1708–17. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 162.Peehl DM, Skowronski RJ, Leung GK, et al. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–10. [PubMed] [Google Scholar]
- 163.Oades GM, Dredge K, Kirby RS, Colston KW. Vitamin D receptor-dependent antitumour effects of 1,25-dihydroxyvitamin D3 and two synthetic analogues in three in vivo models of prostate cancer. BJU Int. 2002;90:607–16. doi: 10.1046/j.1464-410x.2002.02964.x. [DOI] [PubMed] [Google Scholar]
- 164.Gregory KJ, Zhao B, Bielenberg DR, et al. Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells. PLoS One. 2010;5:e13428. doi: 10.1371/journal.pone.0013428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dimopoulos MA, Germenis A, Savides P, et al. Genetic markers in carcinoma of the prostate. Eur Urol. 1984;10:315–16. doi: 10.1159/000463818. [DOI] [PubMed] [Google Scholar]
- 166.Ahn J, Albanes D, Berndt SI, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis. 2009;30:769–76. doi: 10.1093/carcin/bgp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Corder EH, Friedman GD, Vogelman JH, Orentreich N. Seasonal variation in vitamin D, vitamin D-binding protein, and dehydroepiandrosterone: risk of prostate cancer in black and white men. Cancer Epidemiol Biomarkers Prev. 1995;4:655–9. [PubMed] [Google Scholar]
- 168.Poynter JN, Jacobs ET, Figueiredo JC, et al. Genetic variation in the vitamin D receptor (VDR) and the vitamin D-binding protein (GC) and risk for colorectal cancer: results from the Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2010;19:525–36. doi: 10.1158/1055-9965.EPI-09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hibler EA, Hu C, Jurutka PW, et al. Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2012;21:368–75. doi: 10.1158/1055-9965.EPI-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou L, Zhang X, Chen X, et al. GC Glu416Asp and Thr420Lys polymorphisms contribute to gastrointestinal cancer susceptibility in a Chinese population. Int J Clin Exp Med. 2012;5:72–9. [PMC free article] [PubMed] [Google Scholar]
- 171.Flohil SC, De Vries E, Van Meurs JBJ, et al. Vitamin D-binding protein polymorphisms are not associated with development of (multiple) basal cell carcinomas. Exp Dermat. 2010;19:1103–5. doi: 10.1111/j.1600-0625.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 172.Niino M, Fukazawa T, Kikuchi S, Sasaki H. Therapeutic potential of vitamin D for multiple sclerosis. Curr Med Chem. 2008;15:499–505. doi: 10.2174/092986708783503159. [DOI] [PubMed] [Google Scholar]
- 173.Niino M, Kikuchi S, Fukazawa T, et al. No association of vitamin D-binding protein gene polymorphisms in Japanese patients with MS. J Neuroimmunol. 2002;127:177–9. doi: 10.1016/s0165-5728(02)00099-1. [DOI] [PubMed] [Google Scholar]
- 174.Steckley JL, Dyment DA, Sadovnick AD, et al. Genetic analysis of vitamin D related genes in Canadian multiple sclerosis patients. Canadian Collaborative Study Group. Neurology. 2000;54:729–32. doi: 10.1212/wnl.54.3.729. [DOI] [PubMed] [Google Scholar]
- 175.Rithidech KN, Honikel L, Milazzo M, et al. Protein expression profiles in pediatric multiple sclerosis: potential biomarkers. Mult Scler. 2009;15:455–64. doi: 10.1177/1352458508100047. [DOI] [PubMed] [Google Scholar]
- 176.Hollsberg P, Haahr S, Larsen PM, Fey SJ. MS and the group-specific component. Acta Neurol Scand. 1988;78:158–60. doi: 10.1111/j.1600-0404.1988.tb03638.x. [DOI] [PubMed] [Google Scholar]
- 177.Lindblom B, Wetterling G, Link H. Distribution of group-specific component subtypes in multiple sclerosis. Acta Neurol Scand. 1988;78:443–4. doi: 10.1111/j.1600-0404.1988.tb03683.x. [DOI] [PubMed] [Google Scholar]
- 178.Simon KC, Munger KL, Xing Y, Ascherio A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16:133–8. doi: 10.1177/1352458509355069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Orton SM, Ramagopalan SV, Para AE, et al. Vitamin D metabolic pathway genes and risk of multiple sclerosis in Canadians. J Neurol Sci. 2011;305:116–20. doi: 10.1016/j.jns.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 180.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 181.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 182.Palma AS, De Carvalho M, Grammel N, et al. Proteomic analysis of plasma from Portuguese patients with familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:339–49. doi: 10.1080/17482960801934239. [DOI] [PubMed] [Google Scholar]
- 183.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40:173–7. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 184.Lange V. Genetic markers for schizophrenic subgroups. Psychiatr Clin (Basel) 1982;15:133–44. doi: 10.1159/000283933. [DOI] [PubMed] [Google Scholar]
- 185.Papiha SS, Roberts DF, McLeish L. Group-specific component (Gc) subtypes and schizophrenia. Clin Genet. 1982;22:321–6. doi: 10.1111/j.1399-0004.1982.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 186.Saha N, Tsoi WF. Serum protein markers in Chinese schizophrenics–haptoglobin types and transferrin and group-specific component subtypes. Clin Genet. 1990;37:54–8. doi: 10.1111/j.1399-0004.1990.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 187.Beckman L, Beckman G, Perris C. Gc serum groups and schizophrenia. Clin Genet. 1980;17:149–52. doi: 10.1111/j.1399-0004.1980.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 188.Rudduck C, Franzen G, Hansson A, Rorsman B. Gc serum groups in schizophrenia. Hum Hered. 1985;35:11–4. doi: 10.1159/000153507. [DOI] [PubMed] [Google Scholar]
- 189.Fananas L, Moral P, Marti Tusquets JL, Bertranpetit J. Genetic markers in schizophrenia: ACP1, ESD, TF and GC polymorphisms. Hum Hered. 1990;40:136–40. doi: 10.1159/000153920. [DOI] [PubMed] [Google Scholar]
- 190.Sato Y, Kikuyama M, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in Parkinson's disease. Neurology. 1997;49:1273–8. doi: 10.1212/wnl.49.5.1273. [DOI] [PubMed] [Google Scholar]
- 191.Suzuki M, Yoshioka M, Hashimoto M, et al. 25-Hydroxyvitamin D, vitamin D receptor gene polymorphisms, and severity of Parkinson's disease. Mov Disord. 2012;27:264–71. doi: 10.1002/mds.24016. [DOI] [PubMed] [Google Scholar]
- 192.Milman N, Thymann M, Graudal N, Morling N. Plasma vitamin D-binding protein (GC) factors, immunoglobulin G heavy chain (GM) allotypes and immunoglobulin kappa light chain (KM1) allotype in patients with sarcoidosis and in healthy control subjects. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:97–100. [PubMed] [Google Scholar]
- 193.Tokuda N, Mano T, Levy RB. 1,25-Dihydroxyvitamin D3 antagonizes interferon-gamma-induced expression of class II major histocompatibility antigens on thyroid follicular and testicular Leydig cells. Endocrinology. 1990;127:1419–27. doi: 10.1210/endo-127-3-1419. [DOI] [PubMed] [Google Scholar]
- 194.Thomasset M. [Vitamin D and the immune system] Pathol Biol (Paris) 1994;42:163–72. [PubMed] [Google Scholar]
- 195.Pani MA, Regulla K, Segni M, et al. A polymorphism within the vitamin D-binding protein gene is associated with Graves' disease but not with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2002;87:2564–7. doi: 10.1210/jcem.87.6.8562. [DOI] [PubMed] [Google Scholar]
- 196.Kurylowicz A, Ramos-Lopez E, Bednarczuk T, Badenhoop K. Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves' disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes. 2006;114:329–35. doi: 10.1055/s-2006-924256. [DOI] [PubMed] [Google Scholar]
- 197.Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–6. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 198.Davies PD. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985;66:301–6. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- 199.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187–90. doi: 10.1136/thx.40.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grange JM, Davies PD, Brown RC, et al. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985;66:187–91. doi: 10.1016/0041-3879(85)90035-2. [DOI] [PubMed] [Google Scholar]
- 201.Chan TY, Poon P, Pang J, et al. A study of calcium and vitamin D metabolism in Chinese patients with pulmonary tuberculosis. J Trop Med Hyg. 1994;97:26–30. [PubMed] [Google Scholar]
- 202.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–8. [PubMed] [Google Scholar]
- 203.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007;62:1003–7. doi: 10.1136/thx.2006.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46:443–6. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 205.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 206.Martineau AR, Leandro AC, Anderson ST, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35:1106–12. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Petrini M, Emerson DL, Galbraith RM. Linkage between surface immunoglobulin and cytoskeleton of B lymphocytes may involve Gc protein. Nature. 1983;306:73–4. doi: 10.1038/306073a0. [DOI] [PubMed] [Google Scholar]
- 208.Bahr GM, Eales LJ, Nye KE, et al. An association between Gc (vitamin D-binding protein) alleles and susceptibility to rheumatic fever. Immunology. 1989;67:126–8. [PMC free article] [PubMed] [Google Scholar]
- 209.Carter CA, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–43. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- 210.Kuroda MJ. Macrophages: do they impact AIDS progression more than CD4 T cells? J Leukoc Biol. 2010;87:569–73. doi: 10.1189/jlb.0909626. [DOI] [PubMed] [Google Scholar]
- 211.Yamamoto N, Ushijima N, Koga Y. Immunotherapy of HIV-infected patients with Gc protein-derived macrophage activating factor (GcMAF) J Med Virol. 2009;81:16–26. doi: 10.1002/jmv.21376. [DOI] [PubMed] [Google Scholar]
- 212.Pronk JC, Frants RR, Crusius B, et al. No predictive value of GC phenotypes for HIV infection and progression to AIDS. Hum Genet. 1988;80:181–2. doi: 10.1007/BF00702864. [DOI] [PubMed] [Google Scholar]
- 213.Goudsmit J, de WF, Paul DA, et al. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986;2:177–80. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- 214.Lange JM, Paul DA, Huisman HG, et al. Persistent HIV antigenaemia and decline of HIV core antibodies associated with transition to AIDS. Br Med J (Clin Res Ed) 1986;293:1459–62. doi: 10.1136/bmj.293.6560.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.de WF, Goudsmit J, Paul DA, et al. Risk of AIDS related complex and AIDS in homosexual men with persistent HIV antigenaemia. Br Med J (Clin Res Ed) 1987;295:569–72. doi: 10.1136/bmj.295.6598.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alonso A, Montesino M, Iturralde MJ, et al. GC subtyping and HIV infection in a Spanish population: no evidence of an association between GC subtypes and AIDS. Hum Hered. 1990;40:34–7. doi: 10.1159/000153900. [DOI] [PubMed] [Google Scholar]
- 217.Cleve H, Weidinger S, Gurtler LG, Deinhardt F. AIDS: no association with the genetic systems GC (D-binding protein), ORM (orosomucoid = alpha-1-acid glycoprotein), and A2HS (alpha-2-HS-glycoprotein) Infection. 1988;16:31–5. doi: 10.1007/BF01646929. [DOI] [PubMed] [Google Scholar]
- 218.Putkonen P, Albert J, Karlsson A, et al. Group specific component and susceptibility to HIV infection and progression to AIDS. Scand J Infect Dis. 1988;20:11–4. doi: 10.3109/00365548809117211. [DOI] [PubMed] [Google Scholar]
- 219.Eales LJ, Nye KE, Parkin JM, et al. Association of different allelic forms of group specific component with susceptibility to and clinical manifestation of human immunodeficiency virus infection. Lancet. 1987;1:999–1002. doi: 10.1016/s0140-6736(87)92269-0. [DOI] [PubMed] [Google Scholar]
- 220.Nagasawa H, Sasaki H, Uto Y, et al. Association of the macrophage activating factor (MAF) precursor activity with polymorphism in vitamin D-binding protein. Anticancer Res. 2004;24:3361–66. [PubMed] [Google Scholar]
- 221.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 222.Dong X, Lutz W, Schroeder TM, et al. Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci USA. 2005;102:16007–12. doi: 10.1073/pnas.0506516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bondy B. Genetics in psychiatry: are the promises met? World J Biol Psychiatry. 2011;12:81–8. doi: 10.3109/15622975.2010.546428. [DOI] [PubMed] [Google Scholar]
- 224.Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin d genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60:1624–31. doi: 10.2337/db10-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hodge SE, Anderson CE, Neiswanger K, et al. Association studies between Type 1 (insulin-dependent) diabetes and 27 genetic markers: lack of association between Type 1 diabetes and Kidd blood group. Diabetologia. 1983;25:343–7. doi: 10.1007/BF00253199. [DOI] [PubMed] [Google Scholar]
- 226.Eichner JE, Cauley JA, Ferrell RE, et al. Genetic variation in two bone-related proteins: is there an association with bone mineral density or skeletal size in postmenopausal women? Genet Epidemiol. 1992;9:177–84. doi: 10.1002/gepi.1370090304. [DOI] [PubMed] [Google Scholar]
- 227.Rosberger DF, Werner PA, Steinman R, et al. Group specific component (Gc) and HIV diseases. Dis Markers. 1988;6:269–74. [PubMed] [Google Scholar]