Summary
Background and objective
Among general populations, a healthy lifestyle has been associated with lower risk of death. This study evaluated this association in individuals with CKD.
Design, setting, participants, & measurements
A total of 2288 participants with CKD (estimated GFR < 60 ml/min per 1.73 m2 or microalbuminuria) in the Third National Health and Nutrition Examination Survey were included. A weighted healthy lifestyle score was calculated (range, −4 to 15, with 15 indicating healthiest lifestyle) on the basis of the multivariable Cox proportional hazards model regression coefficients of the following lifestyle factors: smoking habit, body mass index (BMI), physical activity, and diet. Main outcome was all-cause mortality, ascertained through December 31, 2006.
Results
After median follow-up of 13 years, 1319 participants had died. Compared with individuals in the lowest quartile of weighted healthy lifestyle score, adjusted hazard ratios (95% confidence intervals) of all-cause mortality were 0.53 (0.41–0.68), 0.52 (0.42–0.63), and 0.47 (0.38–0.60) for individuals in the second, third, and fourth quartiles, respectively. Mortality increased 30% among individuals with a BMI of 18.5 to <22 kg/m2 versus 22 to <25 kg/m2 (P<0.05); decreased mortality was associated with never-smoking versus current smoking (0.54 [0.41–0.70]) and regular versus no physical activity (0.80 [0.65–0.99]). Diet was not significantly associated with mortality.
Conclusions
Compared with nonadherence, adherence to a healthy lifestyle was associated with lower all-cause mortality risk in CKD. Examination of individual components of the healthy lifestyle score, with adjustment for other components, suggested that the greatest reduction in all-cause mortality was related to nonsmoking.
Introduction
More than 26 million individuals in the United States are estimated to have CKD (1). In the general population, it is well established that adherence to a healthy lifestyle is associated with lower risk of adverse outcomes (2–7). Exploring the association of lifestyle with outcomes in CKD is important because this population is at higher risk for cardiovascular events and death (8–11). Furthermore, there is evidence of reverse epidemiology for certain risk factors, such as body mass index (BMI), in individuals with CKD (12,13).
Although current guidelines for CKD management recommend dietary and lifestyle modifications, these have largely been based on general population studies (14). Few studies have evaluated select lifestyle factors in CKD populations and have reported adverse effects of smoking and physical inactivity (15,16). However, the benefits of adherence to multiple components of a healthy lifestyle have not been systematically evaluated in individuals with CKD. For this reason, we conducted a study to assess the association of four lifestyle factors (diet, physical activity, BMI, and smoking) with all-cause mortality among participants with CKD in the Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File.
Materials and Methods
Study Population and Baseline Data
NHANES III was a cross-sectional, stratified, clustered, multistage probability sample survey of the civilian, noninstitutionalized population in the United States, conducted by the National Center for Health Statistics (NCHS) between 1988 and 1994. The survey protocol was approved by the NCHS institutional review board. All participants provided informed consent. Participants underwent a home interview followed by an extensive physical examination and blood and urine sampling at a mobile examination center (17).
Self-reported information on sociodemographic characteristics and presence of medical conditions was collected during the home interview. Blood and urine samples and BP were obtained during the physical examination. Hypertension was defined as a BP >140 mmHg/>90 mmHg or the use of antihypertensive medications. Diabetes was defined as a history of diabetes, use of insulin or other medication to treat diabetes, a fasting blood glucose level ≥126 mg/dl, or a random blood glucose level ≥200 mg/dl. CKD was defined by an estimated GFR (eGFR) < 60 ml/min per 1.73 m2, calculated using the CKD Epidemiology Collaboration equation for creatinine (developed from the pooling of several cohorts with GFR measured by iothalamate [18 ]), or the presence of microalbuminuria (urine albumin-to-creatinine ratio [UACR] ≥ 30 mg/g). We used the formula for correction of serum creatinine recommended in the NHANES III Data File Documentation (19).
Exposure Ascertainment: Healthy Lifestyle Factors
Four different lifestyle factors were considered (BMI, diet, physical activity, and cigarette smoking) on the basis of their association with mortality in the general population and current recommendations for cardiovascular health promotion (14,20). BMI was calculated as weight in kilograms divided by height in meters squared. Participants were asked about the frequency of leisure time physical activity (e.g., walking a mile without stopping, running or jogging, riding a bicycle, swimming). The level of physical activity was ascertained using metabolic equivalent intensity levels (moderate exercise ranged from 3 to 5 metabolic equivalents and vigorous exercise, >6 metabolic equivalents) based on the Compendium of Physical Activities (21). The frequency of each physical activity was reported in times per month, which was divided by 4.3 to obtain the number of times per week. Participants were classified as current, past, or never smoker according to responses to the questions “Have you smoked at least 100 cigarettes during your entire life?” and “Do you smoke cigarettes now?” Dietary data were collected using a 24-hour recall. Diet quality was assessed using the 1995 Healthy Eating Index (HEI) (22), which provides a measure of the overall quality of the diet on the basis of the extent to which Food Guide Pyramid recommendations on 10 dietary components (intake of grains, vegetables, fruits, milk, meat, total fat, saturated fat, cholesterol, sodium, and dietary variety) are met. The HEI score ranges from 0 to 100, with higher scores indicating a healthier eating pattern.
Weighted Healthy Lifestyle Score
A weighted healthy lifestyle score was computed by allocating points for each category of the four healthy lifestyle factors based on multivariable Cox proportional hazards regression coefficients (23). We derived the total number of points for each participant by adding the points assigned to his or her healthy lifestyle factors. Scores ranged from −4 to 15, with a higher number indicating a healthier lifestyle (Table 1). Healthy lifestyle factors were defined a priori using an approach similar to that used in general populations studies (3–5,24): (1) BMI 22 to <25 versus 18.5 <22 kg/m2, 25 to <30, or ≥30 kg/m2; (2) physically inactive (no reported leisure time physical activity) versus insufficient physical activity (not inactive but not meeting criteria for recommended physical activity) or recommended physical activity, defined as moderate self-reported leisure-time physical activity at least five times per week, vigorous physical activity at least three times per week, or a combination of the two ([weekly frequency of moderate activity/5] + [weekly frequency of vigorous activity/3] ≥ 1) (16); (3) current smoker versus past or never smoker; and (4) HEI score <54.4 versus 54.5 to <63.8, 63.8 to <73.1, or 73.1–100 (22).
Table 1.
Point-based system to calculate weighted healthy lifestyle score
| Characteristic | Points Based on Regression Coefficients |
|---|---|
| BMI | |
| 18.5 to <22 kg/m2 | −4 |
| 22 to <25 kg/m2 | 0 |
| 25 to <30 kg/m2 | 0 |
| ≥30 kg/m2 | 2 |
| Physical activity | |
| Inactive | 0 |
| Insufficient | 2 |
| Recommended | 3 |
| Smoking | |
| Current | 0 |
| Past | 7 |
| Never | 9 |
| Healthy Eating Index score | |
| <54.4 | 0 |
| 54.5 to <63.7 | 0 |
| 63.8 to <73.1 | 0 |
| 73.1–100 | 1 |
Outcome Ascertainment
Vital status was determined using the NHANES III Linked Mortality Public-use File, which provides follow-up data on vital status in person-months from the date of the NHANES III survey participation through the date of death or December 31, 2006. Mortality was ascertained by the NCHS through a probabilistic match between NHANES III participants and National Death Index death certificate records. Participants who were not matched with any death records were considered to be alive through the follow-up period (25).
Statistical Analyses
NCHS recommendations were followed to account for stratification and clustering of the survey design, as well as oversampling of ethnic minorities and elderly persons (26). Continuous variables were expressed as means ± SEM or medians (interquartile range) if not normally distributed, and categorical variables as weighted percentage. Cox proportional hazards models were used to determine the association between a healthy lifestyle and survival among individuals with CKD, adjusting for important covariates. No censoring variables were used. Because of missing values in at least one of the covariates included in the model, 143 participants were excluded from multivariable analyses (income was missing in 54 participants, education in 12, diabetes in 51, cardiovascular disease in 25, and cholesterol in 6). Sensitivity analyses were performed by conducting stratified survival analyses by eGFR and UACR, by using sex-specific cut-points for UACR (≥17 µg/mg in men and ≥25 µg/mg in women) (27), and by using indicator variables for missing covariates in the fully adjusted models. All tests were two sided, and P<0.05 was considered to represent significant differences for hypothesis testing. The proportional hazards assumption of the Cox models was examined using Schoenfeld residuals, which showed no significant departure from proportionality over time (P>0.05) (28). We used SAS software, version 9.2 (SAS Institute, Inc., Cary, NC), for descriptive statistical analyses and SAS-Callable SUDAAN, version 10.0.1 (RTI International, Research Triangle Park, NC), for survival analyses.
Results
Participant Characteristics
Of the 16,973 NHANES III participants who were examined (men and nonpregnant women ≥18 years of age), we sequentially excluded 1472 individuals with incomplete data on serum creatinine or UACR, 29 participants with eGFR < 15 ml/min per 1.73 m2, 12,992 individuals who did not meet criteria for CKD, 123 participants with incomplete data on dietary variables or BMI, and 69 individuals with BMI < 18.5 kg/m2. The final sample included in this analysis consisted of 2288 men and women aged ≥18 years with CKD. Compared with individuals included in the final cohort, participants who were excluded because of missing serum creatinine or UACR were more likely to be younger (44.5±1.2 versus 59.0±0.9 years [mean ± SEM]; P<0.01) and non-Hispanic black (18.5% versus 11.9%; P=0.003) and to have lower BMI (25.8±0.3 versus 28.0±0.2 kg/m2 [mean ± SEM]; P<0.01) and lower systolic BP (122.7±1.0 versus 136.9±0.9 mmHg; P<0.01). The sex distribution was similar between the two groups (percentage of men, 45% versus 40%; P=0.2); there were also no significant differences in income or education. Compared with participants included in the analysis, the age-adjusted all-cause mortality rate was slightly higher for those excluded (48.7 versus 46.2 per 1000 person-years; P<0.01).
The mean age at the time of the interview was 59 years, 60% of participants were women, 77% were non-Hispanic white, 12% were non-Hispanic black, 4% were Mexican-American, and 7% had other racial/ethnic background. In this sample of NHANES III participants with CKD, 7.8% of individuals adhered to no healthy lifestyle factors, and 29%, 37%, 23%, and 4.2% were found to adhere to one, two, three, and four healthy lifestyle factors, respectively. In general, compared with individuals in the lowest quartile of the weighted healthy lifestyle score, those in the highest quartile were more likely to be older, women, and high-school graduates (Table 2). The overall mean eGFR was 78 ml/min per 1.73 m2. All individuals with eGFR ≥60 ml/min per 1.73 m2 had a UACR ≥ 30 mg/g. Individuals in the lowest quartile had the highest mean eGFR of all four groups (88 ml/min per 1.73 m2). However, they had the highest prevalence of micro- and macroalbuminuria combined (87%) compared with all other groups (65%–72%). Participants in the highest quartile of the weighted healthy lifestyle score had the highest BMI, weekly physical activity frequency, and healthy eating index; 82% of individuals in the highest quartile were never smokers compared with 4.7% in the first quartile (Table 2).
Table 2.
Characteristics of Participants in the Third National Health and Nutrition Examination Survey with CKD by Healthy Lifestyle Score
| Demographic and Clinical Variables | Weighted Healthy Lifestyle Score Quartiles | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Points | −4 to 6 | 7–9 | 10–11 | 12–15 |
| Mean age (yr) | 51.8±1.1 | 59.3±1.9 | 63.5±1.4 | 61.4±1.4 |
| Sex (%) | ||||
| Male | 46.6 | 39.0 | 43.6 | 32.4 |
| Female | 53.4 | 61.0 | 56.4 | 67.6 |
| Race/ethnicity (%) | ||||
| Non-Hispanic white | 73.4 | 78.4 | 80.5 | 75.5 |
| Non-Hispanic black | 14.0 | 11.7 | 10.2 | 11.4 |
| Mexican-American | 3.2 | 3.9 | 3.5 | 4.8 |
| Other | 9.4 | 6.0 | 5.8 | 8.1 |
| Annual household income ≥$20,000 (%) | 47.8 | 53.0 | 56.0 | 57.4 |
| Graduated from high school (%) | 61.5 | 60.0 | 59.6 | 66.4 |
| Mean CKD-EPI eGFR (ml/min per 1.73 m2) | 88.4±1.7 | 78.3±2.4 | 71.9±2.1 | 74.4±1.7 |
| Urine albumin-to-creatinine ratio (%) | ||||
| <30 mg/g | 13.3 | 28.1 | 34.7 | 34.1 |
| 30 to <300 mg/g | 75.8 | 62.3 | 58.5 | 55.2 |
| ≥300 mg/g | 10.9 | 9.5 | 6.8 | 10.7 |
| CKD KDOQI stage (%) | ||||
| 1 | 51.6 | 37.9 | 24.2 | 30.7 |
| 2 | 24.4 | 19.3 | 28.5 | 23.9 |
| 3 | 23.3 | 40.1 | 44.7 | 43.7 |
| 4 | 0.8 | 2.6 | 2.5 | 1.7 |
| Mean body mass index (kg/m2) | 26.3±0.5 | 24.7±0.3 | 29.1±0.4 | 30.9±0.4 |
| Median physical activity, times per week | ||||
| Moderate | 0.9 (0–6.0) | 0.7 (0–2.5) | 1.8 (0.4–6.7) | 5.5 (1.3–8.3) |
| Vigorous | 1.8 (0.7–2.7) | 0.6 (0.4–1.6) | 1.6 (0.3–3.2) | 2.2 (0.8–4.4) |
| Smoking (%) | ||||
| Never | 4.7 | 46.6 | 38.8 | 82.1 |
| Past | 12.2 | 53.4 | 61.2 | 17.9 |
| Current | 83.0 | 0 | 0 | 0 |
| Mean Healthy Eating Index score | 59.9±0.7 | 62.5±0.8 | 66.5±0.8 | 70.8±0.6 |
| Healthy Eating Index score > 64 (%) | 35.8 | 43.7 | 59.8 | 71.0 |
| Mean systolic BP (mmHg) | 132±1.5 | 135±1.4 | 140±1.4 | 140±1.5 |
| Mean diastolic BP (mmHg) | 76±0.8 | 74±0.8 | 77±0.8 | 77±0.6 |
| Cardiovascular disease (%)a | 11.6 | 19.5 | 22.1 | 12.9 |
| Diabetes mellitus (%) | 17.8 | 19.5 | 25.9 | 28.8 |
| Cancer (%) | 5.2 | 11.7 | 7.8 | 6.9 |
| Family history of premature CHD (%)b | 10.7 | 12.5 | 7.3 | 13.0 |
| Ideal waist circumference (%)c | 44.6 | 38.3 | 64.7 | 76.3 |
| Cholesterol level < 200 mg/dl (%) | 58.1 | 60.8 | 67.5 | 72.4 |
| Statin use (%) | 2.6 | 1.4 | 3.0 | 4.5 |
| ACE inhibitor/ARB use (%) | 6.2 | 12.4 | 15.4 | 11.3 |
Percentages are weighted. Means are expressed with SEM. Medians are expressed with interquartile range. CKD-EPI, CKD Epidemiology Collaboration; eGFR, estimated GFR; KDOQI, Kidney Disease Outcome Quality Initiative; CHD, coronary heart disease; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker.
Includes history of myocardial infarction, heart failure, or stroke.
First-degree relative with history of myocardial infarction at age <50 years.
Waist circumference <88 cm in women and <102 cm in men.
Healthy Lifestyle Factors by eGFR
Compared with individuals with eGFR ≥90 ml/min per 1.73 m2, individuals with eGFR of 15 to <60 had a higher median weighted healthy lifestyle score (10 versus 8.8); this was consistent across age groups (Table 3). Compared with individuals in the highest eGFR strata, those in the lowest eGFR strata were more likely to adhere to the recommended level of physical activity (42% versus 34%) and less likely to be current smokers (11% versus 34%).
Table 3.
Distribution of healthy lifestyle factors by estimated GFR among participants in Third National Health and Nutrition Examination Survey with CKD
| Variable | Data per CKD-EPI–Estimated GFR | ||
|---|---|---|---|
| ≥90 ml/min per 1.73 m2 | 60 to <90 ml/min per 1.73 m2 | 15to - <60 ml/min per 1.73 m2 | |
| Weighted percentage | 35.9 | 24.3 | 39.7 |
| Median weighted healthy lifestyle score (range, −4 to 15) | |||
| All ages | 8.1 (2.7–10.9) | 9.8 (4.9–11.2) | 10.0 (8.0–11.4) |
| 18–57 yr | 7.6 (2.6–10.8) | 10.3 (3.7–11.7) | 9.3 (2.7–11.3) |
| 58–74 yr | 8.8 (3.9–11.1) | 9.6 (4.6–11.0) | 10.0 (8.1–11.3) |
| >74 yr | 6.8 (2.4–10.8) | 9.1 (6.7–11.0) | 10.1 (8.1–11.4) |
| Mean body mass index (kg/m2) | 28.0±0.4 | 28.6±0.5 | 27.5±0.2 |
| Recommended physical activity (%) | 34.1 | 33.8 | 42.1 |
| Mean Healthy Eating Index score | 61.4±0.6 | 66.1±0.8 | 67.9±0.6 |
| Current smoker (%) | 33.6 | 21.9 | 11.2 |
Percentages are weighted. Means are expressed with SEM. Medians are expressed with interquartile range. CKD-EPI, CKD Epidemiology Collaboration; eGFR, estimated GFR.
Association of Healthy Lifestyle with All-Cause Mortality
During a median follow-up of 13 years, 1319 patients died. Compared with individuals in the lowest quartile of the weighted healthy lifestyle score, the adjusted hazard ratio of all-cause mortality were 0.53 (95% confidence interval [CI], 0.41–0.68), 0.52 (95% CI, 0.42–0.63), and 0.47 (95% CI, 0.38–0.60) for individuals in the second, third, and fourth quartiles, respectively (Table 4). The risk of all-cause death was no different between individuals in the second, third, and forth quartiles.
Table 4.
Risk of all-cause mortality by lifestyle factors among participants in Third National Health and Nutrition Examination Survey with CKD
| Variable | Age-Adjusted All-Cause Mortality Rate per 1000 Person-Year (95% CI) (%) | Demographic Characteristic–Adjusted HR (95% CI) (n=2223)a | P Value | Fully Adjusted HR (95% CI) (n=2145)b | P Value |
|---|---|---|---|---|---|
| Body mass index | |||||
| 18.5 to <22 kg/m2 | 61.2 (60.5–61.9) | 1.19 (0.97–1.46) | 0.09 | 1.30 (1.03–1.64) | 0.03 |
| 22 to <25 kg/m2 | 56.5 (55.9–57.2) | Referent | Referent | ||
| 25 to <30 kg/m2 | 55.9 (55.4–56.3) | 0.97 (0.81–1.17) | 0.77 | 1.00 (0.82–1.22) | 1.00 |
| ≥30 kg/m2 | 53.8 (53.5–54.1) | 0.96 (0.77–1.21) | 0.73 | 0.87 (0.71–1.07) | 0.17 |
| Physical activity | |||||
| Inactive | 65.1 (64.4–65.7) | Referent | Referent | ||
| Insufficient | 54.2 (53.7–54.6) | 0.76 (0.60–0.96) | 0.02 | 0.86 (0.67–1.10) | 0.22 |
| Recommended | 51.7 (51.3–52.2) | 0.73 (0.57–0.92) | 0.01 | 0.80 (0.65–0.99) | 0.04 |
| Cigarette smoking | |||||
| Current smoker | 92.2 (91.7–92.8) | Referent | Referent | ||
| Past smoker | 59.2 (58.7–59.7) | 0.63 (0.51–0.78) | <0.01 | 0.61 (0.50–0.76) | <0.01 |
| Never smoker | 47.3 (46.4–48.2) | 0.53 (0.41–0.67) | <0.01 | 0.54 (0.41–0.70) | <0.01 |
| Healthy Eating Index score | |||||
| <54.5 | 65.2 (64.5–66.0) | Referent | Referent | ||
| 54.5 to <63.7 | 60.1 (59.5–60.7) | 0.92 (0.74–1.14) | 0.43 | 1.02 (0.80–1.31) | 0.86 |
| 63.7 to <73.1 | 58.5 (58.0–59.0) | 0.93 (0.74–1.14) | 0.55 | 1.02 (0.78–1.33) | 0.87 |
| 73.1–100 | 49.5 (49.1–49.8) | 0.81 (0.62–1.06) | 0.12 | 0.94 (0.71–1.25) | 0.66 |
| Weighted healthy lifestyle score, quartiles | |||||
| 1 (−4 to 6 points) | 85.1 (83.9–86.4) | Referent | Referent | ||
| 2 (7–9 points) | 54.5 (54.1–54.9) | 0.59 (0.46–0.75) | <0.01 | 0.53 (0.41–0.68) | <0.01 |
| 3 (10–11 points) | 51.5 (51.0–52.0) | 0.58 (0.48–0.70) | <0.01 | 0.52 (0.42–0.63) | <0.01 |
| 4 (12–15 points) | 47.7 (47.5–48.0) | 0.54 (0.44–0.67) | <0.01 | 0.47 (0.38–0.60) | <0.01 |
HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), annual household income (<$20,000 versus ≥$20,000), and education (high school, yes versus no).
In addition to demographic factors, adjusted for CKD Epidemiology Collaboration–estimated GFR, microalbuminuria (<30, 30 to >300, ≥300 mg/g), diabetes, cardiovascular disease, cancer, systolic BP, serum cholesterol (<200 versus ≥200 mg/dl), use of statin (yes versus no), and use of an angiotensin-converting enzyme inhibitor (yes versus no). Models for each individual component of the healthy lifestyle score were adjusted for the other three components.
Multivariable regression analyses of each component of the healthy lifestyle score (including adjustment for the other three factors) demonstrated an independent association of abstinence from cigarette smoking and recommended physical activity with all-cause mortality (Table 4). Compared with current smokers, the risk of death was 46% lower in never smokers and 39% lower in former smokers (P<0.01). Adherence to recommended physical activity was associated with a 20% lower risk of death compared with physical inactivity (P=0.04). Using a BMI of 22 to <25 kg/m2 as the referent category, we observed a 30% increased risk of death among individuals with a BMI of 18.5 to <22 kg/m2 (P=0.03). The multivariable-adjusted risk of death was not significantly different between the referent category and individuals with a BMI of 25 to <30 or ≥30 kg/m2 (P>0.05). Because of the observed association between all-cause mortality and BMI, we conducted an additional analysis evaluating cardiovascular mortality and BMI. Compared with individuals with a BMI of 22 to <25 kg/m2, participants with a BMI of 18.5 to <22 kg/m2 had a 33% increased risk of cardiovascular death (hazard ratio, 1.33 [95% CI, 1.00–1.76]; P=0.049). This risk was not significantly different between the referent category and overweight or obese participants (P>0.05, results not shown). The age-adjusted all-cause mortality rates were lower for individuals within the highest compared with the lowest HEI quartile (49.5 versus 65.2 per 1000 person-years). However, there was no significant association between diet and all-cause mortality after multivariable adjustment (Table 4).
Sensitivity Analyses
Adjusted survival analyses stratified by eGFR (<60 or ≥60 ml/min per 1.73m2), UACR (≥30 or <30 mg/g), and a combination of both (eGFR <60 ml/min per 1.73 m2 and UACR ≥30 mg/g) revealed results similar to those observed in the main cohort (Figure 1). According to use of sex-specific UACR cut-points, 496 additional participants were classified as having CKD; compared with individuals in the lowest quartile of the weighted healthy lifestyle score, the adjusted hazard ratios of all-cause mortality were 0.57 (95% CI, 0.46–0.71), 0.54 (95% CI, 0.44–0.66), and 0.48 (95% CI, 0.40–0.59) for individuals in the second, third, and fourth quartiles, respectively; these values were not significantly different from those in the main cohort. Furthermore, the results of the main analyses did not change after we used indicator variables for missing covariates in the fully adjusted model (data not shown).
Figure 1.
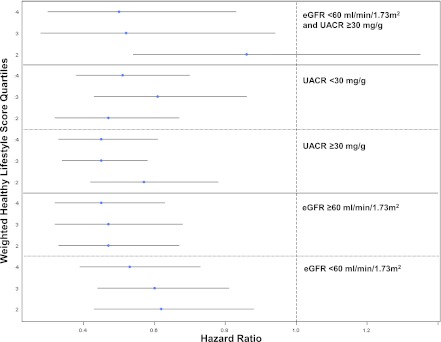
Risk of all-cause mortality by weighted healthy lifestyle score quartiles (using the first quartile as the reference category) in subgroups stratified by estimated GFR (eGFR) and urine albumin-to-creatinine ratio (UACR) in participants in the Third National Health and Nutrition Examination Survey with CKD. Error bars represent 95% confidence intervals.
Discussion
Less than one third of this cohort of individuals with mild-to-moderate CKD was adherent to three or more healthy lifestyle factors. Factors associated with a high weighted healthy lifestyle score included female sex, older age, and higher socioeconomic status. Moreover, participants with lower eGFR had higher healthy lifestyle scores, which could be related to the receipt of lifestyle modification counseling among individuals with advanced CKD. Persons in the highest quartile of the weighted healthy lifestyle score had a 53% lower risk of death compared with those in the lowest quartile. The most significant association with improved survival was observed with abstinence from smoking. Additionally, regular versus no physical activity was associated with a 20% reduction in mortality, whereas a BMI of 18.5 to <22 kg/m2 was associated with a 30% increased mortality.
To our knowledge, this study is the first to examine combinations of healthy lifestyle factors in relation to all-cause mortality in CKD. Several studies of general populations suggest that adherence to multiple healthy lifestyle factors is associated with improved clinical outcomes (5–7,20,29). An analysis of the Nurses’ Health Study (7) demonstrated that a healthy lifestyle was associated with lower risk of death. Similarly, the Health Professionals Follow-up Study demonstrated that a healthy lifestyle was associated with lower risk of coronary heart disease events (3). Our findings suggest that the beneficial effects of a healthy lifestyle may potentially be extended to the CKD population, which is at higher cardiovascular morbidity and mortality risk (8,9,15,30).
However, in contrast to these general population studies, which showed a dose effect between adherence to multiple lifestyle factors and adverse outcomes, we did not find additional benefit of adherence to more than one factor. The reasons for these differences are not clear but may be due to inherent differences between CKD and non-CKD populations or to differences in the outcomes evaluated and methods used to assess the exposure. Nonetheless, our findings support current CKD guidelines recommending lifestyle modifications regarding nonsmoking and physical activity for individuals with CKD (14,31–34). Further work is needed to investigate regarding optimal diet and BMI.
Several prior studies have examined how individual lifestyle factors influence outcomes in CKD. In the Cardiovascular Health Study (15), lower levels of physical activity were associated with increased cardiovascular mortality in elderly individuals with CKD. Similarly, a recent analysis of NHANES III reported that physical activity was associated with lower mortality in CKD (16). Very few studies have evaluated the relationship between smoking and mortality in CKD. Our findings regarding abstinence from smoking are consistent with analyses from the Cardiovascular Health Study reporting lower risk of cardiovascular death in CKD (15).
In contrast to studies on general populations that have reported increased morbidity and mortality in obese individuals (7,35), we found that the mortality risk for obese and overweight persons was similar to that for participants with a BMI of 22 to <25 kg/m2. Moreover, we report a 30% increased mortality risk among participants with a BMI of 18.5 to <22 kg/m2, which is within the lower half of what is considered to be the ideal BMI range. This is consistent with findings from a prospective study of a non-CKD population, which reported increased mortality risk with a BMI <21 kg/m2 (24). The reasons for these findings are unclear. However, underweight patients undergoing maintenance hemodialysis experience a similar increased risk of death, which has been attributed to protein-energy malnutrition and inflammation (36). The association between BMI and outcomes has been less extensively studied in predialysis CKD. Although one study in male veterans reported a significant inverse association between BMI and all-cause mortality (37), BMI was not found to be an independent predictor of death in analyses of data from the Modification of Diet in Renal Disease (13) and the Reasons for Geographic and Racial Differences in Stroke Studies (12). Future research is needed to further evaluate the relationship between BMI and outcomes in patients with CKD and to determine what constitutes an ideal BMI for this population.
The association between a healthy diet and survival has not been previously studied in predialysis CKD. However, in general populations it is well established that a diet that includes high intake of vegetables, fruits, nuts, whole grains, legumes, and fish is associated with lower risk of cardiovascular morbidity and mortality (2–5,29). Evidence suggests that this benefit may be mediated by favorable effects on BP, glucose, and lipids (38–40). We found that a diet rich in fruits and vegetables and low in saturated fat and sodium was associated with lower rates of age-adjusted all-cause mortality in individuals with CKD. However, this association was no longer significant after adjustment for demographic factors. It is possible that the HEI may not capture dietary components that are important for individuals with CKD. Prospective studies are needed to further evaluate this association.
Strengths of our study include the large sample size and the prospective design with a median follow-up of 13 years. However, our findings should be interpreted in the context of the limitations inherent to this type of analysis. First, measurement errors are possible, particularly with the use of self-reported questionnaires to ascertain medical history and physical activity, which have not been validated against medical records or objective measures of physical activity, respectively. In addition, the 24-hour dietary recall used to assess the diet component was not validated against food diaries among NHANES III participants. However, the HEI has been validated using selected biomarkers of dietary intake (41). Second, CKD was defined on the basis of a single eGFR and UACR measurement, which might lead to misclassification of the selected study sample, and information on certain important covariates (e.g., use of low-dose aspirin) was not available. Finally, individuals excluded from the analysis because of missing serum creatinine or UACR had significantly different demographic and clinical characteristics; this may have resulted in bias.
Adherence to a healthy lifestyle was associated with lower all-cause mortality risk in individuals with CKD, with the greatest reduction in all-cause mortality related to nonsmoking. These findings reinforce the importance of smoking abstinence counseling in patients with CKD. Our findings also suggest that regular physical activity and avoidance of low BMI might improve patient survival. Further work is needed to confirm these findings and to investigate what the optimal diet and BMI are in this population.
Disclosures
None.
Acknowledgments
The research reported in this study was supported by a National Institutes of Health Research Supplement to Promote Diversity in Health-Related Research to the Chronic Renal Insufficiency Cohort Study (A.C.R.). Support was also provided for the authors by the Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service (HSR&D), Career Development Award (M.J.F.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Risk Factor Paradox in CKD and ESRD: Does a Healthy Lifestyle Matter?,” on pages 515–517.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Akesson A, Weismayer C, Newby PK, Wolk A: Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med 167: 2122–2127, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Chiuve SE, McCullough ML, Sacks FM, Rimm EB: Healthy lifestyle factors in the primary prevention of coronary heart disease among men: Benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 114: 160–167, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB: Primary prevention of stroke by healthy lifestyle. Circulation 118: 947–954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM: Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA 306: 62–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC: Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 343: 16–22, 2000 [DOI] [PubMed] [Google Scholar]
- 7.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB: Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 337: a1440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D: Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 56: 2214–2219, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS, Sarnak MJ: Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis 48: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kramer H, Shoham D, McClure LA, Durazo-Arvizu R, Howard G, Judd S, Muntner P, Safford M, Warnock DG, McClellan W: Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 58: 177–185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madero M, Sarnak MJ, Wang X, Sceppa CC, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Body mass index and mortality in CKD. Am J Kidney Dis 50: 404–411, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease Outcomes Quality Initiative (K/DOQI) : K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 15.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T: Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 4: 1901–1906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and Collection Procedures. Vital Health Stat 1: 1–78, 1994 [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics : NHANES III Laboratory Data File Documentation: Ages One and Older. Catalog No. 76300, Hyattsville, MD, Centers for Disease Control and Prevention, 1996 [Google Scholar]
- 20.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee : Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121: 586–613, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS: Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 32[Suppl]: S498–S504, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy ET, Ohls J, Carlson S, Fleming K: The Healthy Eating Index: Design and applications. J Am Diet Assoc 95: 1103–1108, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Sullivan LM, Massaro JM, D’Agostino RB, Sr: Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 23: 1631–1660, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF: Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355: 763–778, 2006 [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics: Office of Analysis and Epidemiology, The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality follow-up through 2006: Matching Methodology. May 2009. Available at http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf Accessed July 20, 2012
- 26.National Center for Health Statistics : Analytical and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, 1988-1994, Hyattsville, MD, National Center for Health Statistics, 1996 [Google Scholar]
- 27.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld DA: Partial residuals for the proportional hazards regression model. Biometrika 69: 239–241, 1982 [Google Scholar]
- 29.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA: Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 292: 1433–1439, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA: Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl S24–S31, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Chadban S, Howell M, Thomas M, Jerums G, Alan C, Campbell D, Nicholls K, Tong A, Mangos G, Stack A, McIsaac R, Girgis S, Colagiuri R, Craig J: National Evidence Based Guideline for Diagnosis, Prevention and Management of Chronic Kidney Disease in Type 2 Diabetes, Canberra, Diabetes Australia and the NHMRC, 2009 [Google Scholar]
- 32.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, Burns K, Manns B, White C, Madore F, Moist L, Klarenbach S, Barrett B, Foley R, Jindal K, Senior P, Pannu N, Shurraw S, Akbari A, Cohn A, Reslerova M, Deved V, Mendelssohn D, Nesrallah G, Kappel J, Tonelli M, Canadian Society of Nephrology : Guidelines for the management of chronic kidney disease. CMAJ 179: 1154–1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewington A, Kanagasundaram S: Renal Association Clinical Practice Guidelines on acute kidney injury. Nephron Clin Pract 118[Suppl 1]: c349–c390, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Mactier R, Davies S, Dudley C, Harden P, Jones C, Kanagasundaram S, Lewington A, Richardson D, Taal M, Andrews P, Baker R, Breen C, Duncan N, Farrington K, Fluck R, Geddes C, Goldsmith D, Hoenich N, Holt S, Jardine A, Jenkins S, Kumwenda M, Lindley E, MacGregor M, Mikhail A, Sharples E, Shrestha B, Shrivastava R, Steddon S, Warwick G, Wilkie M, Woodrow G, Wright M: Summary of the 5th edition of the Renal Association Clinical Practice Guidelines (2009-2012). Nephron Clin Pract 118: C27–C70, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ: Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211–2219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD: Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis 42: 864–881, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49: 581–591, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, Tinker L, Howard BV, Lloyd-Jones D, Van Horn L: Diet quality and the risk of cardiovascular disease: The Women’s Health Initiative (WHI). Am J Clin Nutr 94: 49–57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB: Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 119: 1093–1100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu FB, Willett WC: Optimal diets for prevention of coronary heart disease. JAMA 288: 2569–2578, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Weinstein SJ, Vogt TM, Gerrior SA: Healthy Eating Index scores are associated with blood nutrient concentrations in the third National Health And Nutrition Examination Survey. J Am Diet Assoc 104: 576–584, 2004 [DOI] [PubMed] [Google Scholar]


