Summary
Background and objectives
There exists gross disparity in national deceased donor kidney transplant availability and practice: waiting times exceed 6 years in some regions, but some patients receive kidneys before they require dialysis. This study aimed to quantify and characterize preemptive deceased donor kidney transplant recipients and compare their outcomes with patients transplanted shortly after dialysis initiation.
Design, setting, participants, & measurements
Using the Scientific Registry of Transplant Recipients database, first-time adult deceased donor kidney transplant recipients between 1995 and 2011 were classified as preemptive, early (on dialysis≤1 year), or late recipients. Random effects logistic regression and multivariate Cox proportional hazards regression were used to identify characteristics of preemptive deceased donor kidney transplant and evaluate survival in preemptive and early recipients, respectively.
Results
Preemptive recipients were 9.0% of the total recipient population. Patients with private insurance (adjusted odds ratio=3.15, 95% confidence interval=3.01–3.29, P<0.001), previous (nonkidney) transplant (adjusted odds ratio=1.94, 95% confidence interval=1.67–2.26, P<0.001), and zero-antigen mismatch (adjusted odds ratio=1.45, 95% confidence interval=1.37–1.54, P<0.001; Caucasians only) were more likely to receive preemptive deceased donor kidney transplant, even after accounting for center-level clustering. African Americans were less likely to receive preemptive deceased donor kidney transplant (adjusted odds ratio=0.44, 95% confidence interval=0.41–0.47, P<0.001). Overall, patients transplanted preemptively had similar survival compared with patients transplanted within 1 year after initiating dialysis (adjusted hazard ratio=1.06, 95% confidence interval=0.99–1.12, P=0.07).
Conclusions
Preemptive deceased donor kidney transplant occurs most often among Caucasians with private insurance, and survival is fairly similar to survival of recipients on dialysis for <1 year.
Introduction
A careful balance of utility and equity is critical in deceased donor kidney transplantation (DDKT) policy: it is a life-prolonging therapy with varying survival benefits among recipients (1–3), and there are longstanding sex, racial, socioeconomic, and geographic differences in DDKT rates (4–6). One aspect of DDKT with particularly striking disparities is its timing vis-à-vis dialysis initiation. Despite average national waiting times exceeding 3 years (7,8) and times to transplantation in some geographic areas exceeding 6 years (9), it is possible for candidates in most regions of the United States to receive DDKT even before requiring maintenance dialysis (10,11).
As a general principle (certain regions elect to use slightly divergent organ allocation systems) (12), the Organ Procurement and Transplantation Network policy mandates that a recovered kidney is first offered to the suitably matched local candidate with the longest waiting time, defined as time since listing for transplant (13). One national policy circumvents this allocation system: the mandatory sharing of zero-antigen mismatched kidneys. Although the strength of this policy was recently diminished (extraregion sharing is required only to pediatric candidates and adults with plasma reactive antigen [PRA] >20%) (13,14), the candidate at the top of the waiting list will still be bypassed if there exists a local candidate with zero-antigen mismatches, regardless of waiting time or dialysis requirement. In this manner, a preemptive candidate—one who has not yet required dialysis—may preferentially receive a kidney over a candidate on dialysis who has been waiting much longer.
Despite the allowance (and sometimes preference) for preemptive DDKT, its relative utility compared with DDKT after a short period on dialysis remains unknown. Kidney transplantation significantly improves life expectancy and reduces costs for dialysis patients (2,15), but this improvement has not been shown in predialysis CKD. Although time on dialysis (dialysis vintage) strongly associates with recipient mortality (16–18), no study has shown that preemptive DDKT improves on DDKT among those patients with short dialysis vintage. Early preemptive transplantation (an increasingly common practice) (10) may be redundant with residual native kidney function and even hasten native kidney function decline (19,20). Preemptive DDKT may, thus, allocate an organ to a candidate who would otherwise remain dialysis-free for years, wasting the limited potential years of allograft function.
Given uncertain utility, the parity of preemptive DDKT must be closely examined. At an individual level, candidates in similar health with similar waiting times may not have similar access to preemptive DDKT: the phenotype of a preemptive DDKT recipient is typically privately insured, Caucasian, and college educated (11). Area of listing is also important: some regions of the United States allow waiting time accrual only after dialysis is initiated, rendering preemptive DDKT impossible (12). Finally, the zero-antigen mismatch policy (13,14), historically affecting 15% of DDKTs (21), may preferentially benefit certain ethnic groups. HLA distributions are vastly different in the donor and candidate pools (7), facilitating zero-antigen mismatch transplants primarily among Caucasian recipients and potentially exacerbating racial inequity in DDKT (21–25).
In this study, we sought to quantify the practice of preemptive DDKT and determine patient-, center-, and transplant-related factors that characterized these transplants, including the impact of zero-mismatch allocation on racial disparities in transplant timing. In addition, we compared the patient and graft survival of preemptive DDKT recipients with those patients receiving DDKT after relatively short dialysis vintage to get a sense of the effects of restricting organ offers only to those patients on dialysis.
Materials and Methods
Study Population
First-time kidney-only adult DDKT recipients from January 1, 1995 to May 31, 2011 were identified through the Scientific Registry of Transplant Recipients (SRTR) (n=121,853). Recipients were classified as preemptive kidney transplant recipients, early transplant recipients (transplanted with 1 year of dialysis or less), or late transplant recipients (transplanted after more than 1 year of dialysis). This cut point was chosen to minimize bias between the preemptive and early recipient groups, because it is likely that DDKT recipients of longer dialysis vintage are increasingly different in ways that registry data cannot capture. Mortality data were augmented through linkage with the Social Security Death Master File; graft survival data were augmented through linkage with Centers for Medicare and Medicaid Services and the Organ Procurement and Transplantation Network data.
Characterization of Preemptive DDKT Recipients and Their Transplant Centers
Differences in baseline variables by DDKT timing were assessed by t and chi-squared tests for continuous and categorical variables, respectively. Characteristics independently associated with preemptive DDKT were modeled using random effects logistic regression within the entire population (preemptive, early transplant, and late transplant recipients), specifying a random intercept for transplant center. This technique allows the baseline rate of preemptive DDKT to vary by center, reflecting the fact that different centers have different balances of organ supply and demand. Covariates considered for adjustment included all baseline characteristics listed in Table 1, excluding recipient wait time (because of the high correlation with transplant timing categories). The final model contained characteristics with significant adjusted association (P<0.05) and one donor variable (median candidate waiting time at transplant) included for mechanistic relevance and face validity. The intraclass correlation of latent responses was used to estimate the proportion of variation explained by variation across centers. To test whether racial variations in preemptive DDKT rates were similar by key drivers of preemptive DDKT, the interactions between race, private insurance, and zero-antigen mismatch were tested. Finally, the cumulative distribution of preemptive DDKT was plotted against the cumulative distribution of transplant centers (using a Lorenz curve) (26) to assess whether preemptive DDKT was performed universally or at only a few select centers. This distribution was compared with the Lorenz curve of late transplants (i.e., transplants among recipients who had been on dialysis for more than 1 year).
Table 1.
Characteristics of first-time deceased donor kidney transplant recipients by transplant timing
| Preemptive Transplants | ≤1-Yr Dialysis Vintage | >1-Yr Dialysis Vintage | |
|---|---|---|---|
| N | 10,992 (9.0%) | 14,428 (11.8%) | 96,433 (79.1%) |
| Recipient age (yr) | 52.7 (12.5) | 50.6 (13.2) | 50.9 (13.0) |
| Sex (%) | |||
| Male | 8.3 | 12.0 | 79.7 |
| Female | 10.2 | 11.6 | 78.3 |
| Race/ethnicity (%) | |||
| Caucasian | 13.3 | 16.4 | 70.3 |
| African American | 4.7 | 6.7 | 88.6 |
| Other | 5.2 | 8.5 | 86.3 |
| Body mass index (kg/m2) | 27.1 (4.9) | 26.8 (4.9) | 27.2 (5.0) |
| Last PRA (%) | |||
| ≤40 | 9.3 | 12.4 | 78.3 |
| >40 | 7.6 | 8.5 | 83.9 |
| Hepatitis C status (%) | |||
| Negative | 9.0 | 11.7 | 79.4 |
| Positive | 9.9 | 14.5 | 75.6 |
| Previous (nonkidney) transplant (%) | |||
| No | 8.7 | 11.6 | 80.0 |
| Yes | 26.6 | 26.6 | 46.8 |
| Poor functional status (%) | |||
| No | 9.0 | 11.5 | 79.4 |
| Yes | 8.8 | 15.0 | 76.2 |
| Insurance type (%) | |||
| Public | 5.4 | 8.4 | 86.3 |
| Private | 17.6 | 20.0 | 62.4 |
| Etiology of renal disease (%) | |||
| GN | 9.3 | 12.7 | 78.0 |
| Diabetes | 6.6 | 11.6 | 81.8 |
| Polycystic kidney disease | 16.2 | 15.1 | 68.7 |
| Hypertension | 6.0 | 8.2 | 85.8 |
| Vasculitis | 6.1 | 7.9 | 86.0 |
| Congenital | 11.3 | 14.8 | 73.4 |
| Interstitial disease | 14.1 | 12.7 | 73.2 |
| Neoplasm | 10.0 | 10.6 | 79.3 |
| Transplant-related | 26.2 | 22.7 | 51.1 |
| Miscellaneous | 14.8 | 19.7 | 65.5 |
| Blood type (%) | |||
| O | 7.8 | 9.7 | 82.5 |
| A | 10.5 | 14.4 | 75.1 |
| B | 7.7 | 8.9 | 83.4 |
| AB | 12.4 | 18.9 | 68.7 |
| Waiting time (yr) | 1.3 (1.5) | 0.8 (1.1) | 2.2 (1.7) |
| Center median wait (yr) | 3.4 (1.5) | 3.2 (1.5) | 3.5 (1.6) |
| Cold ischemia time (h) | 18.1 (9.0) | 18.8 (8.9) | 19.1 (9.2) |
| Donor age (yr) | 36.5 (16.8) | 36.8 (17.2) | 38.1 (17.1) |
| Zero HLA mismatch (%) | |||
| No | 8.4 | 11.0 | 80.6 |
| Yes | 13.6 | 18.0 | 68.4 |
| ECD transplant (%) | |||
| No | 9.3 | 12.1 | 78.6 |
| Yes | 7.7 | 10.7 | 81.6 |
| DCD (%) | |||
| No | 9.1 | 12.0 | 78.9 |
| Yes | 8.5 | 9.5 | 82.0 |
| CDC high risk (%) | |||
| No | 9.0 | 11.9 | 79.1 |
| Yes | 10.3 | 10.7 | 79.0 |
For continuous variables, values reflect the cell-wise mean (SD). For categorical variables, percentage reflects the row-wise percentage. P values<0.05 for all comparisons except donor age between preemptive and early transplant recipients (P=0.20). PRA, percent reactive antibody, ECD, expanded criteria donor; DCD, declared cardiac death donor; CDC, Centers for Disease Control and Prevention.
Outcomes by Transplant Timing
Patient survival (irrespective of graft function) and graft survival (censored at death) were evaluated within preemptive and early postdialysis recipients using multivariate Cox proportional hazards regression, adjusting for all variables included in the model of preemptive transplant timing (except blood type) as well as region. The proportional hazards assumption was evaluated using complementary log-log plots and testing the interaction of transplant timing with year post-transplant. Interactions were tested between transplant timing (preemptive versus early) and recipient age, diabetes, and zero-antigen mismatch kidneys. Because of a significant interaction between age and transplant timing, subgroup analysis was performed.
Sensitivity Analyses
Sensitivity analyses for patient survival models were performed by three distinct methods. First, the Cox analysis was repeated adjusting for variables (and their functional form) included in the SRTR 3-year patient survival models, namely 5 donor factors (age, ethnicity, creatinine, diabetes, and hypertension), 10 recipient factors (age, sex, ethnicity, body mass index, previous transplant, etiology of kidney disease, hepatitis C [HCV] status, history of malignancy, peak PRA, and insurance type), and 8 transplant-related factors (cold ischemia time, whether an organ was pumped, donor after cardiac death, expanded criteria donor, weight ratio of donor to recipient, zero-antigen mismatch transplant, median center waiting time, and transplant year). Second, a propensity score for preemptive transplantation (i.e., modeling the likelihood that a patient with a given set of characteristics would receive a preemptive transplant) was used to match cases (preemptive DDKT recipients) with controls (early postdialysis DDKT recipients). The propensity score was modeled on all variables included in the base model as well as United Network for Organ Sharing region; matching was performed using 1:1 nearest neighbor matching without replacement and a caliper of 0.1. Cox proportional hazards regression was then fit to this population, adjusting for continuous propensity score to mitigate any residual confounding (27). Third, cases and controls were matched on individual variables with the following specification: exact matches for sex, race/ethnicity, diabetes, HCV status, private insurance, and zero-antigen mismatch status, and iterative, expanding radius matching for donor age, recipient age, transplant year, and waiting time as previously described (28,29). The adjusted Cox model was fit on this matched sample as well. All analyses were performed using STATA 12.0/MP (StataCorp, College Station, TX).
Results
Frequency and Characteristics of Preemptive and Early DDKT
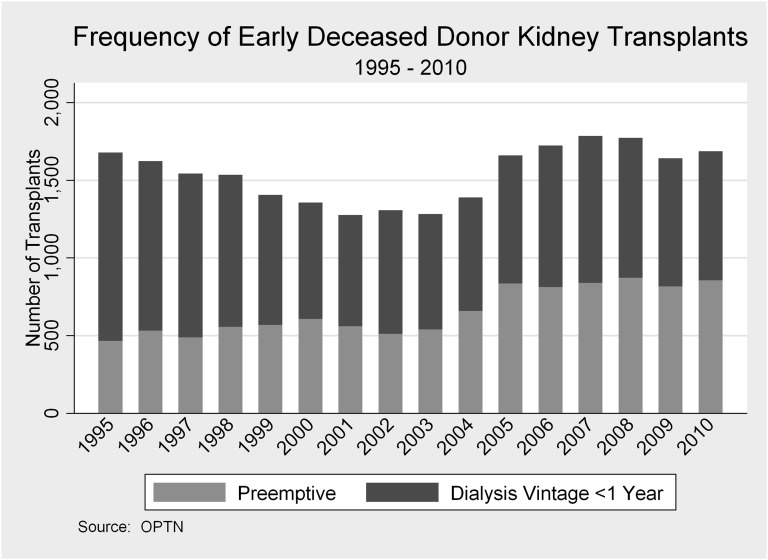
Of 121,853 first-time adult DDKT recipients, 10,992 (9.0%) received a kidney preemptively, and 14,428 (11.8%) received a kidney early postdialysis (within 1 year of starting dialysis) (Table 1). The relative frequency of preemptive and early transplants varied over the years (Figure 1). In 1995, 7.3% of all DDKTs were preemptive, and 19.1% were early; in 2010, 9.7% were preemptive, and only 9.4% were early postdialysis transplants. On average, preemptive recipients were slightly older (52.7, 50.6, and 50.9 years in preemptive, early, and late recipients, respectively, P<0.001 for all comparisons) and more often female and Caucasian. More previous recipients of a nonkidney organ transplant underwent DDKT preemptively. Mean waiting time for preemptive transplant recipients was slightly longer than the time among early postdialysis recipients (1.3 versus 0.8 years [P<0.001] among patients who received a transplant), and the median waiting time at the centers where these patients were listed was also longer (3.4 versus 3.2 years [P<0.001] among those patients added to the waiting list). Nearly one fifth of preemptive and early transplants were zero-antigen mismatch (19.0% and 19.2%, respectively) compared with only 10.6% of late transplants. There was little meaningful difference in kidney quality between the preemptive, early transplants, and late transplants in terms of cold ischemia time, expanded criteria donor, and donor after cardiac death.
Figure 1.
Deceased donor kidney transplantation in recipients with less than 1 year dialysis vintage, 1995–2010.
Independent Associations with Preemptive DDKT
Factors independently associated with preemptive DDKT included more recent transplant year, zero-antigen mismatch, older recipient age, female sex, positive HCV status, non-O blood type, and certain etiologies of ESRD (Table 2). In addition, having private insurance was associated with 3.2-fold higher odds of receiving preemptive DDKT (95% confidence interval [CI]=3.01–3.29, P<0.001), and a previous (nonkidney) transplant was associated with 1.9-fold higher odds (95% CI=1.67–2.26, P<0.001). Compared with Caucasians, African Americans had less than one half the odds of preemptive DDKT (adjusted odds ratio [aOR]=0.44, 95% CI=0.41–0.47, P<0.001). The association between positive HCV status and preemptive DDKT (aOR=1.31, 95% CI=1.20–1.44, P<0.001) was slightly larger when accounting for HCV status of the donor organ (aOR=1.46, 95% CI=1.33–1.60, P<0.001); in other words, HCV-positive recipients of preemptive transplants were less likely to receive an HCV-positive organ than those patients who were first on dialysis (16.2% versus 30.5%, P<0.001).
Table 2.
Independent associations with preemptive deceased donor kidney transplantation
| Adjusted Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Recipient factors | ||
| Recipient age (per decade) | 1.12 (1.10–1.14) | <0.001 |
| Female sex | 1.33 (1.27–1.39) | <0.001 |
| Ethnicity | ||
| Caucasian | Reference | |
| African American | 0.44 (0.41–0.47) | <0.001 |
| Other | 0.53 (0.49–0.57) | <0.001 |
| Impaired functional status | 0.88 (0.82–0.96) | 0.003 |
| Last PRA greater than 40% | 0.77 (0.72–0.83) | <0.001 |
| Hepatitis C | 1.31 (1.20–1.44) | <0.001 |
| Previous nonkidney transplant | 1.94 (1.67–2.26) | <0.001 |
| Private insurance | 3.15 (3.01–3.29) | <0.001 |
| Etiology of renal disease | ||
| GN | Reference | |
| Diabetes | 0.69 (0.64–0.74) | <0.001 |
| Polycystic kidney disease | 1.36 (1.26–1.46) | <0.001 |
| Hypertension | 0.75 (0.70–0.81) | <0.001 |
| Vasculitis | 0.68 (0.60–0.77) | <0.001 |
| Congenital | 1.28 (1.12–1.46) | <0.001 |
| Interstitial disease | 1.35 (1.22–1.50) | <0.001 |
| Neoplasm | 1.04 (0.86–1.27) | 0.70 |
| Transplant-related | 1.20 (0.98–1.48) | 0.08 |
| Miscellaneous | 1.51 (1.39–1.63) | <0.001 |
| Blood type | ||
| O | Reference | |
| A | 1.21 (1.15–1.27) | <0.001 |
| B | 1.10 (1.02–1.18) | 0.01 |
| AB | 1.56 (1.02–1.18) | <0.001 |
| Donor factors | ||
| Transplant year | 1.03 (1.03–1.04) | <0.001 |
| ECD transplant | 0.90 (0.85–0.98) | 0.01 |
| DCD transplant | 0.88 (0.81–0.96) | 0.005 |
| Zero-antigen mismatch transplant | 1.45 (1.37–1.54) | <0.001 |
| Donor age (per yr increase) | 0.96 (0.95–0.98) | <0.001 |
| Cold ischemia time (per 12 h) | 0.89 (0.87–0.92) | <0.001 |
| Center median wait time (per yr) | 0.98 (0.94–1.03) | 0.50 |
CI, confidence interval; PRA, percent reactive antibody, ECD, expanded criteria donor; DCD, declared cardiac death donor.
There was a significant interaction between zero-antigen mismatch transplants and race (P=0.05 for interaction): in Caucasians, zero-antigen mismatch was associated with 1.4-fold higher odds of preemptive DDKT (95% CI=1.35–1.53, P<0.001), whereas in African Americans, the association was not seen (aOR=1.15, 95% CI=0.93–1.42, P=0.19). There was also a significant interaction between private insurance and race (P=0.007 for interaction): private insurance was a somewhat weaker predictor of preemptive DDKT in Caucasians (aOR=3.06, 95% CI=2.91–3.21, P<0.001) than African Americans (aOR=3.56, 95% CI=3.22–3.92, P<0.001).
Center Effect in Rates of Preemptive Transplantation
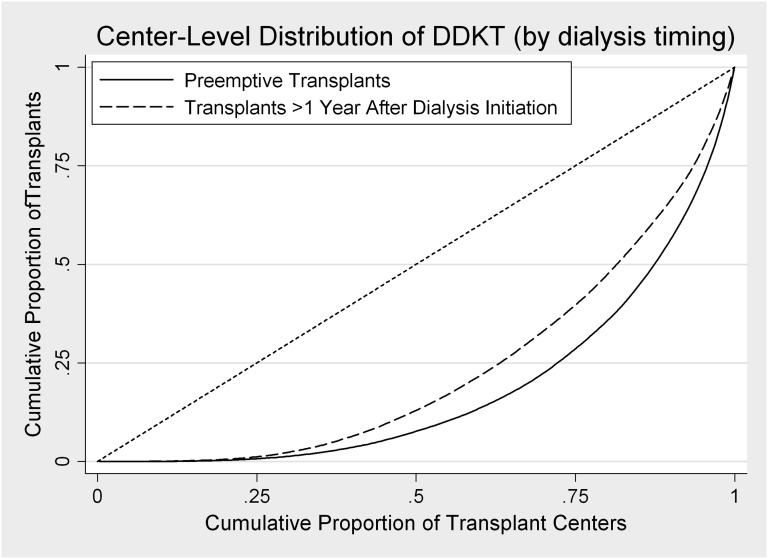
After adjusting for median center waiting time and other donor-, recipient-, and transplant-related factors, variation between transplant centers explained 10.4% of the variation in transplant timing overall. Interestingly, median center waiting time was not associated with preemptive transplantation in adjusted analysis (Table 2). Compared with late DDKT (i.e., in recipients with dialysis vintage>1 year), preemptive DDKT was slightly more concentrated among fewer transplant centers, although this difference was small (Figure 2).
Figure 2.
Distribution of deceased donor transplants across centers by transplant timing (preemptive versus late), 1995–2011. The diagonal reference line represents equal numbers of transplants at each center; the farther the curve is shifted to the lower right corner, the more concentrated the practice. Thus, the majority of preemptive transplants occurs at fewer centers than the majority of late transplants.
Patient Survival Rates by Transplant Timing
In adjusted analysis, there was little difference between preemptive and early postdialysis transplant timing in terms of patient survival (adjusted hazard ratio [aHR]=1.06, 95% CI=0.99–1.12, P=0.07) (Table 3); both were associated with significantly better survival than late DDKT. The magnitude of this relationship was close to null in all sensitivity analyses (SRTR-adjusted: aHR=1.09, 95% CI=1.03–1.16; propensity score: aHR=1.06, 95% CI=0.99–1.14; matched controls: aHR=1.05, 95% CI=0.97–1.13). The interaction between timing and recipient age was significant (P=0.05 for interaction term): there was no association between transplant timing and mortality among younger recipients (aHR=1.03, 95% CI=0.96–1.10, P=0.50), but there was a slightly higher risk of mortality associated with early postdialysis DDKT among older recipients (aHR=1.19, 95% CI=1.05–1.35, P=0.007).
Table 3.
Independent associations of early (dialysis vintage≤1 year) versus preemptive deceased donor kidney transplant timing with patient mortality and death-censored graft loss, 1995–2011
| Adjustment Type | Population Analyzed | Death (Hazard Ratio, 95% Confidence Interval) | Death-Censored Graft Loss (Hazard Ratio,95% Confidence Interval) |
|---|---|---|---|
| Multivariablea | Full populationb | 1.06 (0.99–1.12, P=0.07) | 1.23 (1.15–1.32, P<0.001) |
| SRTR | Full populationb | 1.09 (1.03–1.16, P=0.005) | 1.25 (1.17–1.33, P<0.001) |
| Propensity | Propensity matchedc | 1.06 (0.99–1.14, P=0.06) | 1.21 (1.12–1.30, P<0.001) |
| Multivariablea | 10-variable matchedd | 1.05 (0.97–1.13, P=0.20) | 1.26 (1.17–1.36, P<0.001) |
| Multivariablea | Recipients under 65 yr | 1.03 (0.96–1.10, P=0.50) | 1.25 (1.16–1.34, P<0.001) |
| Multivariablea | Recipients ages 65 yr and older | 1.19 (1.05–1.35, P=0.007) | 1.12 (0.92–1.35, P=0.30) |
Adjusted for all variables listed in Table 2 with the exception of blood type.
All early or preemptive deceased donor transplant recipients (n=25,420).
Propensity score-matched population (n=18,976).
Matched on sex, race/ethnicity, diabetes status, hepatitis C status, private insurance status, zero-antigen mismatch status, donor age, recipient age, transplant year, and waiting time (n=18,168).
Graft Survival Rates by Transplant Timing
Adjusted death-censored graft survival rates were worse in early postdialysis DDKT recipients compared with preemptive DDKT recipients, although both preemptive and early DDKT recipients had better graft survival than late DDKT recipients (5-year graft survival: 88.5%, 83.9%, and 81.3% in preemptive, early, and late DDKT recipients, respectively). Compared with preemptive DDKT, early DDKT recipients experienced a 23% higher risk of graft loss (aHR=1.23, 95% CI=1.15–1.32, P<0.001); this association held in all sensitivity analyses except within older DDKT recipients (Table 3). The increased risk of graft loss associated with early DDKT became slightly more pronounced the farther out a recipient was from transplant (P=0.001 for interaction).
Discussion
In this study of DDKT timing in the United States, preemptive DDKT was common (9% of all DDKT), and it was more common among certain recipients (namely, Caucasians with private insurance). Older adults and women were also more likely to receive DDKT preemptively, although measures of association were smaller. Not surprisingly, zero-antigen mismatch was strongly associated with preemptive DDKT; however, this association was limited to Caucasian patients. Center-level factors contributed only marginally to the variation in transplant timing. Recipients of preemptive DDKT had fairly comparable survival with DDKT recipients with short dialysis vintages, but rates of graft loss among preemptive DDKT recipients were higher. Older age significantly modified the association between transplant timing and outcomes, with preemptive recipients ages 65 years and older experiencing slightly higher rates of post-transplant mortality and equivalent rates of graft survival.
The interaction between race and zero-antigen mismatch DDKT in the association with preemptive transplantation is a novel observation. Zero-antigen mismatch organs account for approximately 19% of all preemptive DDKT, and among Caucasians, they confer a 40% higher odds of preemptive transplant timing. Among African Americans, however, there is no significant association. This finding may reflect both the delay that many African Americans experience in being listed for transplant as well as the relative paucity of zero-antigen mismatch transplants among this ethnic group. However, the interaction—and furthermore, the strong main effect of race, even after accounting for factors like age, PRA, and presence of private insurance, similar to those effects reported over a decade ago (11)—raises the specter of continued inequity in DDKT transplantation timing.
Analysis of the estimated utility of preemptive DDKT showed mixed results. Whereas patient survival was fairly comparable between preemptive and early transplant groups (except among those patients≥65 years old), preemptive graft survival was slightly better (except among those patients≥65 years old). The difference in graft survival, although small (only 7% at 10 years), persisted in most sensitivity analyses and increased over time. It may be that the difference reflects residual native kidney function. Another possibility is that dialysis exposure induces a proinflammatory state, resulting in increased rejection and shorter allograft survival in the nonpreemptive recipient (30,31). With both of these explanations, however, one would expect a more pronounced benefit in the early post-transplant period, which was not observed. A possible alternative is that preemptive transplantation is a surrogate for better health care access: better medical insurance facilitates continuous access to immunosuppressive medications and more regular medical follow-up and allows for more timely diagnosis of rejections, drug reactions, and toxicity. This finding is supported by the increased frequency of preemptive DDKT among older recipients who, by the availability of Medicare, have more uniform access to health care. However, one might expect that a surrogate for better health care would also translate to improved patient survival.
The differences seen between younger and older patients by transplant timing are worth noting. Older adults had better overall survival but equivalent graft survival after preemptive transplantation. It is possible that any negative physiologic effect of dialysis is greater among older adults, thus favoring preemptive transplantation. However, it is also possible that unmeasured bias increases with age. Older preemptive recipients may be relatively healthier, and the competing risks of death before transplantation are greater, resulting in greater survivor bias.
There are several strengths to this study. It is a large, nationally representative study encompassing over 15 years of follow-up. We used a novel comparison group (recipients transplanted early in their dialysis course), providing a sense of what might happen if candidates were required to wait until dialysis initiation before DDKT eligibility. Finally, we performed multiple sensitivity analyses, including propensity-matched and iterative radius-matched samples, which address treatment selection bias, or the likelihood that recipients of early and preemptive DDKT differ in both observed and unobserved ways related to both their transplant timing and subsequent outcomes.
There are also important limitations, primarily owing to the observational, registry-based nature of this study, and the analyses of patient and graft survival warrant cautious interpretation. Comparisons by transplant timing are fraught with unmeasured confounders: early recipients are not perfect counterfactuals (what would have happened had DDKT been delayed?) for preemptive recipients. Preemptive recipients may have different attitudes to DDKT, affecting compliance with immunosuppression, and they may have different levels of baseline health unmeasured in registry data. The time of origin is transplantation: we do not credit the postdialysis transplant recipients with postdialysis survival until the time of transplant (lead time bias favoring preemptive transplants), and we do not account for candidates who died before transplantation (survival bias favoring postdialysis transplants). Similarly, we cannot know the duration of the preemptive transplant recipient’s native kidney function had he or she not received a transplant, thus preventing a comparison from the onset of dialysis requirement.
In summary, we find continued evidence of disparity in DDKT timing. African Americans are much less likely to receive preemptive DDKT than Caucasians, and zero-antigen mismatch sharing seems to exclusively facilitate preemptive DDKT timing among Caucasians. Given the long DDKT wait times and the fairly comparable survival between preemptive and early DDKT recipients, the utility of preemptive transplantation from a societal standpoint may be low. In addition, at the individual level, restricting preemptive DDKT may provide additional impetus for identifying live donors. Continued efforts to ensure the equitable distribution of DDKT, including the timing of DDKT, are needed.
Disclosures
None.
Acknowledgments
The authors thank the Organ Procurement and Transplantation Network (OPTN) for provision of the data. The OPTN is supported by Health Resources and Services Administration Contract 234-2005-370011C.
This work was funded by the National Kidney Foundation of Maryland, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant K08DK092287 (to M.E.G.), and National Institutes of Health Grants K23AG032885 (to D.L.S.; cofunded by the American Federation of Aging Research) and R21DK085409 (to D.L.S.).
Parts of this study were presented at the American Transplant Congress Annual Meeting in Boston, MA, on June 4, 2012.
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services. Also, mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Meier-Kriesche HU, Schold JD, Gaston RS, Wadstrom J, Kaplan B: Kidneys from deceased donors: Maximizing the value of a scarce resource. Am J Transplant 5: 1725–1730, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Segev DL: Evaluating options for utility-based kidney allocation. Am J Transplant 9: 1513–1518, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA: Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 20: 621–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Held PJ, Pauly MV, Bovbjerg RR, Newmann J, Salvatierra O, Jr: Access to kidney transplantation. Has the United States eliminated income and racial differences? Arch Intern Med 148: 2594–2600, 1988 [DOI] [PubMed] [Google Scholar]
- 7.The Organ Procurement and Transplantation Network: Data Reports, 2011. Available at: http://optn.transplant.hrsa.gov/latestData/viewDataReports.asp Accessed August 17, 2011
- 8.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 9.Leichtman AB, McCullough KP, Wolfe RA: Improving the allocation system for deceased-donor kidneys. N Engl J Med 364: 1287–1289, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Grams ME, Massie AB, Coresh J, Segev DL: Trends in the timing of pre-emptive kidney transplantation. J Am Soc Nephrol 22: 1615–1620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 12.The Organ Procurement and Transplantation Network: Catalogue of All Current OPTN Member Variances, April: Variance List 9B, 2010.
- 13.The Organ Procurement and Transplantation Network: Policies. Organ Distribution: Allocation of Deceased Kidneys, 2011. Available at: http://optn.transplant.hrsa.gov/policiesAndBylaws/policies.asp Accessed August 18, 2011
- 14.Ellison MD, Creger JH, Fiol BS, McBride MA, Davies DB, Stockdreher DD: Expanded mandatory sharing of zero-antigen mismatched kidneys: First-year effects following policy revision. Transplant Proc 29: 3437–3438, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Wong G, Howard K, Chapman JR, Chadban S, Cross N, Tong A, Webster AC, Craig JC: Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One 7: e29591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier-Kriesche H, Port FK, Ojo AO, Leichtman AB, Rudich SM, Arndorfer JA, Punch JD, Kaplan B: Deleterious effect of waiting time on renal transplant outcome. Transplant Proc 33: 1204–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Kaplan B: Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation 74: 1377–1381, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Meier-Kriesche HU, Schold JD: The impact of pretransplant dialysis on outcomes in renal transplantation. Semin Dial 18: 499–504, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Akkina SK, Connaire JJ, Snyder JJ, Matas AJ, Kasiske BL: Earlier is not necessarily better in preemptive kidney transplantation. Am J Transplant 8: 2071–2076, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ishani A, Ibrahim HN, Gilbertson D, Collins AJ: The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. Am J Kidney Dis 42: 1275–1282, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ting A, Edwards LB: Human leukocyte antigen in the allocation of kidneys from cadaveric donors in the United States. Transplantation 77: 610–614, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lazda VA, The Medical Advisory Committee : The impact of HLA frequency differences in races on the access to optimally HLA-matched cadaver renal transplants. Transplantation 53: 352–357, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Gaston RS, Ayres I, Dooley LG, Diethelm AG: Racial equity in renal transplantation. The disparate impact of HLA-based allocation. JAMA 270: 1352–1356, 1993 [PubMed] [Google Scholar]
- 24.Rebellato LM, Arnold AN, Bozik KM, Haisch CE: HLA matching and the United Network for Organ Sharing Allocation System: Impact of HLA matching on African-American recipients of cadaveric kidney transplants. Transplantation 74: 1634–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Zachary AA, Braun WE, Hayes JM, McElroy JB, Novick AC, Schulak JA, Sharp WV: Effect of HLA matching on organ distribution among whites and African-Americans. Transplantation 57: 1115–1119, 1994 [PubMed] [Google Scholar]
- 26.Massie AB, Zeger SL, Montgomery RA, Segev DL: The effects of DonorNet 2007 on kidney distribution equity and efficiency. Am J Transplant 9: 1550–1557, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Stuart EA: Matching methods for causal inference: A review and a look forward. Stat Sci 25: 1–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, McBride MA, Montgomery RA: Perioperative mortality and long-term survival following live kidney donation. JAMA 303: 959–966, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL: Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 365: 318–326, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Mange KC, Joffe MM, Feldman HI: Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 344: 726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Mange KC, Joffe MM, Feldman HI: Dialysis prior to living donor kidney transplantation and rates of acute rejection. Nephrol Dial Transplant 18: 172–177, 2003 [DOI] [PubMed] [Google Scholar]