Summary
Background and objectives
Elevated serum vitamin D with hypercalciuria can result in nephrocalcinosis and nephrolithiasis. This study evaluated the cause of excess 1,25-dihydroxycholecalciferol (1α,25(OH)2D3) in the development of those disorders in two individuals.
Design, setting, participants, & measurements
Two patients with elevated vitamin D levels and nephrocalcinosis or nephrolithiasis were investigated at the National Institutes of Health (NIH) Clinical Center and the NIH Undiagnosed Diseases Program, by measuring calcium, phosphate, and vitamin D metabolites, and by performing CYP24A1 mutation analysis.
Results
Both patients exhibited hypercalciuria, hypercalcemia, low parathyroid hormone, elevated vitamin D (1α,25(OH)2D3), normal 25-OHD3, decreased 24,25(OH)2D, and undetectable activity of 1,25(OH)2D-24-hydroxylase (CYP24A1), the enzyme that inactivates 1α,25(OH)2D3. Both patients had bi-allelic mutations in CYP24A1 leading to loss of function of this enzyme. On the basis of dbSNP data, the frequency of predicted deleterious bi-allelic CYP24A1 variants in the general population is estimated to be as high as 4%–20%.
Conclusions
The results of this study show that 1,25(OH)2D-24-hydroxylase deficiency due to bi-allelic mutations in CYP24A1 causes elevated serum vitamin D, hypercalciuria, nephrocalcinosis, and renal stones.
Introduction
Although nephrocalcinosis and nephrolithiasis are distinct entities, evidence suggests a common underlying mechanism (1). Nephrocalcinosis refers to the diffuse precipitation of calcium salts within the tubules, tubular epithelium, and/or interstitial tissue of the kidney (2). It involves the medulla in 98% of cases, and is readily detected by ultrasonography or computed tomography (CT). Nephrolithiasis refers to renal stones, generally visible on plain radiographs, ultrasounds, or CT scans; the majority of renal stones are composed of calcium salts (3). Because calcium precipitation is common to both nephrocalcinosis and nephrolithiasis, the two entities often occur together (4). In addition, both nephrolithiasis and nephrocalcinosis frequently result from metabolic abnormalities such as hyperphosphaturia, hyperoxaluria, hypocitruria, hyperuricosuria, defective urinary acidification, and especially hypercalciuria (5). Approximately 50% of patients with nephrolithiasis (6) and 14%–27% of non-stone formers (7) have hypercalciuria.
Previous classifications of hypercalciuria have been revised in view of recent evidence of intrinsic alterations of calcium metabolism associated with this phenomenon. Calcium metabolism is regulated by parathyroid hormone (PTH), calcitonin, and 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), the active form of vitamin D. Today, hypercalciuria is considered a complex trait, governed by several genetic mechanisms.
One of those mechanisms involves 1α,25(OH)2D3, which acts on calcium and phosphate to stimulate mobilization from bone, reabsorption by the kidney, and absorption by the intestine, 1α,25(OH)2D3 is formed through 1-hydroxylation of 25(OH)D3 (cholecalciferol) by the gene product of CYP27B1, which is present in many tissues; 25(OH)D3 is formed by 25-hydroxylation of vitamin D in the liver (Figure 1) (8). Excessive 1α,25(OH)2D3 leads to absorptive hypercalcemia and/or hypercalciuria (9), as first described in 1928 by Kreitmair and Moll (10).
Figure 1.
Vitamin D metabolism. The first step of activation, 25-hydroxylation by CYP2R1 and CYP27A1, occurs in the liver. The second step, 1α-hydroxylation by CYP27B1 to yield active vitamin D (i.e., 1α,25(OH)2D3), occurs in the kidney. Inactivation of vitamin D occurs via the C-23 and C-24 oxidation pathways, catalyzed by CYP24A1 in the kidney. Calcitroic acid has no biologic activity. Larger type emphasizes important metabolites.
Both major vitamin D metabolites, 25(OH)D3 and 1α,25(OH)2D3, are inactivated by 24-hydroxylation, a process catalyzed by 1,25(OH)2D-24-hydroxylase (CYP24A1), a mitochondrial cytochrome P-450 mixed-function oxidase present largely in intestine and kidney. Bi-allelic mutations in CYP24A1 and reduced activity of the enzyme have been associated with elevated levels of 1α,25(OH)2D3 in individuals given large amounts of vitamin 25(OH)D3 (11). In addition, a recent report demonstrated reduced 1,25(OH)2D-24-hydroxylase activity in patients with monoallelic CYP24A1 mutations (12).
We present a young boy with nephrocalcinosis and an adult male patient with nephrolithiasis, both of whom have molecular and biochemical defects in CYP24A1, leading to elevated levels of 1α,25(OH)2D3 and hypercalciuria.
Materials and Methods
Patients
Patients were enrolled in clinical protocol 76-HG-0238, “Diagnosis and Treatment of Patients with Inborn Errors of Metabolism and Other Genetic Disorders” and were evaluated at the National Institutes of Health (NIH) Clinical Center. Written, informed consent was obtained. Patient 2 was enrolled in the NIH Undiagnosed Diseases Program (UDP) (13,14), dedicated to investigating the basic defects underlying undiagnosed disorders.
Cell Culture
Primary fibroblasts from the patients were grown from forearm punch skin biopsies. Normal control fibroblasts were racially matched and randomly selected from our fibroblast bank. The cells were maintained in high-glucose (4.5 g/L) DMEM supplemented with 15% FBS, 2 mM l-glutamine, nonessential amino acid solution, and penicillin-streptomycin, as previously described (15,16).
Mutation Screening and Genotyping
Genomic DNA was isolated from PBMCs using the Gentra Puregene Blood Kit (Qiagen, Valencia, CA). All CYP24A1 exons and flanking introns were PCR-amplified and directly sequenced using standard protocols (Applied Biosystems, Foster City, CA). Data regarding position, coding region sequence, and exon-intron boundaries of CYP24A1 were obtained using the Ensembl Genome Browser (http://www.ensembl.org/), the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/mapview/), and the UCSC Genome Browser (http://genome.ucsc.edu/), which were used to design primers (available upon request).
Estimated Frequency of CYP24A1 Mutations
The dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) was searched (March 2012) for nonsynonymous variants within the CYP24A1 gene and their minor allele frequencies (MAFs). The effect of missense variations on protein function was evaluated using pathogenicity prediction programs, including POLYPHEN (Polymorphism Phenotyping; http://genetics.bwh.harvard.edu/pph/), PANTHER (Protein Analysis Through Evolutionary Relationships; http://www.pantherdb.org/), pMut (http://mmb2.pcb.ub.es:8080/PMut/), SIFT (Sorting Intolerant from Tolerant; http://sift.jcvi.org/), Blosum62 (Blocks of Amino Acid Substitution Matrix; ftp://ftp.ncbi.nih.gov/blast/matrices/BLOSUM62), and SNAP (Screening for Non-Acceptable Polymorphisms; http://cubic.bioc.columbia.edu/services/snap/) (Supplemental Methods). Missense variants were tested on 100 ethnically matched control individuals (i.e., a Caucasian DNA panel) (Coriell Institute of Medical Research).
Western Blot Analyses
Fibroblasts were washed twice with cold phosphate-buffered saline (PBS) and lysed with radioimmunoprecipitation assay buffer (50 mM Tris HCl pH 8 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitors (Complete Mini, EDTA-Free; Roche Diagnostics). Supernatants (10 μg) obtained after 30 minutes of centrifugation (15,000 rpm at 4°C) were boiled in reducing SDS buffer and electrophoresed on 4%–20% gradient polyacrylamide gels (Invitrogen). After transfer to nitrocellulose membranes, the proteins were blocked with 4% BSA and probed with rabbit anti-CYP24A1 antibody (sc-66851; Santa Cruz) and rabbit anti-β actin (clone EP1123Y; Chemicon). Appropriate IRDye 800 CW-conjugated secondary antibodies (Li-Cor Biosciences) were used. The antigen-antibody complexes were detected with the Li-Cor Odyssey infrared imaging system.
Biochemical Studies
Serum 24,25-(OH)2D levels were measured by RIA after lipid extraction and purification by high-performance liquid chromatography (HPLC). The intra-assay and interassay coefficients of variation (CVs) were 9.3% and 11.8% , respectively (Heartland Assays LLC) (17). 1,25(OH)2 vitamin D testing was performed by immunoextraction and liquid chromatography–tandem mass spectrometry, similar to published methods (18). 1,25(OH)2D2 interassay imprecision was 8.5% and 8% at levels of 67 and 219 pg/ml, respectively. Intact human fibroblast growth factor-23 (FGF-23) was measured using a commercially available ELISA kit (Kainos, Tokyo, Japan). The CV for the FGF-23 assay was <5%, as reported by the manufacturer.
1,25(OH)2D-24-Hydroxylase Assay
Fibroblasts were seeded into a 35-mm dish at 2×105 cells per well and incubated at 37°C. After 3 hours, 1 mM 1α,25(OH)2D3 in ethanol (Wako Pure Chemicals, Osaka, Japan) was added to a final concentration of 1 µM. After further incubation for 24 hours, culture medium and cells were recovered separately. Each metabolite was analyzed as described below. To obtain the metabolites of 1α,25(OH)2D3 using recombinant CYP24A1, a reconstituted system containing the membrane fraction of recombinant Escherichia coli cells expressing human CYP24A1 was utilized (19,20). The reaction mixture—containing 10 μM 1α,25(OH)2D3, 2.0 μM adrenodoxin, 0.2 μM adrenodoxin reductase, 20 nM CYP24A1, 1 mM nicotinamide adenine dinucleotide phosphate, 100 mM Tris HCl (pH 7.4), and 1 mM EDTA—was incubated at 37°C for 30 minutes. 1α,25(OH)2D3 and its metabolites were extracted using chloroform/methanol (3:1, v/v). The organic phase was dried under reduced pressure. The resultant residue was dissolved in acetonitrile and applied to an HPLC column under the following conditions: column, YMC-Pack ODS-AM (5 μm, 4.6×300 mm; YMC Co., Kyoto, Japan); ultraviolet detection, 265 nm; flow rate, 1.0 ml/min−1; column temperature, 40°C; and mobile phase, linear gradient of 20%–100% acetonitrile aqueous solution per 25 minutes followed by 100% acetonitrile for 20 minutes.
Results
Patients
Patient 1 (family I, patient I-2.2) is a 9-year-old boy with nephrocalcinosis diagnosed by routine renal ultrasonography performed because of a urinary tract infection at 3 years of age. Initial investigations outside the NIH showed hypercalciuria up to 8 mg/kg per day (normal <4.0 mg/kg per day) and increased plasma 1α,25(OH)2D3 to 86 pg/ml (normal 18–64 pg/ml). Repeated 24-hour urine evaluations revealed significant hypercalciuria, and no hyperuricosuria or oxaluria (Table 1), elevated serum calcium levels and appropriately suppressed intact PTH, as well as normal serum 25(OH)D2, elevated 1α,25(OH)2D3, and decreased 24,25-(OH)2D (Table 2). Renal ultrasonography performed at the NIH showed extensive medullary nephrocalcinosis. A dual energy x-ray absorptiometry scan yielded Z-scores of −1.4 for the spine, 0.2 for the femoral neck, and −1.3 for the forearm. The patient’s unaffected dizygotic twin brother did not have kidney stones or nephrocalcinosis, and neither did any other family member.
Table 1.
Patients’ 24-hour urine values
| Urine pH | Calcium Excretiona | Calcium/ Creatinine Ratioa | Phosphate Excretion (g/24 h) | Fractional Excretion of PO4 (%) | |
|---|---|---|---|---|---|
| Patient 1 | 6.5 | 9.3 mg/kg per day | 0.42 | 0.51 | 12 |
| Patient 2 | 6.0 | 160–405 mg/d | 0.33 | 0.97 | 34 |
These laboratory values were elevated compared with normal values.
Table 2.
Patients’ blood laboratory values
| Ionized Ca2+ (mmol/L) | Parathyroid Hormone (pg/ml) | Plasma Creatinine (mg/dl) | 25-OHD3 (ng/ml) | 1α,25(OH)2D3 (pg/ml) | 24,25(OH)2D (ng/ml) | |
|---|---|---|---|---|---|---|
| Patient 1 | 1.23–1.34 | 3 | 0.4 | 71 | 79–115 | 0.64 |
| Patient 2 | 1.32–1.41 | 3–10 | 1.2–1.3 | 39–59 | 83–160 | 0.33 |
| Normal reference rangea | 1.12–1.32 | 16–87 | 0.5–1.2 | 10–80 | 18–64 | 1.2–2.6b |
In the authors’ laboratories.
Obtained for this study.
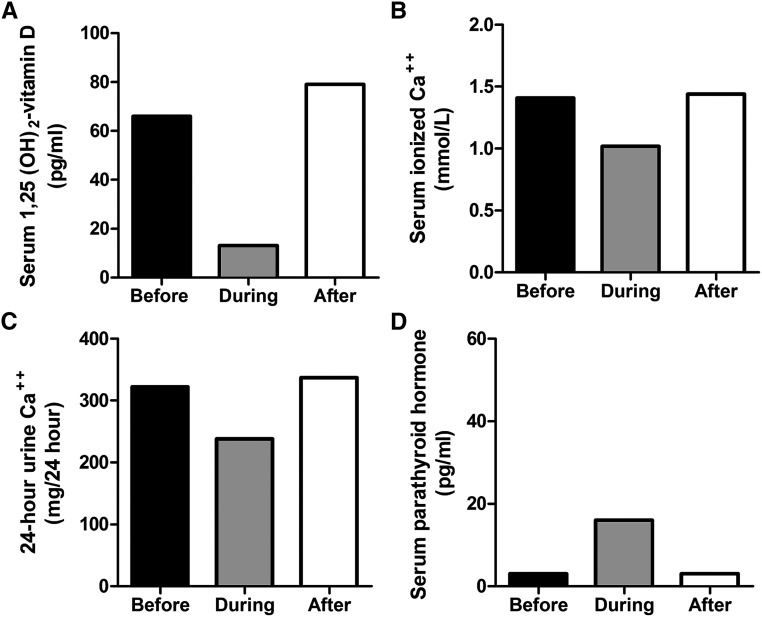
Patient 2 (family II, patient II-2.2), a 38 year-old man, had recurrent kidney stones since age 25 years, hypercalcemia, hypercalciuria, and marked elevation of 1α,25(OH)2D3. His 24-hour urine evaluations revealed hypercalciuria without hyperuricosuria or oxaluria (Table 1), high serum calcium levels and suppressed intact PTH, normal serum 25(OH)D3, high 1α,25(OH)2D3, and low 24,25-(OH)2D (Table 2). He had an elevated fractional excretion of phosphate (Table 1). An abdominal CT scan performed at age 34 years demonstrated a large renal calculus (25×34 mm) in the left kidney. Treatments included extracorporeal shockwave lithotripsy, percutaneous nephrolithotomy with subsequent residual stone fragments, Harrington rod placement for scoliosis, thiazide diuretics, sodium cellulose phosphate, and, after his admission to the NIH Clinical Center, ketoconazole. Stone analysis of patient 2, performed at the Mayo Clinic, demonstrated 100% calcium phosphate (brushite). A gallium scan showed no evidence of granulomatous disease. The dual energy x-ray absorptiometry Z-scores and T-scores were −2.8 and −2.8 in the spine, −1.3 and −1.8 in the femoral neck, and −2.8 and −3.0 in the forearm, respectively,. The plasma FGF-23 level, a possible contributing factor to the patient’s hyperphosphaturia, was 64 RU/ml, within the normal range (63±45 SD). The patient had decreased urine calcium after administration of sodium cellulose phosphate, confirming the presence of increased enteric calcium absorption (data not shown). On ketoconazole therapy (800 mg per day in divided doses), the patient’s serum 1,25(OH)2D3, serum calcium, and urine calcium decreased, and the PTH returned to normal (Figure 2). His male sibling (II-2.1) had a questionable history of renal stones.
Figure 2.
Effect of ketoconazole treatment in patient 2 on 1,25 vitamin D levels, hypercalcemia, hypercalciuria, and parathyroid hormone. Patient 2 was treated with ketoconazole and the dosage was escalated to 800 mg per day in divided doses. The effects monitored before the initiation of therapy, approximately 1 month after the initiation of therapy, and approximately 1 month after discontinuation. The following levels are displayed: (A) 1α,25(OH)2D3, (B) serum ionized calcium, (C) 24-hour urine calcium, and (D) serum parathyroid hormone.
Both probands adhered to low calcium and oxalate diets with no excess sun exposure and copious fluid intake. Multivitamins, vitamin D supplementations, and vitamin D fortified food had been largely eliminated in both patients.
Biochemical Studies
Fibroblast 1,25(OH)2D-24-hydroxylase activity was assayed by exposing cultured cells to 1α,25(OH)2D3 and measuring the 24-hydroxylated derivative. HPLC profiles of both patients’ fibroblasts showed no identifiable metabolites of 1α,25(OH)2D3, indicating negligible1,25(OH)2D-24-hydroxylase activity compared with non-stone forming controls (Figure 3A) (20). Moreover, CYP24A1 protein expression was reduced in the fibroblasts of both patient 1 and patient 2 (Figure 3, B and C) compared with a normal control.
Figure 3.
CYP24A1 activity in patient 1 and patient 2. CYP24A1 activity was measured in fibroblasts from patients 1 and 2 and compared with values for normal non-stone formers. (A) CYP24A1 activity was measured in fibroblasts from patients 1 and 2, and compared with that of normal non-stone formers (all fibroblasts are the same passage number 3). Fibroblasts from patient 1 (P1) and patient 2 (P2) produced no metabolites of 1α,25(OH)2D3. In contrast, normal fibroblasts produced metabolites that have the same retention time (18.4 minutes) of metabolites using recombinant CYP24A1 (as indicated by the arrow). Our previous study revealed that this peak contained 1-α,24R,25(OH)2D3 and 1-α,23S,25-(OH)2D3 in the ratio of 4:1 (19). Although we found no other metabolites in normal fibroblasts, further metabolites may be observed with increasing reaction time. The shoulder on the main substrate peak is the 6-s-cis form of 1- α,25(OH)2D3 generated by rotation around the 6,7 carbon bond. The interconversion between the 6-s-trans- and 6-s-cis-forms has a low energy barrier and therefore occurs rapidly in solution at room temperature. (B) CYP24A1 protein amount is reduced in patient 1 and patient 2. Western blot of whole fibroblast lysates probed with CYP24A1 antibody shows reduced protein amount in patients compared with normal. (C) β-actin serves as loading control quantification by densitometry.
Molecular Analyses
We focused our molecular analysis on candidate genes involved in vitamin D metabolism. CYP27B1 sequence analysis revealed no mutations in either patient, eliminating the possibility of constitutively upregulated production of 1α,25(OH)2D3.
However, sequencing of CYP24A1 in patient 1 revealed a heterozygous 3-bp deletion (c.428_430del; p.E143del) inherited from his mother (I-1.2) and predicted to remove a highly conserved glutamate at position 143 (Figure 4; I-2.2). Patient 1 also had a heterozygous transition of T to C (c.443T>C), inherited from his father (I-1.1) and altering a moderately conserved amino acid (p.L148P). His unaffected twin (I-2.1) harbored only the p.E143del mutation. Patient 2 exhibited bi-allelic mutations in CYP24A1, including the p.E143del deletion that he inherited from his father (Figure 4; II-1.1). The second mutation was a heterozygous transition in exon 9 (c.1226T>C), changing a moderately conserved amino acid (p.L409S); his mother (II-1.2) and brother (II-2.1) were heterozygous for this variant.
Figure 4.
Family pedigree and molecular analysis. The pedigrees of the two families are illustrated. Affected members are shown in black squares, whereas unaffected members are denoted in white solid objects. Patient 1 (I-2.2) harbored a 3-bp deletion, p.E143del, inherited from his mother (I-1.2), and a p.L148P missense mutation that was inherited from his father (I-1.1). Patient 2 (II-2.2) also had the same p.E143del inherited from his father (II-1.1), and a p.L409S inherited from his mother (II-1.2).
Frequency of CYP24A1 Mutations
A search of dbSNP for CYP24A1 variants identified 37 nonsynonymous single nucleotide polymorphisms (SNPs). Reported MAFs within this group varied between 0.001 and 0.075 (19 SNPs); 16 SNPs had not reported MAFs, (Table 3 and Supplemental Table). We applied six pathogenicity prediction programs (Blossum 62, POLYPHEN, SIFT, Pmut, Panther, and SNAP) to estimate the deleteriousness of each variant (Supplemental Table 1).
Table 3.
Reported CYP24A1 mutations and likely deleterious nonsynonymous variants in dbSNP
| Reported Disease-Causing Mutations | dbSNP ID | Minor Allele Frequencya |
|---|---|---|
| p.L148Pb | rs139763321 | — |
| p.R396Wc | rs114368325 | 0.001 |
| p.E143delb,c,d | — | 0.002d |
| p.L409Sb,c | rs6068812 | 0.003 |
| p.E151× | — | — |
| p.R159Qc | — | — |
| p.E322Kc | — | — |
| p.A475fsX490c | — | — |
| c.732+1G>A (splice site mutation)d | — | — |
| c.733–2A>G (splice site mutation)d | — | — |
| Deleterious variants in dbSNP | ||
| p.R120H | rs114476330 | 0.009 |
| p.P126S | rs148084028 | — |
| p.R157W | rs35873579 | 0.035 |
| p.P375L | rs189801930 | — |
| p.M374T | rs6022990 | 0.075 |
| p.C380Y | rs150006710 | 0.001 |
| p.R396Qc | rs143934667 | — |
| p.Y407N | rs140189382 | — |
| p.R439H | rs141152573 | 0.001 |
| p.V457I | rs112596218 | 0.002 |
| p.R481C | rs143523685 | — |
| p.A510V | rs116065115 | 0.011 |
| Total deleterious minor allele frequency | 0.140 |
Minor allele frequency, as reported in dbSNP (March 2012). Dashes indicate no minor allele frequency reported in dbSNP or not computed in Exome Sequencing Project cohort populations (4548 control chromosomes, National Heart Lung and Blood Institute Exome Sequencing Project).
Decreased CYP24A1 enzyme activity; reported in this study, measured in fibroblasts.
Enzyme activity decreased, measured in mutants expressed in vitro.
Thirteen of the SNPs were considered likely deleterious (i.e., evaluated by POLYPHEN to have an 80%–90% chance of causing a functional defect in enzyme activity)s (Table 3). The cumulative allele frequency of these variants was 0.140. If the variant with unusually high MAF, p.M374T, was discarded, then the cumulative frequency was 0.065. These estimates do not include the 16 variants in dbSNP considered neutral or possibly deleterious by POLYPHEN, or those listed without frequencies (Supplemental Table 1). Published disease-causing mutations were also included in Supplemental Table 1; they were not reported in dbSNP as of March 2012.
Discussion
Nephrolithiasis represents a major global health problem with a lifetime prevalence estimated at 10%–15%, depending upon age, sex, race, and geographic location (21–23). Family history of stones has been reported in close to 40% of patients with nephrolithiasis (24,25). In contrast, the frequency of nephrocalcinosis is not available because most patients are asymptomatic, but detection of nephrocalcinosis is increasing due to the performance of routine diagnostic renal ultrasonography (26,27). Prevention of nephrocalcinosis and nephrolithiasis could have widespread beneficial effects, but prophylaxis should be based on understanding the etiologies.
Both nephrocalcinosis and nephrolithiasis exhibit hypercalciuria as a manifestation of abnormal calcium handling, which can be related to the level of 1α,25(OH)2D3, or calcitriol. This hormone stimulates the synthesis of epithelial calcium channels, calbindin 9, and the calcium adenosine triphosphatase pump to upregulate calcium absorption by the kidney and intestine. Elevated 1α,25(OH)2D3 can also adversely affect the kidney, because calcium ions alter mitochondrial structure and metabolism, causing damage to renal epithelial cells, tubular necrosis, and calcium deposition (10). In the intestine, excess 1α,25(OH)2D3 leads to hyperabsorptive hypercalcemia and hypercalciuria.
Because vitamin D 24-hydroxylase is the key regulator in preventing the development of high levels of 1α,25(OH)2D3 (28), we pursued a defect in this enzyme as the cause of nephrocalcinosis and nephrolithiasis in our patients. Several lines of evidence supported this hypothesis. First, both of our patients had increased 1α,25(OH)2D3 and very low levels of 24,25-(OH) 2D in the blood (Table 2). Low PTH detected in our patients is not commonly seen in patients with high urine calcium and high serum calcium. Second, the cultured fibroblasts of both patients showed diminished 1,25(OH)2D-24-hydroxylase activity (Figure 3A) and reduced amounts of Cyp24A1 protein (Figure 3, B and C). The occurrence of small concentrations of 24,25(OH)2D in serum could be explained by the presence of another enzyme, Cyp27A1, which catalyzes multiple hydroxylation steps involving vitamin D metabolites (29). The naturally occurring 24,25(OH)2D has the 24(R) configuration (30)(Figure 1).
Finally, our patients had bi-allelic mutations in the CYP24A1 gene. Patient 1 has a p.E143del, a known deleterious change, and a second variant, p.L148P. Residue 148 directly interacts with the enzyme’s substrate, and the L148P change decreases enzyme activity by 25%–50% (31,32). Patient 2 has the p.E143del as well as the previously reported p.L409S mutation (31). p.L409S weakens the binding of 1,25-dihydroxyvitamin D to 1,25(OH)2D-24-hydroxylase (32,33). The p.E143del mutation was reported by Schlingmann et al. in patients with increased sensitivity to vitamin D supplementation (11), and the same mutation has been found in patients with nephrolithiasis (34). Our pathogenicity and frequency assessment of the nonsynonymous CYP24A1 variants reported in dbSNP predicts that bi-allelic pathogenic defects in CYP24A1 may account for a significant portion of all calcium-containing renal stone patients.
Human CYP24A1 contains 514 amino acids and possesses both 23- and 24-hydroxylating activity (Figure 1) (8). The purified CYP24A1 has an absorption spectrum characteristic of P450 enzymes (35). The 1,25(OH)2D-24-hydroxylase molecule resides within the mitochondria of renal tubular cells of normal kidney (36) and is expressed by most 1α25(OH)2D3-responsive tissues (37). After the intestine and kidney, the skin has the highest CYP24A1 expression (38). The abundance of this enzyme in tissues plays a critical role in the removal of vitamin D metabolites (39). Studies of the Cyp24a1-null mouse also support a catabolic role for CYP24A1, because the clearance of 1α25(OH)2D3 is dramatically reduced in these mice; the plasma t1/2 increased from 6 to 60 h when CYP24A1 was absent. (31,37) In addition, mutant rat Cyp24a1 had less hydroxylating activity than the wild-type enzyme (40).
Despite the fact that CYP24A1 has equivalent Km values for 25(OH)D3 and 1α,25(OH)2D3 (41), our patients had normal levels of 25(OH)D3 in the face of elevated 1α,25(OH)2D3. This could be explained by the fact that 25(OH)D3 can undergo hydroxylation by two different enzymes, CYP27B1 and CYP24A1, whereas 1α,25(OH)2D3 undergoes catabolic hydroxylation only by CYP24A1.
Besides nephrocalcinosis and nephrolithiasis, osteopenia may be a clinical manifestation of 1,25(OH)2D-24-hydroxylase deficiency. Our pediatric patient manifested osteopenia of the spine and radius, and our adult patient had osteoporosis of the spine and forearm as well as osteopenia of the femoral neck. This phenomenon may be because 1α,25(OH)2D3 stimulates osteoclastic resorption of bone (42), a finding long recognized to occur in vitamin D intoxication (43).
Our adult patient with nephrolithiasis attempted several different therapies. Thiazide diuretics and sodium cellulose phosphate apparently had beneficial effects on the hypercalciuria. Ketoconazole, an inhibitor of 25-hydroxyvitamin D-1α-hydroxylase, normalized calcium, vitamin D, and PTH levels and may be an effective treatment for patients with 24-hydroxylase deficiency (12). However, additional studies are needed to assess the efficacy and safety of this regimen (44) because ketoconazole also inhibits other P450 enzymes, including the steroidogenic pathways producing testosterone, cortisol, and aldosterone (45). Extended use of ketoconazole in 1,25(OH)2D-24-hydroxylase deficiency may be problematic, although its long-term safety and efficacy in Cushing’s syndrome (up to 83 months) appears encouraging (46,47).
Previous reports have shown CYP24A1 mutations in children whose high vitamin D intake led to idiopathic infantile hypercalcemia (IIH) (11,48), and others hypothesized that CYP24A1 dysregulation causes hypercalcemia and nephrolithiasis (31). We have shown that CYP24A1 mutations are, in fact, associated with both nephrocalcinosis and nephrolithiasis, and have extended the phenotypic spectrum of CYP24A1 defects. Our findings are derived from investigations into patients with suspected novel metabolic disorders; we did not specifically target patients with renal disorders or disorders of vitamin D metabolism.
Genome-wide association studies have demonstrated the influence of CYP24A1 variants on vitamin D concentrations (49). In our patients, bi-allelic mutations in CYP24A1 (50) represent an autosomal recessive disorder involving enzyme deficiency. The sum of the MAFs of published mutations plus mutations reported as deleterious in dbSNP is 0.140. According to the Hardy-Weinberg principal [(p2) + (2pq) + (q2) = 1], the frequency of a recessive disorder with this allele frequency will be (0.140 (2)), or 1960 per 100,000 individuals in the general population. This includes the p.M374T mutation with an MAF of 0.075 (Table 3). If this most common variant is disregarded, the frequency of 1,25(OH)2D-24-hydroxylase deficiency is estimated at 420 per 100,000. The lifetime risk of renal stones in the general population is 10%, or 10,000 per 100,000, so the estimated frequency of kidney stones due to 1,25(OH)2D-24-hydroxylase deficiency will be between 420 and 1960 per 10,000, or 4%–20%. This may be an overestimate; however, some CYP24A1 polymorphisms may be associated with mild and more severe diseases, including IIH (31). Nevertheless, recognition of 1,25(OH)2D-24-hydroxylase has important implications (51), particularly in patients who have high urine calcium, high serum calcium, and low PTH, a pattern that is not commonly seen by experienced clinicians. Further studies involving a larger number of kidney stone formers are needed to determine if the hydroxylated forms of vitamin D (not 25(OH)D3 levels) should be routinely measured in patients with nephrocalcinosis, nephrolithiasis, and hypercalciuria of undetermined etiology.
In summary, we demonstrate that one cause of nephrocalcinosis and nephrolithiasis is elevated vitamin D due to 1,25(OH)2D-24-hydroxylase deficiency. Our analysis of whole exome sequencing data suggests that between 4% and 20% of all calcium-containing kidney stone patients may have this enzyme deficiency. Identification of patients with vitamin 1,25(OH)2D-24-hydroxylase deficiency could prompt salutary avoidance of vitamin D–supplemented dietary products. Our study was limited by having clinical and molecular data on only two patients, but the findings provide a basis for future investigations into the mechanism of nephrolithiasis related to vitamin D metabolism.
Disclosures
None.
Acknowledgments
The authors thank Genia Dubrovsky, Patra Yeetong, and Dimitre Simeonov for their technical assistance in sequencing analysis.
This work was supported by the Intramural Research Program, National Human Genome Research Institute, National Institute of Dental and Craniofacial Diseases, and Undiagnosed Diseases Program, and Office of Rare Disorders Research, National Institutes of Health, Bethesda, Maryland.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05360512/-/DCSupplemental.
References
- 1.Sayer JA, Carr G, Simmons NL: Nephrocalcinosis: Molecular insights into calcium precipitation within the kidney. Clin Sci (Lond) 106: 549–561, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Vervaet BA, Verhulst A, D’Haese PC, De Broe ME: Nephrocalcinosis: New insights into mechanisms and consequences. Nephrol Dial Transplant 24: 2030–2035, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G: Urolithiasis through the ages: Data on more than 200,000 urinary stone analyses. J Urol 185: 1304–1311, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Miller NL, Humphreys MR, Coe FL, Evan AP, Bledsoe SB, Handa SE, Lingeman JE: Nephrocalcinosis: Re-defined in the era of endourology. Urol Res 38: 421–427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stechman MJ, Loh NY, Thakker RV: Genetics of hypercalciuric nephrolithiasis: Renal stone disease. Ann N Y Acad Sci 1116: 461–484, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cioppi F, Taddei L, Brandi ML, Croppi E: Idiopathic hypercalciuria and calcium renal stone disease: Our cases. Clin Cases Miner Bone Metab 6: 251–253, 2009 [PMC free article] [PubMed]
- 7.Curhan GC, Willett WC, Speizer FE, Stampfer MJ: Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 59: 2290–2298, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Sakaki T, Kagawa N, Yamamoto K, Inouye K: Metabolism of vitamin D3 by cytochromes P450. Front Biosci 10: 119–134, 2005 [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF: Metabolism of vitamin D: Current status. Am J Clin Nutr 29: 1258–1270, 1976 [DOI] [PubMed] [Google Scholar]
- 10.Scarpelli DG, Tremblay G, Pearse AGE: A comparative cytochemical and cytologic study of vitamin D induced nephrocalcinosis. Am J Pathol 36: 331–353, 1960 [PMC free article] [PubMed] [Google Scholar]
- 11.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M: Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R: Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: Effects of ketoconazole therapy. J Clin Endocrinol Metab 97: E423–E427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gahl WA, Tifft CJ: The NIH Undiagnosed Diseases Program: Lessons learned. JAMA 305: 1904–1905, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G, Wolfe L, Groden C, Godfrey R, Nehrebecky M, Wahl C, Landis DM, Yang S, Madeo A, Mullikin JC, Boerkoel CF, Tifft CJ, Adams D: The National Institutes of Health Undiagnosed Diseases Program: Insights into rare diseases. Genet Med 14: 51–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashima A: Establishment of fibroblast cultures. Curr Protoc Cell Biol Chapter 2: Unit 2.1, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, Zivony-Elboum Y, Gumruk F, Cetin M, Khayat M, Boerkoel CF, Kfir N, Huang Y, Maynard D, Dorward H, Berger K, Kleta R, Anikster Y, Arat M, Freiberg AS, Kehrel BE, Jurk K, Cruz P, Mullikin JC, White JG, Huizing M, Gahl WA: NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet α-granules. Nat Genet 43: 732–734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horst RL, Littledike ET, Gray RW, Napoli JL: Impaired 24,25-dihydroxyvitamin D production in anephric human and pig. J Clin Invest 67: 274–280, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strathmann FG, Laha TJ, Hoofnagle AN: Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 57: 1279–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe D, Sakaki T, Kusudo T, Kittaka A, Saito N, Suhara Y, Fujishima T, Takayama H, Hamamoto H, Kamakura M, Ohta M, Inouye K: Metabolism of 2 alpha-propoxy-1 alpha,25-dihydroxyvitamin D3 and 2 alpha-(3-hydroxypropoxy)-1 alpha,25-dihydroxyvitamin D3 by human CYP27A1 and CYP24A1. Drug Metab Dispos 33: 778–784, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kusudo T, Sakaki T, Abe D, Fujishima T, Kittaka A, Takayama H, Hatakeyama S, Ohta M, Inouye K: Metabolism of A-ring diastereomers of 1alpha,25-dihydroxyvitamin D3 by CYP24A1. Biochem Biophys Res Commun 321: 774–782, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri A: Epidemiology of urolithiasis: An update. Clin Cases Miner Bone Metab 5: 101–106, 2008 [PMC free article] [PubMed] [Google Scholar]
- 22.Curhan GC: Epidemiology of stone disease. Urol Clin North Am 34: 287–293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero V, Akpinar H, Assimos DG: Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol 12: e86–e96, 2010 [PMC free article] [PubMed] [Google Scholar]
- 24.Trinchieri A, Mandressi A, Luongo P, Coppi F, Pisani E: Familial aggregation of renal calcium stone disease. J Urol 139: 478–481, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Rimm EB, Stampfer MJ: Family history and risk of kidney stones. J Am Soc Nephrol 8: 1568–1573, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Sas DJ: An update on the changing epidemiology and metabolic risk factors in pediatric kidney stone disease. Clin J Am Soc Nephrol 6: 2062–2068, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Habbig S, Beck BB, Hoppe B: Nephrocalcinosis and urolithiasis in children. Kidney Int 80: 1278–1291, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Masuda S, Prosser DE, Guo YD, Kaufmann M, Jones G: Generation of a homology model for the human cytochrome P450, CYP24A1, and the testing of putative substrate binding residues by site-directed mutagenesis and enzyme activity studies. Arch Biochem Biophys 460: 177–191, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sawada N, Sakaki T, Ohta M, Inouye K: Metabolism of vitamin D(3) by human CYP27A1. Biochem Biophys Res Commun 273: 977–984, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, DeLuca HF, Ikekawa N, Morisaki M, Koizumi N: Determination of stereochemical configuration of the 24-hydroxyl group of 24,25-dihydroxyvitamin D3 and its biological importance. Arch Biochem Biophys 170: 620–626, 1975 [DOI] [PubMed] [Google Scholar]
- 31.Jones G, Prosser DE, Kaufmann M: 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys 523: 9–18, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann M, Prosser DE, Jones G: Bioengineering anabolic vitamin D-25-hydroxylase activity into the human vitamin D catabolic enzyme, cytochrome P450 CYP24A1, by a V391L mutation. J Biol Chem 286: 28729–28737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji HF, Shen L: CYP24A1 mutations in idiopathic infantile hypercalcemia. N Engl J Med 365: 1741; author reply 1742–1743, 2011 [DOI] [PubMed]
- 34.Streeten EA, Zarbalian K, Damcott CM: CYP24A1 mutations in idiopathic infantile hypercalcemia. N Engl J Med 365: 1741–1742, author reply 1742–1743, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Okuda K, Usui E, Ohyama Y: Recent progress in enzymology and molecular biology of enzymes involved in vitamin D metabolism. J Lipid Res 36: 1641–1652, 1995 [PubMed] [Google Scholar]
- 36.Iwata K, Yamamoto A, Satoh S, Ohyama Y, Tashiro Y, Setoguchi T: Quantitative immunoelectron microscopic analysis of the localization and induction of 25-hydroxyvitamin D3 24-hydroxylase in rat kidney. J Histochem Cytochem 43: 255–262, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Akeno N, Saikatsu S, Kawane T, Horiuchi N: Mouse vitamin D-24-hydroxylase: Molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology 138: 2233–2240, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N: Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J Endocrinol 164: 339–348, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Anderson PH, May BK, Morris HA: Vitamin D metabolism: New concepts and clinical implications. Clin Biochem Rev 24: 13–26, 2003 [PMC free article] [PubMed] [Google Scholar]
- 40.Annalora AJ, Bobrovnikov-Marjon E, Serda R, Pastuszyn A, Graham SE, Marcus CB, Omdahl JL: Hybrid homology modeling and mutational analysis of cytochrome P450C24A1 (CYP24A1) of the vitamin D pathway: Insights into substrate specificity and membrane bound structure-function. Arch Biochem Biophys 460: 262–273, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annalora A, Bobrovnikova-Marjon E, Serda R, Lansing L, Chiu ML, Pastuszyn A, Iyer S, Marcus CB, Omdahl JL: Rat cytochrome P450C24 (CYP24A1) and the role of F249 in substrate binding and catalytic activity. Arch Biochem Biophys 425: 133–146, 2004 [DOI] [PubMed] [Google Scholar]
- 42.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH: Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141: 2658–2666, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Davies M, Mawer EB, Freemont AJ: The osteodystrophy of hypervitaminosis D: A metabolic study. Q J Med 61: 911–919, 1986 [PubMed] [Google Scholar]
- 44.Nguyen M, Boutignon H, Mallet E, Linglart A, Guillozo H, Jehan F, Garabedian M: Infantile hypercalcemia and hypercalciuria: New insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. J Pediatr 157: 296–302, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Peehl DM, Seto E, Hsu JY, Feldman D: Preclinical activity of ketoconazole in combination with calcitriol or the vitamin D analogue EB 1089 in prostate cancer cells. J Urol 168: 1583–1588, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Nieman L. Prolonged remission after long-term treatment with steroidogenesis inhibitors in Cushing's syndrome caused by ectopic ACTH secretion. Eur J Endocrinol 166: 531–536, 2012 [DOI] [PMC free article] [PubMed]
- 47.Chou SC, Lin JD: Long-term effects of ketoconazole in the treatment of residual or recurrent Cushing’s disease. Endocr J 47: 401–406, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Dauber A, Nguyen TT, Sochett E, Cole DE, Horst R, Abrams SA, Carpenter TO, Hirschhorn JN: Genetic defect in CYP24A1, the vitamin D 24-hydroxylase gene, in a patient with severe infantile hypercalcemia. J Clin Endocrinol Metab 97: E268–E274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD: Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 376: 180–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K: dbSNP: The NCBI database of genetic variation. Nucleic Acids Res 29: 308–311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenter TO: Take another CYP: Confirming a novel mechanism for “idiopathic” hypercalcemia. J Clin Endocrinol Metab 97: 768–771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]