Summary
Background
Critically ill children are at high risk of underfeeding and AKI, which may lead to further nutritional deficiencies. This study aimed to determine the adequacy of nutrition support during the first 5 days of intensive care unit (ICU) stay.
Design, setting, participants, & measurements
A chart review of pediatric patients admitted to the pediatric ICU for >72 hours between August 2007 and March 2008 was conducted. Patients were classified as having no AKI versus AKI by modified pediatric RIFLE criteria. All nutrition was analyzed. Basal metabolic rate (BMR) was estimated by the Schofield equation and protein needs by American Society for Parenteral and Enteral Nutrition guidelines.
Results
Of the 167 patients, 102 were male and 65 were female (median age 1.4 years). Using the RIFLE criteria, 102 (61%) patients had no AKI, whereas 44 (26%) were classified as category R (risk), 12 (7%) as category I (injury), and 9 (5%) as category F (failure). The median 5-day energy intake was lower relative to estimated BMR. Overall protein provision (19%) was lower than energy provision (55%) compared with estimated needs (P<0.001). I/F patients were more likely to be fasted versus receiving enteral/parenteral nutrition (n=813 patient days) and to receive <90% of BMR (n=832 patient days) than No AKI/R patients.
Conclusions
Underfeeding, common in critically ill children, was accentuated in AKI. Protein underfeeding was greater than energy underfeeding in the first 5 days of PICU stay. Efforts should be made to provide adequate nutrition in ICU patients with AKI.
Introduction
Critically ill children are at high risk of underfeeding early in their intensive care unit (ICU) stay. Hospital undernutrition is a known risk factor for morbidity and mortality in children, adolescents, and adults (1,2). Malnutrition has been shown to affect patient outcome and represents a continuous spectrum ranging from marginal nutrient status to severe metabolic and functional alterations, with different degrees of relative alterations of body weight and body composition (3).
AKI is very common in critically ill children, especially in the first 5 days of pediatric ICU (PICU) stay, and occurs in 80% of patients with cardiorespiratory failure (4). Patients with AKI are at high risk of malnutrition, and the presence of malnutrition in the context of AKI has been linked to increased morbidity and mortality (5–7). Almost half of adult patients with AKI have severe malnutrition on admission to the ICU (6). Physiologic changes due to AKI cause derangements in substrate metabolism and body composition (8), leading to hypercatabolism (9), hypoalbuminemia, loss of lean body mass, and depletion of adipose tissue, even with adequate ingestion of nutrients (10). Muscle protein catabolism and atrophy occur as a result of coexisting systemic inflammation, oxidative stress, insulin resistance, and metabolic acidosis (11). Systemic inflammation has been shown to increase muscle protein breakdown, decrease albumin synthesis, and redirect protein utilization toward hepatic acute-phase protein synthesis rather than anabolism (11). Maintenance of protein balance in such conditions requires that at least adequate energy and protein intake be provided during acute illnesses.
In critically ill children, nutrition support is often deferred until patients are medically stabilized, which may delay adequate nutrition support for several days due to fluid restriction, digestive intolerance, and interruption of feeding for diagnostic and therapeutic procedures (12). However, patient outcome and survival are dependent on adequate nutrition and restoration of energy balance (13), and pediatric patients are highly dependent on nutritional support due to their intrinsic high anabolic drive and lower nutrient reserves compared with adults. AKI, like most other organ dysfunctions, happens very early during pediatric critical illness (4,14) and it is associated with more severe clinical deterioration and organ dysfunction (15). Timely and adequate energy and protein must be provided to critically ill pediatric patients to optimize tissue healing and organ recovery and to limit the effect of critical illness on their growth potential.
The purpose of this study was to determine the adequacy of nutrition support during the first 5 days of ICU stay in patients with AKI and whether there was an association between AKI and nutrition support.
Materials and Methods
All consecutive patients aged <18 years admitted to the Texas Children’s Hospital PICU for >72 hours between August 2007 and March 2008 were eligible. We excluded patients who were admitted to the PICU >48 hours after hospital admission (n=52), patients who had >1 PICU admission (n=18), patients whose medical charts were not available (n=12), and patients with CKD requiring renal replacement therapy (n=7).
The chart review included the following: age, sex, height, admission weight, admission diagnosis, duration of mechanical ventilation, medications, intravenous fluids, length of ICU and hospital stay, and outcome. Nutrition data included the day of initiation of nutrition support, as well as the route, type, and quantity of enteral/parenteral nutrition solutions for 5 days. Actual energy and protein intakes were calculated until oral intake was started. Patients who were NPO (nothing by mouth) on admission were included in the calculations. As patients improved, they were taken off nutrition support and started oral intake. Once oral intake was started, the patient-days were no longer included in the calculations, because it was not possible to evaluate actual oral intake in this retrospective study.
Estimated energy and protein needs were determined according to the Schofield prediction equation (basal metabolic rate [BMR]) (16) as well as the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) guidelines for critically ill children (17), respectively. Moderate/severe acute malnutrition was defined as weight for age ≤2 z-scores and moderate/severe chronic malnutrition or growth stunting was defined as height for age ≤2 z-scores, using the 2000 Centers for Disease Control and Prevention (CDC) growth charts (18). Overweight/obesity was defined as weight for age ≥2 z-scores, using the 2000 CDC growth charts (18).
Patients were classified as having no AKI versus risk, injury, and failure by the modified pediatric RIFLE (pRIFLE) criteria categories R, I, and F, respectively (4). Only the serum creatinine criteria were used for modified pRIFLE designation because urine output data were not available. For the nutrition support outcome data analysis, we combined the patients with no AKI and patients at risk into one group (no AKI/R), and patients with injury and failure into another group (I/F).
Severity of illness was assessed by the Pediatric Risk of Mortality Score (PRISM III score) (19). Organ dysfunction was assessed with the Pediatric Logistic Organ Dysfunction (PELOD) score (20).
The study was approved by the Baylor College of Medicine Institutional Review Board, who granted a waiver of informed consent for this study.
Statistical Analyses
Normally distributed continuous variables were compared using paired and unpaired t tests and ANOVA. Non-normally distributed variables were compared by the Mann–Whitney U test, Wilcoxon signed-rank test, or Kruskal–Wallis test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. Box plots are medians (25th and 75th percentiles) and 95% confidence intervals (CIs). Odds ratios (ORs) with 95% CIs of selected factors associated with morbidity were determined by simple and multiple logistic regression models. The analyses for the ORs were performed for day 5. Values are mean ± SD for normally distributed data, and median (25th–75th percentile) for non-normally distributed data. Statistical significance was defined as P<0.05. Statistical analysis was performed with Statview and JMP software (SAS Institute Inc, Cary, NC).
Results
Of 167 patients, 61% were male. The median age was 1.4 years (25th–75th percentile, 0.3–9.4) . Slightly more than half of these patients (53%; n=89) were aged <2 years. Admission diagnoses were respiratory distress/failure in 50% (n=83), sepsis/septic shock/infection in 18% (n=30), cancer in 8% (n=14), gastrointestinal/hepatic in 4% (n=7), and miscellaneous in 17% (n=29). Sixty-nine percent (n=116) of patients required mechanical ventilation and 16% (n=26) were on vasopressors at admission. Miscellaneous comorbidities or chronic conditions were reported in 60% of patients.
Using the modified pRIFLE criteria, 61% of patients (n=102) were classified as having no AKI, 26% (n=44) as category R, 7% (n=12) as category I, and 5% (n=9) as category F. Height, height percentile, weight, weight percentile, and body mass index did not differ between the No AKI, R, and I/F groups (Table 1).
Table 1.
Demographic and clinical characteristics of patients
| No AKI | Risk | Injury/Failure | |
|---|---|---|---|
| Total (male/female) | 102 (62/40) | 44 (29/15) | 21 (10/11) |
| Age (yr) | 1.7 (0.4–9.5) | 0.8 (0.2–5.5) | 2.0 (0.4–11.4) |
| Height (cm) | 83.0 (62.5–131.0) | 69.0 (56.5–108.2) | 86.0 (60.5–145.1) |
| Height (percentile) | 27.0 (4.0–71.0) | 27.5 (0.5–73.5) | 27.0 (2.8–77.3) |
| Weight (kg) | 11.4 (6.1–32.4) | 7.8 (4.6–23.3) | 12.7 (6.4–40.5) |
| Weight (percentile) | 36.0 (12.0–73.0) | 32.0 (2.5–69.5) | 22.0 (1.8–81.3) |
| IBW (percentile) | 100.0 (91.0–111.0) | 100.0 (90.8–116.5) | 102.0 (85.0–113.8) |
| BMI (kg/m2) | 16.8 (15.0–19.5) | 16.0 (14.4–18.3) | 16.4 (14.3–21.0) |
| PRISM III | 3 (1–7) | 6 (3–11)a | 7 (5–10.3)b |
| PELOD | 10 (1–12) | 11 (1.5–12) | 11 (10–20.3) |
| PICU LOS | 6.5 (5–10) | 8 (5.5–12) | 7 (4–20.8) |
| Hospital LOS | 13 (9–23) | 14 (11–22.5) | 20 (8.8–41.3) |
Data are median (25th–75th percentile). IBW, ideal body weight; BMI, body mass index; PRISM III, Pediatric Risk of Morality Score; PELOD, Pediatric Logistic Organ Dysfunction; PICU, pediatric intensive care unit; LOS, length of stay.
Mann–Whitney test, risk versus no risk (P=0.02).
Mann–Whitney test, injury/failure versus no risk (P=0.02).
Twenty percent (n=33) of all patients and 33% (n=7) of I/F patients were classified as having acute malnutrition (chi-squared 2.0; P=0.04). I/F patients were more likely to show acute malnutrition than patients with no AKI (OR, 3.1; CI, 1.1–9.1; P=0.03) (Table 2). Patients with an R classification (OR, 2.7; CI 1.2–5.9; P=0.02), but not I/F patients (OR, 1.5; CI, 0.5–4.5; P=0.51), were significantly more likely to show chronic malnutrition (growth stunting) than patients with no AKI (Table 2). Eight percent (n=13) of patients were overweight/obese.
Table 2.
ORs in patients with AKI compared with no AKI
| % (n) | % (n) | OR (CI) | P | |
|---|---|---|---|---|
| Acute malnutrition | Normal | Moderate/severe | ||
| No AKI | 86.3 (88) | 13.7 (14) | 1 | |
| Risk | 72.7 (32) | 27.3 (12) | 2.4 (0.99–5.6) | 0.05 |
| Injury/failure | 66.7 (14) | 33.3 (7) | 3.1 (1.1–9.1) | 0.03 |
| Chronic malnutrition | ||||
| No AKI | 82.4 (84) | 17.6 (18) | 1 | |
| Risk | 63.6 (28) | 36.4 (16) | 2.7 (1.2–5.9) | 0.02 |
| Injury/failure | 76.2 (16) | 23.8 (5) | 1.5 (0.5–4.5) | 0.51 |
| PRISM III | ≤6 | >7 | ||
| No AKI | 71.2 (74) | 28.8 (30) | 1 | |
| Risk | 52.3 (23) | 47.7 (21) | 2.3 (1.1–4.7) | 0.03 |
| Injury/failure | 38.1 (8) | 61.9 (13) | 4.0 (1.5–10.7) | 0.01 |
| PELOD | ≤21 | >22 | ||
| No AKI | 98.0 (100) | 2.0 (2) | 1 | |
| Risk | 93.2 (41) | 6.8 (3) | 3.7 (0.6–22.7) | 0.17 |
| Injury/failure | 81.0 (17) | 19.0 (4) | 11.8 (2.0–69.3) | 0.01 |
| Hospital LOS | ≤14 | >15 | ||
| No AKI | 61.8 (63) | 38.2 (39) | 1 | |
| Risk | 52.3 (23) | 47.7 (21) | 1.5 (0.7–3.0) | 0.29 |
| Injury/failure | 33.3 (7) | 66.7 (14) | 3.2 (1.2–8.7) | 0.02 |
| Survival | Alive | Died | ||
| No AKI | 97.1 (99) | 2.9 (3) | 1 | |
| Risk | 100 (44) | 0 (0) | ||
| Injury/failure | 85.7 (18) | 14.3 (3) | 5.5 (1.0–29.4) | 0.05 |
Moderate/severe wasting is weight for age <2 z-scores below normal. Moderate/severe stunting is height for age <2 z-scores below normal, using the 2000 Centers for Disease Control and Prevention growth charts. OR, odds ratio; CI, confidence interval; PRISM III, Pediatric Risk of Mortality Score; PELOD, Pediatric Logistic Organ Dysfunction; LOS, length of hospital stay.
Patients classified as R and I/F had higher PRISM III scores than patients with no AKI (P<0.03). Patients with I/F were significantly more likely to have a PRISM III score >7 (OR, 4.0; CI, 1.5–10.7; P=0.01) and a PELOD score >22 (OR, 11.8; CI, 2.0–69.3; P=0.01) (Table 2). I/F patients were more likely to have a hospital length of stay (LOS) >15 days (OR, 3.2; CI, 1.2–8.7; P=0.02) than patients with no AKI (Table 2). There was no observed PICU mortality. Nonsurvival at hospital discharge was significantly higher in I/F patients (14.3%; n=3) than R patients (0%; n=0) or patients with no AKI (2.9%; n=3) (P=0.05) (Table 2).
Estimated Needs versus Cumulative Actual Energy and Protein Intake
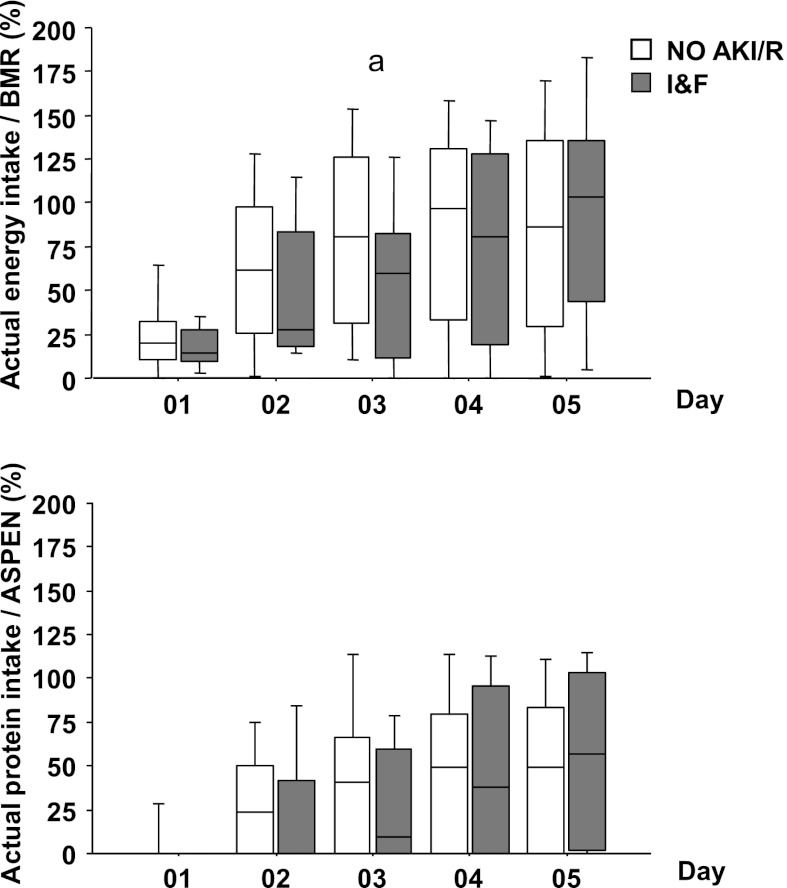
The actual energy intake was significantly lower in the I/F group compared with the No AKI/R group on day 3 only (P<0.03) (Figure 1). Overall, the actual and the percentage of BMR median 5-day energy intake were lower in the I/F patients than in the patients with no AKI (P<0.03) (Table 3). The actual median 5-day energy intake was lower relative to estimated BMR across all groups (No AKI/R, 24.9 kcal/kg per day [9.3–56.3] versus 48.5 kcal/kg per day [38.2–53.9]; I/F, 16.0 kcal/kg per day [5.5–46.5] versus 46.3 kcal/kg per day [33.2–54.5]; all P<0.001) (Table 3). The actual median 5-day protein intake was also lower than protein recommendations in all groups (No AKI/R, 0.50 g/kg per day [0–1.36] versus 2.5 g/kg per day [1.8–2.5]; I/F, 0 g/kg per day [0–1.5] versus 1.8 g/kg per day [1.8–2.5]; all P<0.001) (Table 3). Protein provision was lower compared with energy needs met (No AKI/R, 22.5% protein [0%–60.0%] versus 58.2% BMR [22.4%–114.7%]; I/F, 0.0% [0%–67%] versus 35.4% [14.8%–99.7%]; all P<0.001) (Table 3). Overall protein provision (19% [0%–60%]) was lower than energy provision (55% [22%–113%]) compared with estimated needs (P<0.001).
Figure 1.
Actual energy and protein intake as a percentage of recommended needs on days 1–5. Actual intake as a percentage of recommended energy (Schofield) (top) and protein needs (bottom) in no AKI/risk (No AKI/R) and injury/failure (I/F) groups on days 1–5. No AKI/R, n=146 for days 1–4 and n=138 for day 5; I/F, n=21 for days 1–4 and n=19 for day 5. Box plots are medians (25th–75th percentiles) and 95% confidence intervals. aI/F versus No AKI/R. Mann–Whitney test (P<0.03).
Table 3.
Recommended and actual energy and protein intake in patients with injury/failure versus no AKI/risk in the first 5 days of PICU stay
| No AKI/Risk | Injury/Failure | P Value Injury/Failure versus No AKIa | ||
|---|---|---|---|---|
| n | 722 | 103 | ||
| BMR (kcal/kg per day) | 48.5 (38.2–53.9) | 46.3 (33.2–54.5) | 0.39 | |
| Actual energy intake (kcal/kg per day) | 24.9 (9.3–56.3)b | 16.0 (5.5–46.5)b | 0.03 | |
| BMR (%) | 58.2 (22.4–114.7) | 35.4 (14.8–99.7) | 0.04 | |
| Recommended protein intake (g/kg per day) | 2.5 (1.8–2.5) | 1.8 (1.8–2.5) | 0.75 | |
| Actual protein intake (g/kg per day) | 0.50 (0–1.36)b | 0 (0–1.50)b | 0.09 | |
| Protein needs (%) | 22.5 (0–60.0)c | 0 (0–67.0)c | 0.09 | |
Data are medians (25th–75th percentiles). BMR is estimated by the Schofield equation. PICU, pediatric intensive care unit; BMR, basal metabolic rate.
Mann-Whitney test.
Actual energy or protein intake versus BMR or recommended protein intake, respectively. Wilcoxon signed rank test (P<0.001).
Percentage of protein needs versus percentage BMR. Wilcoxon signed rank test (P<0.001).
I/F patients were more likely to be fasted than patients with no AKI/R (patient-days = 813; OR, 2.3; CI, 1.5–3.4; P<0.001) and to receive <90% of BMR by day 5 of admission (patient-days = 832; OR, 1.6; CI, 1.0–2.6; P=0.04) than R/No AKI patients (Table 4).
Table 4.
ORs for nutrition support in PICU patients with injury/failure compared with no AKI/risk
| Patient-Days | % (n) | % (n) | OR (95% CI) | p |
|---|---|---|---|---|
| Nutrition support status | Oral, enteral, or parental nutrition | NPO | ||
| No AKI/risk | 71.4 (507) | 28.6 (203) | 1 | |
| Injury/failure | 52.4 (54) | 47.6 (49) | 2.3 (1.5–3.4) | <0.001 |
| Caloric intake | ≥90% BMR | <90% BMR | ||
| No AKI/risk | 36.3 (264) | 63.7 (463) | 1 | |
| Injury/failure | 25.7 (27) | 74.3 (78) | 1.6 (1.0–2.6) | 0.04 |
| Protein intake | ≥90% estimated needs | <90% estimated needs | ||
| No AKI/risk | 12.1 (88) | 87.9 (641) | 1 | |
| Injury/failure | 13.3 (14) | 86.7 (91) | 0.9 (0.5–1.6) | 0.71 |
BMR is estimated by the Schofield equation (16), whereas protein intake is estimated by the American Society for Parenteral and Enteral Nutrition guidelines for critically ill children (17). OR, odds ratio; CI, confidence interval; PICU, pediatric intensive care unit; NPO, nothing by mouth; BMR, basal metabolic rate.
Discussion
Our results showed that <68% of energy needs and <35% of protein needs were covered by current nutritional support practices in our PICU. Protein underfeeding was significantly greater than energy underfeeding. Patients with severe AKI (I/F) were sicker and were significantly more likely to not be fed or to meet <90% of estimated energy needs than patients without AKI by day 5. These results indicate not only that the nutritional needs of critically ill children with AKI are not covered by nutrition support practices, but also that adequate nutrition support does not occur until 4–5 days after PICU admission, subjecting critically ill children to an extra burden of starvation and underfeeding.
In the past, nutrition intervention was often postponed in patients with renal insufficiency to delay dialysis (21), because nutrition support may contribute to azotemia, fluid overload, and electrolyte disturbances. Because of the catabolic nature of the illness associated with kidney injury, it is now recognized that standard recommendations for energy and protein are appropriate for ICU patients with AKI (22,23). Most of the available pediatric data for nutritional risk in renal disease come from studies focusing on patients with CKD. Pediatric patients on hemodialysis with severe growth failure have a 3-fold increase in mortality compared with the same age cohorts (24,25). Supplementation of nutrition via intradialytic parenteral nutrition has been successfully used to reverse weight loss and to promote weight gain for pediatric hemodialysis patients (26,27). Enteral nutrition is safe in most adult patients with AKI; moreover, those on renal replacement therapy may require higher protein as intravenous infusions to compensate for protein losses in the dialysate (28). Nutritional recommendations for pediatric AKI are limited to patients receiving renal replacement therapy.
Our study is the first to report on the nutritional status of pediatric patients with AKI not requiring renal replacement therapy. Acute and chronic malnutrition were prevalent in our cohort at PICU admission. Twenty percent (n=33) of all patients and 33% (n=7) of I/F patients exhibited acute malnutrition, whereas about one-fourth of the patients exhibited chronic malnutrition or growth stunting. Patients with I/F were significantly more likely to show moderate/severe acute malnutrition than patients with no AKI (Table 2), similar to adult reports (6). We did not have data on the degree of fluid overload in our patients; however, given that it is a commonly observed finding in the critically ill patient with AKI (29), it is reasonable to suggest that acute malnutrition was underestimated in the AKI group (Table 2). Patients with an R classification had more severe chronic malnutrition for reasons that are unclear. Determination of nutritional status in patients with AKI is quite difficult for multiple reasons. Weights are inconsistently available in the ICU. Biochemical indicators of nutrition such as serum albumin and prealbumin levels can be affected by the fluid status and are both negatively associated with acute phase reactants and thus negatively correlated with inflammation, which is often rampant in the ICU patient (30). Anthropometric measures are time-consuming and require specially trained individuals. Bioimpedance analysis has reliably been used in the outpatient setting of hemodialysis units to determine body composition and might be a useful tool in the pediatric ICU.
The energy needs of critically ill children were not met in our cohort of patients. Actual delivery of energy met <90% of energy needs on two-thirds of all patient-days (Table 4). I/F patients were more likely to be fasted than No AKI/R patients. Our study confirmed that underfeeding was a significant problem in PICU patients, as reported by others (13,31,32) and was due to underdelivery. Furthermore, suboptimal nutrition support is also secondary to imprecise determination of nutritional needs in critically ill children who have variable levels of energy metabolism due to the acute nature of the underlying disease process and degree of malnutrition (12).
The goals of nutrition support in AKI are identical to those suggested for other critically ill patients in the ICU (23,33), and include prevention of protein-energy wasting, preservation of lean body mass and nutritional status, avoidance of metabolic derangements and complications, improvement of wound healing, and support of immune function (7,33). Previous studies have shown that prediction equations are not accurate to predict energy expenditure in critically ill children (34,35), because the dynamic metabolic responses to injury and illness in critically ill children render the prediction of energy needs more difficult (36). With indirect calorimetry, Metha et al. (37) found that slightly more than half of patients were hypometabolic, about 25% were normometabolic, and 17% were hypermetabolic. In the absence of measured energy expenditure, estimation of energy needs should be based on predicted BMR (31,38).
More importantly, protein underfeeding was noted to a greater degree than energy deficits. On almost 87% of patient-days, <90% of protein needs were met in all patients. Although there were no significant differences in protein intake between I/F and No AKI/R patients, on average significantly more I/F patients remained NPO during the first days of PICU stay. This suggests a hesitation from the clinical team to provide adequate protein intake in patients with severe AKI for concerns of azotemia. R and I/F patients in our study were sicker as assessed by the PRISM III and PELOD scores, which might have lead to delayed feeding but does not explain the underfeeding and underprescription of protein. In fact, protein energy malnutrition is associated with increased mortality and morbidity in children (39) and adults (40), including a higher risk for infection, poor wound healing, longer dependency on mechanical ventilation, and increased LOS in adults (41,42). Critically ill children with higher protein store depletion have been shown to be at higher risk for multiorgan system failure, whereas children with fat store depletion had higher probability of death compared with nutritionally normal children (43). On the other hand, critically ill children who received better nutritional support showed significant improvement in physiologic stability and outcome (39). This observation holds true for patients with AKI as well. Low serum prealbumin levels, a marker of nutritional status, were correlated with increased mortality in adults with AKI (44), and sustained improvement in prealbumin levels was associated with improved survival in adult hemodialysis patients (45). Systemic administration of protein in the form of amino acids early in the course of experimental AKI in small animal models of nephrotoxic and septic AKI had anti-inflammatory and antiapoptotic effects in the renal tubular epithelium and may reverse AKI (46,47). Adults with oliguric AKI who were given intravenous amino acid infusions have a faster rate of renal recovery and higher survival than patients who were given isocaloric carbohydrates alone (48). Thus, contrary to old beliefs, adequate protein feeding seems to be beneficial in AKI. Unfortunately, our results suggest that there still seems to be a bias against protein prescription in patients with AKI. Our patients with more severe AKI (I/F) were sicker on admission and had worse outcome as manifested by increased hospital LOS and mortality. This group was also more severely malnourished on presentation and received less nutrition support, suggesting a link between poor nutritional status and worse outcome. Future studies should investigate the relationship between underfeeding and worse outcome in pediatric patients with AKI.
There are several imitations to our study. The data were collected retrospectively from patient charts and were limited to one PICU only, and therefore may not be generalized to other PICUs. Fluid overload and oliguria are both well recognized risk factors in the ICU (29,49,50). There were no data available on the fluid balance and urine volume of patients. We only obtained PELOD scores on PICU admission and were not able to track status of organ dysfunction during the whole study period. In addition, we did not evaluate nutrition outcome, because patients were infrequently weighed in the acute phase of PICU stay and weight would have been questionable in many patients in view of changing fluid status. Therefore, we were not able to evaluate the progression of malnutrition during PICU stay. Furthermore, energy expenditure was calculated from prediction equations instead of using indirect calorimetry. Prediction equations have limitations and cannot accurately predict the effect of drugs, illness, injuries, and individual variability.
Underfeeding practices common in critically ill children are accentuated in AKI. Protein underfeeding was greater than energy underfeeding in the first 5 days of PICU stay. Efforts should be made to develop better nutritional assessment, to provide adequate nutrition in ICU patients with AKI, and to prospectively evaluate nutritional risk and outcomes during AKI.
Disclosures
None.
Acknowledgments
The study was carried out at Texas Children's Hospital, with internal funding by Baylor College of Medicine.
This work was presented in poster form at the 2012 European Society for Clinical Nutrition and Metabolism (ESPEN) Congress, held September 8–11, 2012, in Barcelona, Spain.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Nutrition as Medical Therapy in Pediatric Critical Illness,” on pages 513–514.
References
- 1.Coss-Bu JA, Klish WJ, Walding D, Stein F, Smith EO, Jefferson LS: Energy metabolism, nitrogen balance, and substrate utilization in critically ill children. Am J Clin Nutr 74: 664–669, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Delgado AF, Okay TS, Leone C, Nichols B, Del Negro GM, Vaz FA: Hospital malnutrition and inflammatory response in critically ill children and adolescents admitted to a tertiary intensive care unit. Clinics (Sao Paulo) 63: 357–362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterlow JC: Classification and definition of protein-calorie malnutrition. BMJ 3: 566–569, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wooley JA, Btaiche IF, Good KL: Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract 20: 176–191, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A: Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: A prospective cohort study. J Am Soc Nephrol 10: 581–593, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Fiaccadori E, Cremaschi E, Regolisti G: Nutritional assessment and delivery in renal replacement therapy patients. Semin Dial 24: 169–175, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Maursetter L, Kight CE, Mennig J, Hofmann RM: Review of the mechanism and nutrition recommendations for patients undergoing continuous renal replacement therapy. Nutr Clin Pract 26: 382–390, 2011 [DOI] [PubMed] [Google Scholar]
- 9.KDOQI. National Kidney Foundation : KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 47[Suppl 3]: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Berbel MN, Pinto MP, Ponce D, Balbi AL: Nutritional aspects in acute kidney injury. Rev Assoc Med Bras 57: 600–606, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Muscaritoli M, Molfino A, Bollea MR, Rossi Fanelli F: Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care 12: 378–383, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Lambe C, Hubert P, Jouvet P, Cosnes J, Colomb V: A nutritional support team in the pediatric intensive care unit: changes and factors impeding appropriate nutrition. Clin Nutr 26: 355–363, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Briassoulis G, Zavras N, Hatzis T: Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition 17: 548–557, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM: Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med 10: 562–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care 15: R146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schofield WN: Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39[supp]: 5–41, 1985 [PubMed] [Google Scholar]
- 17.Mehta NM, Compher C, A.S.P.E.N. Board of Directors : A.S.P.E.N. Clinical Guidelines: Nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr 33: 260–276, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat 246: 1–190, 2002 [PubMed] [Google Scholar]
- 19.Pollack MM, Patel KM, Ruttimann UE: PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 24: 743–752, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F: Validation of the paediatric logistic organ dysfunction (PELOD) score: Prospective, observational, multicentre study. Lancet 362: 192–197, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kalista-Richards M: The kidney: Medical nutrition therapy—yesterday and today. Nutr Clin Pract 26: 143–150, 2011 [DOI] [PubMed] [Google Scholar]
- 23.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G, A.S.P.E.N. Board of Directors. American College of Critical Care Medicine. Society of Critical Care Medicine : Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 33: 277–316, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR: Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 17: 450–455, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C: Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36: 811–819, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein SL, Baronette S, Gambrell TV, Currier H, Brewer ED: nPCR assessment and IDPN treatment of malnutrition in pediatric hemodialysis patients. Pediatr Nephrol 17: 531–534, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Orellana P, Juarez-Congelosi M, Goldstein SL: Intradialytic parenteral nutrition treatment and biochemical marker assessment for malnutrition in adolescent maintenance hemodialysis patients. J Ren Nutr 15: 312–317, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Fiaccadori E, Maggiore U, Giacosa R, Rotelli C, Picetti E, Sagripanti S, Melfa L, Meschi T, Borghi L, Cabassi A: Enteral nutrition in patients with acute renal failure. Kidney Int 65: 999–1008, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL: Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55: 316–325, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H, Ma S, Hao C, Gu Y, Lin S, Ding F: The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol 12: 30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, Duggan C: Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr 34: 38–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RM, Preedy VR, Baker AJ, Grimble G: Nutritional support in critically ill children. Clin Nutr 22: 365–369, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, Lindholm B, Teplan V, Fouque D, Guarnieri G, ESPEN : ESPEN Guidelines on Parenteral Nutrition: Adult renal failure. Clin Nutr 28: 401–414, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Vazquez Martinez JL, Martinez-Romillo PD, Diez Sebastian J, Ruza Tarrio F: Predicted versus measured energy expenditure by continuous, online indirect calorimetry in ventilated, critically ill children during the early postinjury period. Pediatr Crit Care Med 5: 19–27, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Framson CM, LeLeiko NS, Dallal GE, Roubenoff R, Snelling LK, Dwyer JT: Energy expenditure in critically ill children. Pediatr Crit Care Med 8: 264–267, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Chwals WJ, Bistrian BR: Predicted energy expenditure in critically ill children: Problems associated with increased variability. Crit Care Med 28: 2655–2656, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan CP: Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med 12: 398–405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briassoulis G, Tsorva A, Zavras N, Hatzis T: Influence of an aggressive early enteral nutrition protocol on nitrogen balance in critically ill children. J Nutr Biochem 13: 560, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Pollack MM, Ruttimann UE, Wiley JS: Nutritional depletions in critically ill children: Associations with physiologic instability and increased quantity of care. JPEN J Parenter Enteral Nutr 9: 309–313, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Biolo G: Can we increase protein synthesis by anabolic factors? Am J Kidney Dis 37[Suppl 2]: S115–S118, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P: Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 79: 613–618, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Heyland DK: Making inferences from scientific research. Nutr Clin Pract 13: S1–S7, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Briassoulis G, Venkataraman S, Thompson AE: Energy expenditure in critically ill children. Crit Care Med 28: 1166–1172, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Perez Valdivieso JR, Bes-Rastrollo M, Monedero P, de Irala J, Lavilla FJ: Impact of prealbumin levels on mortality in patients with acute kidney injury: An observational cohort study. J Ren Nutr 18: 262–268, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM, French Study Group for Nutrition in Dialysis : Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: A 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18: 2583–2591, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Hu YM, Pai MH, Yeh CL, Hou YC, Yeh SL: Glutamine administration ameliorates sepsis-induced kidney injury by downregulating the high-mobility group box protein-1-mediated pathway in mice. Am J Physiol Renal Physiol 302: F150–F158, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Kim YS, Jung MH, Choi MY, Kim YH, Sheverdin V, Kim JH, Ha HJ, Park DJ, Kang SS, Cho GJ, Choi WS, Chang SH: Glutamine attenuates tubular cell apoptosis in acute kidney injury via inhibition of the c-Jun N-terminal kinase phosphorylation of 14-3-3. Crit Care Med 37: 2033–2044, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Abel RM, Beck CH, Jr, Abbott WM, Ryan JA, Jr, Barnett GO, Fischer JE: Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med 288: 695–699, 1973 [DOI] [PubMed] [Google Scholar]
- 49.Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL: Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 80: 760–767, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL: Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 13: 253–258, 2012 [DOI] [PubMed] [Google Scholar]