Summary
Background and objectives
Women have traditionally been advised not to breastfeed while taking tacrolimus, based on theoretical risks of neonatal immunosuppression and assumed secretion into breast milk, rather than clinical data suggesting neonatal absorption. The aim of this study was to assess tacrolimus levels in breast milk and neonatal exposure during breastfeeding.
Design, setting, participants, & measurements
An observational cohort study was performed in two tertiary referral high-risk obstetric medicine clinics. Fourteen women taking tacrolimus during pregnancy and lactation, and their 15 infants, 11 of whom were exclusively breast-fed, were assessed. Tacrolimus levels were analyzed by liquid chromatography–tandem mass spectrometry. Samples from mothers and cord blood were collected at delivery and from mothers, infants, and breast milk postnatally where possible.
Results
All infants with serial sampling had a decline in tacrolimus level, which was approximately 15% per day (ratio of geometric mean concentrations 0.85; 95% confidence interval, 0.82–0.88; P<0.001). Breast-fed infants did not have higher tacrolimus levels compared with bottle-fed infants (median 1.3 μg/L [range, 0.0–4.0] versus 1.0 μg/L [range, 0.0–2.3], respectively; P=0.91). Maximum estimated absorption from breast milk is 0.23% of maternal dose (weight-adjusted).
Conclusions
Ingestion of tacrolimus by infants via breast milk is negligible. Breastfeeding does not appear to slow the decline of infant tacrolimus levels from higher levels present at birth. Women taking tacrolimus should not be discouraged from breastfeeding if monitoring of infant levels is available.
Introduction
Women have traditionally been advised, by several guidelines, including the American Academy of Pediatrics, not to breastfeed while taking tacrolimus. These recommendations were based on theoretical risks of neonatal immunosuppression and assumed secretion into breast milk, rather than on clinical data suggesting absorption via breast milk. The risk of preterm delivery is higher in women with transplants (1) and the benefits of breast milk are diverse and substantial, particularly for preterm infants. Large registry series have not identified any increase in birth defects or neonatal complications in the offspring of mothers taking tacrolimus during pregnancy despite evidence of placental transfer (2).
In view of the increasing numbers of women taking tacrolimus during pregnancy and to provide relevant data for mothers wishing to breastfeed while taking tacrolimus, we performed a study to assess excretion of tacrolimus in breast milk and to evaluate absorption of tacrolimus by breast-fed infants.
Materials and Methods
Fourteen women taking tacrolimus during pregnancy and lactation, and their 15 infants, 11 of whom were exclusively breast-fed, were recruited from two tertiary referral high-risk obstetric antenatal clinics. Maternal blood samples were taken immediately before the next dose of tacrolimus (trough levels). Infant blood samples were taken at the same time as other venous blood sampling when possible. Ethical approval was given by St Thomas’ Research Ethics Committee (09/H0802/68).
Tacrolimus concentrations were measured on EDTA whole blood or breast milk samples. Breast milk samples were stored at −80°C before analysis and whole blood samples were assayed on the day of collection or stored at 2°C–8°C before assay. Breast milk samples (100 μl) were prepared for assay by using solid phase extraction, and whole blood (25 μl) was prepared using a simple protein crash sample preparation (3). Twenty microliters of sample extract were injected for analysis. The analytical range was 1.0–50 μg/L for the whole blood assay and 0.2–5.0 μg/L for the breast milk samples. The imprecision was 10.6% at 2.7 μg/L, 6.5% at 7.3 μg/L, and 5.4% at 12.4 μg/L for the whole blood method and 10.6% at 0.5 μg/L, 4.6% at 1.1 μg/L, and 5.5% at 3.7 μg/L for breast milk. The limits of quantitation for whole blood and breast milk were 1.0 μg/L and 0.2 μg/L, respectively.
All statistical analysis was performed using Stata software (version 10.6 or later; (StataCorp, College Station, TX). Inspection of tacrolimus levels indicated a log-normal distribution; therefore, log values were taken to normalize the data. Results are expressed as ratios of the geometric mean concentrations between treatment groups or times of measurement. Interval regression analysis with random-effect modeling for individual clustering was used, with linear adjustment for days postpartum in serial samples, assuming exponential decline in infant tacrolimus levels. A P value of <0.05 was considered to be significant.
Results
Fifteen women were recruited between 2006 and 2011 from Queen Charlotte’s and Chelsea Hospital (Imperial College NHS Healthcare Trust) and St Thomas’ Hospital (Guy’s and St Thomas’ Foundation Trust). One woman delivered and breast-fed two infants in the study period. One woman was excluded because only cord blood was obtained from her infant and no breast milk samples were provided, leaving 14 women and 15 infants for analysis.
Maternal demographics and neonatal outcomes are shown in Table 1. There were no significant differences in gestational age and birth weight between bottle-fed and breast-fed infants. Two infants were bottle-fed, but the mother of one of these infants provided breast milk samples for analysis. One infant received <20 ml of breast milk per day for the first 10 days postpartum only, and is categorized as bottle-fed. The remaining 12 infants were exclusively breast-fed.
Table 1.
Maternal and infant characteristics
| Patient | Maternal Ethnicity | Maternal Underlying Condition | Additional Maternal Immunosuppression | Gestation at Delivery (Completed Weeks) | Birth Weight (g) | Breast-Fed |
|---|---|---|---|---|---|---|
| 1a | Caucasian | Renal transplant (IgA nephropathy) | Azathioprine | 24 | 720 | Yes |
| 1b | 34 | 2300 | Yes | |||
| 2 | Indo-Asian | Renal transplant (APKD) | None | 36 | 1704 | No |
| 3 | African-Caribbean | Renal transplant (reflux nephropathy) | Azathioprine | 37 | 2822 | Yes |
| 4 | Caucasian | Renal transplant (diabetic nephropathy) | None | 36 | 2900 | No |
| 5 | Caucasian | Kidney pancreas transplant | Prednisolone | 35 | 2432 | Yes |
| Sirolimus | ||||||
| 6 | Indo-Asian | Class IV lupus nephritis | Azathioprine | 30 | 1130 | Yes |
| Hydroxychloroquine | ||||||
| 7 | Indian | Class IV lupus nephritis | None | 37 | 2512 | Yes |
| 8 | N/A | Class V lupus nephritis | None | N/A | N/A | Yes |
| 9 | Caucasian | Primary FSGS | None | 36 | 2480 | Yes |
| 10 | Caucasian | Kidney pancreas transplant | None | 37 | 2450 | Yes |
| 11 | Caucasian | Kidney pancreas transplant | Prednisolone | 32 | 1750 | <20 ml/d |
| Azathioprine | ||||||
| 12 | Caucasian | Class V lupus nephritis | Prednisolone | 32 | 1400 | Yes |
| Hydroxychloroquine | ||||||
| 13 | Caucasian | Renal transplant (Wegener’s GN) | None | 39 | 2720 | Yes |
| 14 | Caucasian | Minimal change disease | None | 38 | 2910 | Yes |
APKD, adult polycystic kidney disease; N/A, data not available.
Maternal, cord, and infant blood and breast milk tacrolimus levels are shown in Table 2. Maternal blood levels were significantly higher after delivery (≥ day1) in mothers who bottle-fed compared with those who breast-fed (P<0.001). All infants had samples taken postpartum (range, 1–3 samples per infant) between days 0 and 72. Thirteen infants had serial blood sampling. All infants with serial blood sampling had a decline in tacrolimus level, which was approximately 15% per day (ratio of geometric mean concentrations, 0.85; 95% confidence interval [95% CI], 0.82–0.88; P<0.001). Numbers were too small to allow comparison of rate of decline; however, infants that were breast-fed did not have higher tacrolimus levels compared with bottle-fed infants (ratio of geometric mean concentrations, 0.93; 95% CI, 0.38–2.39; P=0.91). Infant blood tacrolimus levels are shown in Figure 1. Eight breast-fed infants went on to have undetectable tacrolimus levels (<1.0 μg/L) by median day 14 (interquartile range, 11–22) including two infants at 45 and 72 days, which are not shown in the figure. Two bottle-fed infants had undetectable levels at days 8 and 20. The remaining five patients had a decline in tacrolimus levels but further serial sampling was unavailable.
Table 2.
Maternal, cord, and infant blood tacrolimus levels and breast milk levels
| Sample Type | Breast-Fed Infants (n=12) | Bottle-Fed Infants (n=3) | All | |||
|---|---|---|---|---|---|---|
| n | Median (Range) | n | Median (Range) | n | Median (Range) | |
| Gestation (wk) | 36 (24–39) | 35 (32–37) | 36 (24–39) | |||
| Birth weight (g) | 2450 (720–2910) | 2431 (1750–2822) | 2440 (720–2910) | |||
| Maternal blood at delivery (µg/L) | 7 | 6.2 (4.6–11.2) | 1 | 7.3 | 8 | 6.6 (4.6–11.2) |
| Maternal blood day ≥1 (µg/L) | 24 | 4.1 (1.3–7.0)a | 7 | 9.0 (4.9–16)a | 31 | 4.4 (1.3–16) |
| Infant cord blood (µg/L) | 6 | 4.6 (1.0–9.0) | 1 | 4.6 | 7 | 4.6 (1.0–9.0) |
| Infant blood day ≥1 (µg/L) | 24 | 1.3 (0.0–4.0) | 6 | 1.0 (0.0–2.3) | 30 | 1.1 (0.0–4.0) |
| Breast milk (µg/L) | 17 | 0.7 (0.1–1.6) | 5 | 1.3 (0.7–1.6) | 22 | 0.8 (0.1–1.6) |
P<0.001.
Figure 1.
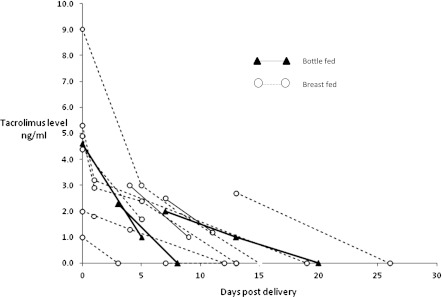
Blood tacrolimus levels in breast-fed and bottle-fed infants.
Twenty-two samples of breast milk were expressed by 11 mothers for 12 infants between days 1 and 72 postpartum, including one sample that contained milk expressed over a 12-hour period. A positive association between concentrations in maternal blood and breast milk samples was identified in the 11 samples in 6 women, when maternal blood levels were taken on the same day (partial correlation 0.685; increase (ratio) in breast milk concentration for a 2-fold increase in maternal blood level: 1.81; 95% CI, 1.14–2.88; P=0.01). Three women (one on two occasions) took breast milk samples predose and at least 4 hours postdose representing theoretical peak and trough levels, and there was no significant change in tacrolimus level. There was no relationship between breast milk concentration and number of days postpartum.
The estimated infant dose, based on the highest breast milk concentration (1.56 µg/L, assuming breast milk ingestion 0.15 L/kg per day and infant weight 2.4 kg) is 0.56 µg/d, which is equivalent to 0.23% of maternal dose (assuming maternal weight of 60 kg and tacrolimus dose 6 mg/d).
Low birth weight at delivery was significantly associated with higher infant tacrolimus levels, assuming a linear effect of additional increments of 500 g in birth weight (ratio of geometric mean concentrations, 0.1.33; 95% CI, 1.01–1.67; P=0.04). Infant tacrolimus levels fell with higher birth weights; for each 500 g increase there was a −25% change in geometric mean tacrolimus level (95% CI, −42% to −1%; P=0.034).
Discussion
Tacrolimus is a macrolide that inhibits calcineurin, thus inhibiting both T lymphocyte signal transduction and IL-2 transcription. Theoretically, neonatal exposure could impair the developing immune system if tacrolimus is given in sufficient quantities; however, we found the drug to be present in only minimal amounts in breast milk. This is the largest study to report serial tacrolimus blood levels in neonates, together with breast milk analysis, and suggests that initial detectable levels are the consequence of placental transfer, rather than ingestion via breast milk.
Tacrolimus is 75%–99% protein-bound, which would suggest low excretion of tacrolimus into breast milk. In addition, its absorption from the gastrointestinal tract is impaired by the consumption of food rich in fat; therefore, the high fat content of breast milk in theory should reduce neonatal absorption (4). Furthermore, drug doses need to be higher in children due to higher volumes of distribution and blood clearance in order to achieve the same tacrolimus concentrations as adults (5). In keeping with predicted pharmacokinetics of tacrolimus, we found that infant tacrolimus levels fell despite ongoing breastfeeding, suggesting that the breast-fed infant of a mother taking tacrolimus is exposed to only minimal amounts of the drug via this route. Three other studies have measured tacrolimus levels in breast milk; similarly, all have identified the drug but only in small amounts, ranging from 0.06% to 0.5% maternal dose (weight adjusted) (6–8).
Placental transfer results in significant neonatal levels that are comparable with maternal levels, and the drug appears to be progressively metabolized by the infant, resulting in a rate of decline of 15% per day. Importantly, breast-fed infant tacrolimus levels were not higher than bottle-fed infants. Maternal blood levels of bottle-fed infants were higher, and it is possible that bottle-fed infants may have had higher tacrolimus levels than breast-fed infants at delivery, although cord blood was not taken in all bottle-fed infants. However, the decline in levels in both breast-fed and bottle-fed infants, together with the complete clearance of tacrolimus in 8 of 12 breast-fed infants (serial sampling was not possible in the remaining 4 infants), suggests that tacrolimus transfer in breast milk is minimal.
Jain et al. measured plasma tacrolimus levels on days 0–3 in seven bottle-fed infants of mothers with liver transplants taking tacrolimus throughout pregnancy (7). Tacrolimus levels on consecutive days were measured in four of their infants, and in keeping with our findings, they were able to eliminate the drug.
We found a large variation in concentrations of tacrolimus in breast milk. These findings, together with those of Gardiner et al. and French et al. (6,8), suggest that there may be a wide range of drug transfer in breast milk despite the low levels of tacrolimus in breast milk. French et al. measured levels in milk and blood from one woman, before and after taking tacrolimus. Predose and postdose milk-to-blood ratios were 0.08 and 0.09 respectively. However, Gardiner et al. also reported that the concentration-time profile of tacrolimus in milk was relatively flat and varied <2-fold during the dosing interval (6). These data, together with the low levels of tacrolimus found in breast milk suggest that restricting feeding to “non-peak drug level” times, as advised by some clinicians, is unnecessary.
Our findings also suggest that tacrolimus levels are higher in infants of lower birth weights, and possibly those delivered at early gestations. As reported by Jain et al., an infant born at 33 weeks’ gestation had higher tacrolimus levels than infants born at later gestations, suggesting that preterm or low birth weight infants may take longer to metabolize tacrolimus, and monitoring of levels in these infants is probably required. Although we did not find a relationship between gestation at delivery and infant blood levels, the small number of infants studied may be limiting.
Limitations of the study include incomplete sample collection in all mother/neonate pairs; however, the unpredictability and circumstances of timing of delivery, variations in length of stay, and mothers’ willingness to provide samples precluded a complete data set. Another limitation is the lack of data regarding neonatal outcome. The long-term consequences of even minimal exposure to tacrolimus are unknown; however, evidence to date together with our findings suggest that exposure to the drug during breastfeeding is relatively insignificant in comparison to in utero exposure and the latter has not been shown to be associated with any long-term adverse effects (1). Furthermore, the majority of women in the study are followed up by nephrologic services; therefore, if any severe infant complications had arisen, they are likely to have been reported.
Infants of mothers receiving tacrolimus in pregnancy may be born with significant levels of the drug, but are exposed to minimal amounts of tacrolimus if they are breast-fed. The advantages of breastfeeding, particularly in preterm infants, needs to be weighed against the theoretical disadvantages of ingestion through breast milk. Although more data are required to confirm drug safety, our findings, together with those of three other studies, suggest that women taking tacrolimus who wish to breastfeed after appropriate counseling should not be discouraged from doing so.
Disclosures
None.
Acknowledgments
We thank Tom Cairns, Paul Seed, Hiten Mistry, Hayley Tarft, Kate Harding, Charlotte Frise, Alexander Wardle, Marcus Martineau, Anthony James, and Carla Rosser.
K.B. is funded by a Doctoral Research Fellowship from the National Institute for Health Research.
Footnotes
L.L. and C.N.P. contributed equally to senior authorship.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Armenti VT, Radomski JS, Moritz MJ, Philips LZ, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 123–134, 2000 [PubMed] [Google Scholar]
- 2.Kainz A, Harabacz I, Cowlrick IS, Gadgil SD, Hagiwara D: Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation 70: 1718–1721, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Keevil BG, McCann SJ, Cooper DP, Morris MR: Evaluation of a rapid micro-scale assay for tacrolimus by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem 39: 487–492, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bekersky I, Dressler D, Mekki QA: Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol 41: 176–182, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Wallemacq PE, Verbeeck RK: Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet 40: 283–295, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Gardiner SJ, Begg EJ: Breastfeeding during tacrolimus therapy. Obstet Gynecol 107: 453–455, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Venkataramanan R, Fung JJ, Gartner JC, Lever J, Balan V, Warty V, Starzl TE: Pregnancy after liver transplantation under tacrolimus. Transplantation 64: 559–565, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French AE, Soldin SJ, Soldin OP, Koren G: Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother 37: 815–818, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


