Summary
Background and objectives
AKI carries a substantial risk of mortality, even after adjustment for comorbidities. Effective risk stratification may lead to more effective therapeutic interventions for high-risk subgroups.
Design, setting, participants, & measurements
This study identified adults who suffered severe in-hospital AKI from January 1, 2004 to August 31, 2010 at three hospitals in the University of Pennsylvania Health System (UPHS). Patients were included if baseline creatinine was ≤1.4 mg/dl for men or ≤1.2 mg/dl for women, and serum creatinine doubled during the hospital admission. Cox proportional hazards models predicting death, dialysis, or a combined endpoint of death or dialysis were fit using data from patients admitted to the Hospital of the University of Pennsylvania (n=4263), and validated at the two other UPHS facilities (n=758, n=1098).
Results
In adjusted analyses, strong predictors of the combined endpoint included intensive care unit location (versus floor), medical service, liver disease, higher creatinine, greater rate of change in creatinine, and greater number of pressor medications. Higher absolute creatinine concentration was associated with greater use of dialysis, but lower overall mortality in adjusted analyses. Harrell’s c-index (95% confidence interval) for the model predicting the combined endpoint was 0.85 (0.84–0.86) in the derivation cohort, and 0.83 (0.80–0.86) and 0.84 (0.82–0.86) in the validation cohorts.
Conclusions
A small group of easily measured clinical factors has good ability to predict mortality and dialysis in severe AKI.
Introduction
AKI is a common complication in hospitalized patients, carrying a significantly increased risk of mortality, even after adjustment for underlying disease (1,2). Many studies examining the predictors of death in AKI are restricted to patients who received acute dialysis (3–9), limiting generalizable inferences regarding the broader AKI population. Other studies have enrolled patients after consultation with a nephrologist (10), restricted to intensive care unit (ICU) settings (2), or after surgical procedures (11–14). Some observational studies lack the patient-level data needed to adequately adjust for comorbid illness, and utilize limited registry data to categorize AKI (15). Small sample sizes of studies with patient-level data have had limited ability to detect novel mediators of mortality or the provision of dialysis after the occurrence of AKI (16,17). Finally, few studies have accurately established the date of the onset of AKI. Using several databases within a comprehensive electronic medical record system of a large academic medical center, we assessed the value of patient-level covariates for predicting the provision of dialysis and short-term mortality among patients experiencing in-hospital AKI.
Materials and Methods
Patients
Patients admitted to any of the three University of Pennsylvania Health System (UPHS) hospitals were considered for inclusion. Patients aged ≥18 years were included if their baseline creatinine (defined as the lowest serum creatinine concentration within 48 hours of hospital admission) was ≤1.4 mg/dl for men and ≤1.2 mg/dl for women, and subsequently doubled during the hospital admission. Patients receiving dialysis within 24 hours of admission were excluded, as were patients who received a renal transplant during the hospitalization. If a patient met inclusion criteria in >1 encounter between January 1, 2004 and August 31, 2010, the first encounter was used. This study was approved by the Institutional Review Board of the University of Pennsylvania.
Defining AKI
AKI onset was defined as the first day a patient met Acute Kidney Injury Network (AKIN) stage 1 creatinine criteria (a 50% increase in serum creatinine or 0.3 mg/dl absolute increase within 48 hours) (18) during the first episode of AKI in which the creatinine doubled. An episode of AKI was considered terminated once the serum creatinine fell to within 10% of the established baseline value.
Covariates
Comorbidity information was determined from admission International Classification of Diseases (ninth revision) codes (19). Laboratory variables were obtained from a laboratory database and reflect measurements made during routine clinical care. Changes in laboratory variables were calculated based on the difference between the day of analysis value and the value 1 day prior. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-Epi equation (20). Medication usage was obtained from an electronic order entry database. Data sources were linked based on medical record number. A linking file to identify patients with >1 medical record number served to integrate data across time and the three study hospitals. The Sequential Organ Failure Assessment (SOFA) score was calculated on a daily basis, but excluded the renal and neurologic components (21).
Outcome Events
The primary outcomes were the implementation of dialytic therapy within 14 days of AKI onset, mortality within 14 days of AKI onset, and the combined endpoint of dialysis or death in this same time frame.
Renal replacement therapy (RRT) was considered initiated on the first day an order for hemodialysis, continuous venovenous hemodialysis, or continuous venovenous hemofiltration appeared in electronic ordering records; each modality has an independent order code. Isolated ultrafiltration methods were not included in the definition. Peritoneal dialysis is not used for AKI at the study institutions.
Date of death was determined from hospital discharge records (in-patient mortality) and via the Social Security Death Master File.
Missing Data and Imputation
Covariates utilized in primary analyses were available for all patients in the final cohort. Laboratory values were permitted to appear in models only if they could be carried forward a maximum of 2 hospital days to account for all missing data. Medical status and surgical status were defined by the service to which the patient was admitted.
Statistical Analyses
Continuous covariates are described in terms of means and SDs for normally distributed variables and medians and interquartile ranges (IQRs) for non-normally distributed variables. Normality was assessed using visual inspection of histograms and confirmed by the Shapiro–Wilk test for normality. Categorical variables are expressed in proportions. Univariate associations between categorical outcomes and continuous variables were assessed using the t test or the Wilcoxon rank sum test as appropriate. Univariable associations between categorical variables were assessed using the chi-squared test.
Intention-to-Treat Cox Regression
Intention-to-treat Cox proportional hazard regression, as described by Hernán et al., was used to assess the associations between covariates and the primary outcome events (22). In this framework, the unit of analysis is the patient-day. One patient can contribute multiple patient-days, all with the same outcome (although differing time-to-outcome), to the model. At each time point, we were interested in predicting the outcome events over the entire subsequent follow-up using the predictors measured up to that time point. To account for intrapersonal correlation, SEMs were adjusted using robust variance estimates. The proportional hazards assumption was evaluated using Schoenfeld residuals and log-log survival plots. All statistical tests were performed in Stata software (version 12.1; StataCorp, College Station, TX).
Predictive Model Derivation
Predictive models were derived in one of the three UPHS hospitals (the Hospital of the University of Pennsylvania). In predicting time to dialysis, patients were censored at death, 14 days after the onset of AKI, or August 31, 2010. When death was the outcome, patients were censored at 14 days after the onset of AKI, or on August 31, 2010 (the last day of full Social Security Death Master File records). Models predicting mortality were derived using data from patient-days before initiation of dialytic therapy, because predictors of death may differ after dialysis initiation due to the effect of dialysis on key laboratory parameters. A parsimonious predictive model was developed utilizing backward selection (with threshold significance P<0.10) from variables identified as significant in univariable analysis as well as age, sex, race, comorbidities, and four biochemical parameters (creatinine, BUN, potassium, and bicarbonate concentrations) regardless of their statistical significance in unadjusted models. A “short model” was created from the six variables in the parsimonious model most strongly associated with the outcome of interest in terms of absolute value of z score in the model in order to assess predictive ability using a simplified multivariable tool.
Predictive Model Validation
Predictive models were validated at Presbyterian Hospital and Pennsylvania Hospital. The former is a medium-sized community hospital with an extensive cardiovascular disease program, whereas the latter is a small community hospital that does not offer continuous dialytic modalities. Predictive ability of models was assessed with Harrell’s c-index using the “somersd” package in Stata (23). P values comparing parsimonious with short model performance were determined by the linear combination of estimators from the two models. The Harrell’s c-index is the time-to-event analog of c-statistics in analyses of binary outcomes (24).
Results
All hospitalizations between January 1, 2004 and August 31, 2010 were evaluated (n=518,089) for possible inclusion. Among those, 297,903 hospitalizations had at least two creatinine measurements. After excluding patients with abnormal baseline creatinine concentrations and other exclusion criteria, 6119 patient-encounters in which a normal baseline creatinine that subsequently doubled during hospitalization were available for analysis. These represented 2% of hospitalizations with ≥2 creatinine measurements, which is consistent with the incidence of severe AKI reported by other studies (1).
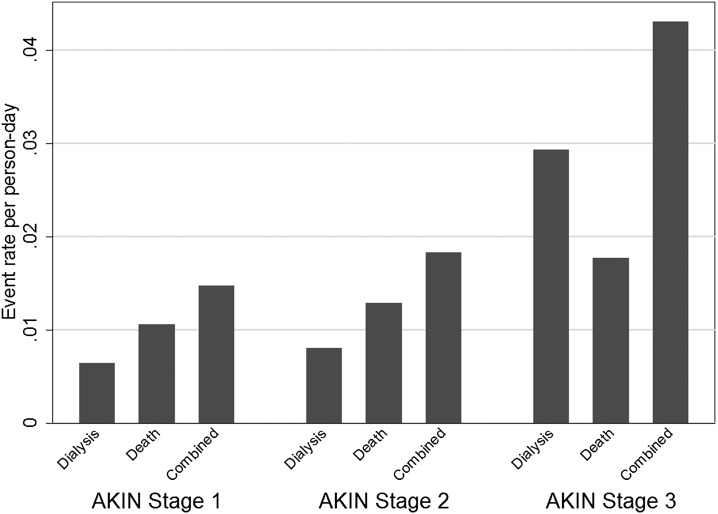
In the AKI cohort, in-hospital, 60-day, and 1-year mortality rates were 64%, 65%, and 72%, respectively, among patients who would receive dialysis and 22%, 28%, and 37%, respectively, among those who were not dialyzed (P<0.001 for all comparisons). Among survivors to hospital discharge, 64% were discharged home and 36% were discharged to other long-term care facilities. Only 16% of patients who received dialysis during the admission were discharged home. Event rates for dialysis, death, and the combined endpoint, across AKIN stages are represented in Figure 1.
Figure 1.
Rates of dialysis, death, and the combined endpoint in the full cohort (n=6119). Patients initiated on dialysis are considered to be AKIN stage 3 at initiation. Patient-days are analyzed distinctly, allowing patients to contribute to multiple AKIN stages. AKIN, Acute Kidney Injury Network.
Baseline Characteristics
Patient characteristics are described in Table 1. Our results showed that 53% of patients were male and 28% described themselves as black. Patients with diabetes comprised more than a quarter of the overall population, with congestive heart failure being the most common comorbidity. Although patients had a normal serum creatinine within the first 48 hours of admission by design, AKI onset occurred early in the hospital course with the median being 3 (IQR, 2–8) days.
Table 1.
Baseline characteristics and unadjusted analyses of patients in the derivation and validation cohorts
| Characteristic | Derivation Cohort HUP (n=4263) | Validation PAH (n=758) | Validation PMC (n=1098) | P Value | |
|---|---|---|---|---|---|
| HUP versus PAH | HUP versus PMC | ||||
| Male sex | 2366 (55.5) | 332 (43.8) | 561 (51.1) | <0.001 | 0.01 |
| Black | 1011 (23.7) | 238 (31.4) | 445 (40.5) | <0.001 | <0.001 |
| Surgical service | 2355 (55.2) | 278 (36.7) | 217 (19.8) | <0.001 | <0.001 |
| HIV/AIDS | 63 (1.5) | 33 (4.4) | 46 (4.2) | <0.001 | <0.001 |
| Malignancy | 408 (9.6) | 144 (19.0) | 135 (12.3) | <0.001 | 0.01 |
| Congestive heart failure | 1478 (34.7) | 239 (31.5) | 501 (45.6) | 0.09 | <0.001 |
| Cardiovascular disease | 433 (10.2) | 49 (6.5) | 121 (11.0) | 0.001 | 0.40 |
| Dementia | 22 (0.5) | 7 (0.9) | 31 (2.8) | 0.17 | <0.001 |
| Diabetes mellitus | 1037 (24.3) | 227 (29.9) | 388 (35.3) | 0.001 | <0.001 |
| Hemiplegia | 1112 (2.6) | 19 (2.5) | 22 (2.0) | 0.84 | 0.24 |
| Metastatic solid tumor | 399 (9.4) | 89 (11.7) | 65 (5.9) | 0.04 | <0.001 |
| Myocardial infarction | 538 (12.6) | 109 (14.4) | 219 (19.9) | 0.18 | <0.001 |
| Liver disease | 847 (19.9) | 102 (13.5) | 140 (12.8) | <0.001 | <0.001 |
| Pulmonary disease | 302 (7.1) | 195 (25.7) | 414 (37.7) | <0.001 | <0.001 |
| Peripheral vascular disease | 594 (13.9) | 94 (12.4) | 167 (15.2) | 0.26 | 0.28 |
| Rheumatic disease | 124 (2.9) | 31 (4.1) | 41 (3.7) | 0.08 | 0.16 |
| Peptic ulcer disease | 113 (2.7) | 17 (2.2) | 27 (2.5) | 0.52 | 0.72 |
| Age (SD) | 59.1 (16.3) | 64.0 (16.5) | 65.6 (14.9) | <0.001 | <0.001 |
| Baseline creatinine, mg/dl (SD) | 0.80 (0.26) | 0.79 (0.26) | 0.84 (0.26) | 0.35 | <0.001 |
| Hospital stay before AKI (d), median (IQR) | 3 (2–8) | 3 (2–7) | 3 (1–6) | 0.26 | <0.001 |
| Onset of AKI to doubling of creatinine (d), median (IQR) | 1 (0–2) | 1 (0–1) | 1 (0–2) | <0.001 | 0.15 |
| Time from AKI to RRT( d), median (IQR)a | 4 (1–9) | 6 (2–9) | 3.5 (1–9) | 0.09 | 0.39 |
Data expressed as n (%) except where noted. HUP, Hospital of the University of Pennsylvania; PAH, Pennsylvania Hospital; PMC, Presbyterian Medical Center; RRT, renal replacement therapy.
Among patients receiving RRT.
Derivation of Predictive Models
A model predicting time to dialysis was developed in the derivation cohort (Table 2). In unadjusted analyses, patients who received dialysis at some point during follow-up were more often male, less often black, and more often on a surgical service. Baseline creatinine was higher in the dialyzed group.
Table 2.
Intention-to-treat Cox proportional hazards model of associations between key covariates and time to initiation of dialytic therapy
| Covariate | Univariable Analysis, Derivation Cohort | Parsimonious Multivariable Model, Derivation Cohort | Short Multivariable Model, Derivation Cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Demographics | ||||||
| Age (per 10 yr) | 0.96 (0.90–1.02) | 0.20 | ||||
| Male sex | 1.52 (1.20–1.92) | 0.001 | ||||
| Black (versus other races) | 0.53 (0.39–0.74) | <0.001 | 0.63 (0.46–0.86) | 0.004 | ||
| Hospital location | ||||||
| Surgical service (versusmedical service) | 1.05 (0.83–1.33) | 0.66 | 0.70 (0.55–0.90) | 0.01 | ||
| ICU (versus other hospital location) | 5.68 (4.55–7.09) | <0.001 | 1.93 (1.52–2.45) | <0.001 | 2.98 (2.35–3.78) | <0.001 |
| Comorbidities (present at admission) | ||||||
| HIV | 0.15 (0.03–0.76) | 0.02 | ||||
| Congestive heart failure | 1.19 (0.95–1.50) | 0.13 | ||||
| Diabetes | 1.06 (0.82–1.37) | 0.64 | 1.27 (1.00–1.62) | 0.05 | ||
| Liver disease | 3.70 (2.95–4.63) | <0.001 | 2.45 (1.94–3.09) | <0.001 | 2.75 (2.18–3.47) | <0.001 |
| Malignancy | 0.72 (0.46–1.11) | 0.14 | ||||
| Metastatic solid tumor | 0.86 (0.53–1.42) | 0.56 | ||||
| Myocardial infarction | 1.13 (0.82–1.55) | 0.46 | ||||
| Peptic ulcer disease | 2.15 (1.32–3.50) | 0.002 | ||||
| Peripheral vascular disease | 1.10 (0.81–1.49) | 0.54 | ||||
| Pulmonary disease | 0.73 (0.45–1.20) | 0.22 | ||||
| Laboratory values | ||||||
| Baseline creatinine (per 0.1 mg/dl) | 1.19 (1.14–1.24) | <0.001 | ||||
| Bicarbonate (per mEq/L) | 0.86 (0.84–0.88) | <0.001 | 0.95 (0.93–0.98) | <0.001 | ||
| BUN (per 10 mg/dl) | 1.27 (1.24–1.30) | <0.001 | 1.07 (1.03–1.10) | <0.001 | ||
| Change in creatinine from day prior (per 0.1 mg/dl) | 1.09 (1.08–1.11) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Creatinine (per mg/dl) | 1.86 (1.75–1.99) | <0.001 | 1.53 (1.41–1.67) | <0.001 | 1.64 (1.54–1.76) | <0.001 |
| Potassium (per mEq/L) | 2.08 (1.94–2.23) | <0.001 | 1.27 (1.16–1.40) | <0.001 | 1.35 (1.23–1.48) | <0.001 |
| Medications | ||||||
| Number of sedatives (per drug) | 1.81 (1.68–1.94) | <0.001 | 1.14 (1.04–1.26) | 0.01 | ||
| Prior aminoglycoside exposure | 1.20 (0.95–1.53) | 0.13 | ||||
| Number of antibiotics (per drug) | 1.42 (1.35–1.49) | <0.001 | 1.12 (1.06–1.19) | <0.001 | ||
| Number of pressors (per drug) | 2.12 (1.98–2.26) | <0.001 | 1.28 (1.18–1.40) | <0.001 | 1.44 (1.33–1.55) | <0.001 |
| Paralytic exposure | 4.55 (3.50–5.92) | <0.001 | 1.40 (1.03–1.90) | 0.03 | ||
| Thiazide diuretic | 2.25 (1.83–2.76) | <0.001 | 1.63 (1.33–2.00) | <0.001 | ||
| Other orders | ||||||
| Ventilated | 3.71 (3.07–4.49) | <0.001 | 1.22 (0.99–1.51) | 0.06 | ||
n=35,462 patient-days, 3951 patients, and 471 outcome events. Data were censored at discharge, death, or 14 days from the onset of AKI, whichever came first. Data excludes patient-days after placement of a do not resuscitate order. HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
After multivariable adjustment, ICU status, liver disease, and creatinine metrics were among the variables most strongly associated with rate of dialysis initiation. Higher serum potassium, BUN, and lower bicarbonate concentrations were independently associated with dialysis initiation as well. Medications associated with severe illness (such as pressors and sedatives) were additionally associated with higher rates of initiation of dialysis, as was the use of thiazide diuretics.
Table 3 describes a model predicting time to 14-day mortality. ICU status, the presence of metastatic cancer, number of pressor medications, higher serum BUN, and a greater rate of change in creatinine (over the past 24 hours) were highly associated with mortality. Surgical patients were significantly less likely to suffer this outcome in adjusted analyses. A higher absolute serum creatinine concentration was protective, as was the utilization of thiazide diuretics.
Table 3.
Intention-to-treat Cox proportional hazards model of associations between key covariates and time to all-cause mortality
| Covariate | Univariable Analysis, Derivation Cohort | Parsimonious Multivariable Model, Derivation Cohort | Short Multivariable Model, Derivation Cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Demographics | ||||||
| Age (per 10 yr) | 1.05 (0.99–1.12) | 0.08 | 1.11 (1.04–1.19) | 0.003 | ||
| Male sex | 1.19 (0.99–1.43) | 0.06 | ||||
| Black (versus other races) | 0.70 (0.55–0.89) | 0.003 | 0.81 (0.63–1.03) | 0.09 | ||
| Hospital location | ||||||
| Surgical service (versus medical service) | 0.51 (0.42–0.61) | <0.001 | 0.39 (0.32–0.47) | <0.001 | 0.36 (0.30–0.43) | <0.001 |
| ICU (versus other hospital location) | 4.39 (3.70–5.21) | <0.001 | 2.07 (1.69–2.53) | <0.001 | 2.97 (2.46–3.59) | <0.001 |
| Comorbidities (present at admission) | ||||||
| HIV | 0.87 (0.36–2.09) | 0.75 | ||||
| Congestive heart failure | 0.93 (0.77–1.12) | 0.45 | 0.80 (0.65–0.99) | 0.04 | ||
| Diabetes | 0.75 (0.59–0.94) | 0.01 | ||||
| Liver disease | 1.90 (1.56–2.32) | <0.001 | 1.48 (1.21–1.81) | <0.001 | ||
| Malignancy | 1.91 (1.47–2.48) | <0.001 | 1.31 (0.99–1.73) | 0.06 | ||
| Metastatic solid tumor | 2.64 (2.06–3.40) | <0.001 | 2.18 (1.67–2.84) | <0.001 | 2.79 (2.16–3.61) | <0.001 |
| Myocardial infarction | 1.13 (0.87–1.48) | 0.35 | ||||
| Peptic ulcer disease | 1.23 (0.71–2.12) | 0.46 | ||||
| Peripheral vascular disease | 1.02 (0.78–1.33) | 0.91 | ||||
| Pulmonary disease | 0.95 (0.92–0.99) | 0.02 | ||||
| Laboratory values | ||||||
| Baseline creatinine (per 0.1 mg/dl) | 1.04 (1.00–1.08) | 0.05 | ||||
| Bicarbonate (per mEq/L) | 0.88 (0.86–0.89) | <0.001 | 0.95 (0.93–0.97) | <0.001 | ||
| BUN (per 10 mg/dl) | 1.18 (1.16–1.21) | <0.001 | 1.14 (1.10–1.18) | <0.001 | 1.12 (1.09–1.15) | <0.001 |
| Change in creatinine from day prior (per 0.1 mg/dl) | 1.06 (1.05–1.07) | <0.001 | 1.05 (1.04–1.06) | <0.001 | 1.05 (1.04–1.05) | <0.001 |
| Creatinine (per mg/dl) | 1.31 (1.24–1.39) | <0.001 | 0.81 (0.73–0.90) | <0.001 | ||
| Potassium (per mEq/L) | 1.70 (1.57–1.83) | <0.001 | 1.16 (1.06–1.26) | 0.001 | ||
| Medications | ||||||
| Number of sedatives (per drug) | 1.69 (1.59–1.79) | <0.001 | ||||
| Prior aminoglycoside exposure | 0.92 (0.74–1.13) | 0.42 | 0.77 (0.61–0.96) | 0.01 | ||
| Number of antibiotics (per drug) | 1.42 (1.36–1.48) | <0.001 | 1.08 (1.02–1.14) | 0.01 | ||
| Number of pressors (per drug) | 1.83 (1.72–1.94) | <0.001 | 1.38 (1.26–1.50) | <0.001 | 1.55 (1.43–1.67) | <0.001 |
| Paralytic exposure | 4.60 (3.64–5.80) | <0.001 | 1.95 (1.53–2.50) | <0.001 | ||
| Thiazide diuretic | 0.67 (0.52–0.87) | 0.002 | 0.60 (0.47–0.78) | <0.001 | ||
| Other orders | ||||||
| Ventilated | 3.65 (3.12–4.27) | <0.001 | 1.43 (1.20–1.70) | <0.001 | ||
n=35,028 patient-days, 3901 patients, and 778 outcome events. Data were censored at 14 days from the onset of AKI or August 31, 2010, whichever came first. Data excludes patient-days after initiation of dialysis or placement of a do not resuscitate order. HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
Table 4 describes a model of the time to the combined endpoint of dialysis or death within 14 days, and demonstrates similar associations.
Table 4.
Intention-to-treat Cox proportional hazards model of associations between key covariates and time to all-cause mortality or initiation of dialysis
| Covariate | Univariable Analysis, Derivation Cohort | Parsimonious Multivariable Model, Derivation Cohort | Short Multivariable Model, Derivation Cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Demographics | ||||||
| Age (per 10 yr) | 1.01 (0.96–1.06) | 0.89 | 1.05 (0.99–1.11) | 0.08 | ||
| Male sex | 1.31 (1.12–1.54) | 0.001 | ||||
| Black (versus other races) | 0.60 (0.49–0.74) | <0.001 | 0.68 (0.55–0.84) | <0.001 | ||
| Hospital location | ||||||
| Surgical service (versus medical service) | 0.68 (0.58–0.79) | <0.001 | 0.48 (0.41–0.57) | <0.001 | 0.45 (0.39–0.53) | <0.001 |
| ICU (versus other hospital location) | 4.82 (4.16–5.57) | <0.001 | 1.91 (1.61–2.27) | <0.001 | 3.12 (2.67–3.65) | <0.001 |
| Comorbidities (present at admission) | ||||||
| HIV | 0.47 (0.20–1.08) | 0.22 | ||||
| Congestive heart failure | 1.07 (0.91–1.25) | 0.37 | 0.86 (0.73–1.02) | 0.08 | ||
| Diabetes | 0.93 (0.77–1.11) | 0.23 | ||||
| Liver disease | 2.61 (2.22–3.07) | <0.001 | 1.92 (1.63–2.27) | <0.001 | 2.12 (1.81–2.50) | <0.001 |
| Malignancy | 1.35 (1.06–1.72) | 0.01 | ||||
| Metastatic solid tumor | 1.78 (1.40–2.26) | <0.001 | 1.67 (1.31–2.13) | <0.001 | ||
| Myocardial infarction | 1.13 (0.91–1.42) | 0.33 | ||||
| Peptic ulcer disease | 1.61 (1.08–2.39) | 0.02 | ||||
| Peripheral vascular disease | 1.11 (0.89–1.37) | 0.61 | ||||
| Pulmonary disease | 0.82 (0.59–1.13) | 0.25 | ||||
| Laboratory values | ||||||
| Baseline creatinine (per 0.1 mg/dl) | 1.11 (1.07–1.14) | <0.001 | ||||
| Bicarbonate (per mEq/L) | 0.87 (0.85–0.88) | <0.001 | 0.96 (0.94–0.97) | <0.001 | ||
| BUN (per 10 mg/dl) | 1.23 (1.21–1.26) | <0.001 | 1.09 (1.06–1.12) | <0.001 | ||
| Change in creatinine from day prior (per 0.1 mg/dl) | 1.08 (1.07–1.10) | <0.001 | 1.04 (1.03–1.04) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| Creatinine (per mg/dl) | 1.66 (1.58–1.76) | <0.001 | 1.26 (1.17–1.35) | <0.001 | 1.42 (1.35–1.50) | <0.001 |
| Potassium (per mEq/L) | 1.92 (1.81–2.03) | <0.001 | 1.24 (1.16–1.33) | <0.001 | ||
| Medications | ||||||
| Number of sedatives (per drug) | 1.73 (1.64–1.82) | <0.001 | 1.08 (1.01–1.16) | 0.02 | ||
| Prior aminoglycoside exposure | 1.03 (0.86–1.22) | 0.83 | ||||
| Number of antibiotics (per drug) | 1.42 (1.37–1.47) | <0.001 | 1.09 (1.05–1.14) | <0.001 | ||
| Number of pressors (per drug) | 1.98 (1.88–2.09) | <0.001 | 1.32 (1.24–1.42) | <0.001 | 1.52 (1.44–1.61) | <0.001 |
| Paralytic exposure | 4.39 (3.60–5.34) | <0.001 | 1.61 (1.30–1.99) | <0.001 | ||
| Thiazide diuretic | 1.35 (1.13–1.61) | 0.002 | ||||
| Other orders | ||||||
| Ventilated | 3.62 (3.17–4.14) | <0.001 | 1.34 (1.15–1.56) | <0.001 | ||
n=35,462 patient-days, 3951 patients, and 1031 outcome events. Data censored at 14 days from the onset of AKI or August 31, 2010, whichever came first. Data exclude patient-days after placement of a do not resuscitate order. HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
Validation of Predictive Models
Table 5 reports the c-statistics for the derivation and validation cohorts. In general, all models demonstrated good discrimination, with models predicting dialysis performing better than models predicting death or the combined endpoint. The c-statistics were similar in the derivation and validation cohorts. The “short models” performed less well than the parsimonious models, but still had significant predictive ability. Models performed equally well in medical versus surgical patients, but demonstrated improved discrimination among ICU patients. The c-statistic for the prediction of dialysis in ICU patients was 0.89 (95% confidence interval [95% CI], 0.88–0.90) versus 0.80 (95% CI, 0.78–0.82) in the non-ICU patients.
Table 5.
c-statistics for derivation and validation cohorts, stratified by outcome of prediction
| Parsimonious Model (95% CI) | Short Model (95% CI) | P Value (Short versus Parsimonious) | |
|---|---|---|---|
| Dialysis | |||
| Derivation cohort (HUP 2004–2010) | 0.89 (0.88–0.91) | 0.88 (0.87–0.89) | <0.001a |
| Validation (PAH 2006–2010) | 0.89 (0.85–0.93) | 0.89 (0.85–0.93) | 0.48 |
| Validation (PMC 2004–2010) | 0.89 (0.86–0.92) | 0.88 (0.85–0.91) | 0.23 |
| Death | |||
| Derivation cohort (HUP 2004–2010) | 0.83 (0.82–0.84) | 0.80 (0.79–0.81) | <0.001a |
| Validation (PAH 2006–2010) | 0.83 (0.80–0.86) | 0.79 (0.76–0.83) | <0.001 |
| Validation (PMC 2004–2010) | 0.82 (0.79–0.85) | 0.79 (0.75–0.82) | <0.001 |
| Dialysis or death | |||
| Derivation cohort (HUP 2004–2010) | 0.85 (0.84–0.86) | 0.82 (0.81–0.83) | <0.001a |
| Validation (PAH 2006–2010) | 0.83 (0.80–0.86) | 0.77 (0.73–0.80) | <0.001 |
| Validation (PMC 2004–2010) | 0.84 (0.82–0.86) | 0.80 (0.78–0.83) | <0.001 |
P value reflects difference in predictive ability between short and parsimonious models. CI, confidence interval; HUP, Hospital of the University of Pennsylvania; PAH, Pennsylvania Hospital; PMC, Presbyterian Medical Center.
P values may be unreliable in cohort from which model was derived
Because this study included patients based on creatinine (not estimated GFR [eGFR]) criteria, some patients had a baseline eGFR <60 ml/min. We examined the parsimonious model performance in this group of individuals (n=529) specifically. In the model predicting dialysis, the c-statistic was 0.85 (95% CI, 0.83–0.88). For the model predicting death, the c-statistic was 0.74 (95% CI, 0.70–0.79). For the combined model, the c-statistic was 0.80 (95% CI, 0.78–0.83).
Finally, the model predicting death was compared with the SOFA model. Overall, the SOFA score performed admirably in this population, but worse than our derived models. The c-statistics for the SOFA score were 0.76 (95% CI, 0.71–0.81) and 0.74 (95% CI, 0.70–0.78) in the validation cohorts (for comparison with the parsimonious model, P<0.001 for all analyses).
Predictors of Death or Dialysis at AKIN Stage 2
Given that predicting death or dialysis earlier in the course of AKI may have particular value, we re-derived and re-validated our models using only covariates from the first day of AKI that met AKIN stage 2 criteria (a doubling of creatinine from baseline) (18). Table 6 reports hazard ratios (HRs) for these models in the derivation cohort. The c-statistics for the parsimonious early model were 0.83 (95% CI, 0.81–0.84) in the derivation cohort and 0.79 (95% CI, 0.74–0.83) and 0.82 (95% CI, 0.79–0.85) in the validation cohorts.
Table 6.
Intention-to-treat Cox proportional hazards model of associations between key covariates and time to all-cause mortality or initiation of dialysis based on covariates measured on the day creatinine doubled from baseline (AKIN stage 2 criteria)
| Covariate | Univariable Analysis, Derivation Cohort | Parsimonious Multivariable Model, Derivation Cohort | Short Multivariable Model, Derivation Cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Demographics | ||||||
| Age (per 10 yr) | 1.00 (0.96–1.04) | 0.98 | ||||
| Male sex | 1.33 (1.17–1.52) | <0.001 | ||||
| Black (versus other races) | 0.64 (0.55–0.76) | <0.001 | 0.80 (0.67–0.95) | 0.01 | ||
| Hospital location | ||||||
| Surgical service (versus medical service) | 0.82 (0.72–0.93) | 0.002 | 0.57 (0.49–0.66) | <0.001 | 0.64 (0.55–0.74) | <0.001 |
| ICU (versus other hospital location) | 4.48 (3.86–5.19) | <0.001 | 1.82 (1.51–2.20) | <0.001 | ||
| Comorbidities (present at admission) | ||||||
| HIV | 0.64 (0.33–1.22) | 0.17 | ||||
| Congestive heart failure | 1.17 (1.03–1.33) | 0.02 | 0.88 (0.76–1.01) | 0.06 | ||
| Diabetes | 0.85 (0.73–0.99) | 0.04 | ||||
| Liver disease | 2.37 (2.08–2.69) | <0.001 | 1.60 (1.39–1.85) | <0.001 | 1.61 (1.40–1.86) | <0.001 |
| Malignancy | 1.47 (1.22–1.78) | <0.001 | ||||
| Metastatic solid tumor | 1.57 (1.31–1.89) | <0.001 | 1.58 (1.25–2.01) | <0.001 | ||
| Myocardial infarction | 1.28 (1.07–1.52) | <0.001 | ||||
| Peptic ulcer disease | 1.73 (1.27–2.34) | <0.001 | ||||
| Peripheral vascular disease | 1.18 (1.00–1.40) | 0.06 | ||||
| Pulmonary disease | 0.84 (0.65–1.09) | 0.19 | ||||
| Laboratory values | ||||||
| Baseline creatinine (per 0.1 mg/dl) | 1.12 (1.10–1.14) | <0.001 | ||||
| Bicarbonate (per mEq/L) | 0,86 (0,85–0.87) | <0.001 | 0.93 (0.92–0.95) | <0.001 | 0.91 (0.89–0.92) | <0.001 |
| BUN (per 10 mg/dl) | 1.17 (1.15–1.20) | <0.001 | 1.12 (1.09–1.15) | <0.001 | 1.15 (1.12–1.18) | <0.001 |
| Change in creatinine from day prior (per 0.1 mg/dl) | 1.05 (1.04–1.06) | <0.001 | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Creatinine (per mg/dl) | 1.58 (1.44–1.73) | <0.001 | ||||
| Potassium (per mEq/L) | 1.80 (1.69–1.91) | <0.001 | 1.23 (1.13–1.33) | <0.001 | ||
| Medications | ||||||
| Number of sedatives (per drug) | 1.66 (1.57–1.74) | <0.001 | ||||
| Prior aminoglycoside exposure | 1.19 (1.03–1.38) | 0.02 | ||||
| Number of antibiotics (per drug) | 1.39 (1.34–1.44) | <0.001 | 1.07 (1.02–1.12) | 0.003 | ||
| Number of pressors (per drug) | 1.87 (1.78–1.97) | <0.001 | 1.34 (1.25–1.44) | <0.001 | 1.69 (1.59–1.80) | <0.001 |
| Paralytic exposure | 3.53 (2.89–4.30) | <0.001 | 1.48 (1.19–1.85) | <0.001 | ||
| Thiazide diuretic | 1.17 (0.98–1.40) | 0.09 | ||||
| Other orders | ||||||
| Ventilated | 3.78 (3.33–4.28) | <0.001 | 1.58 (1.35–1.86) | <0.001 | ||
n=3848 patient-days, 3848 patients, and 954 outcome events. Data were censored at 14 days from the onset of AKI or August 31, 2010, whichever came first. AKIN, Acute Kidney Injury Network; HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
Sensitivity Analyses
We varied model construction in several ways to determine if the same parsimonious predictors of outcomes would arise. We varied our definition of “change in serum creatinine” from the absolute change from the day before the relative change. Parsimonious models were largely unchanged save for the retention of the baseline creatinine variable in the model predicting dialysis (HR per 0.1 mg/dl increase in baseline creatinine, 1.09; 95% CI, 1.02–1.16; P=0.01), the exclusion of creatinine and relative change in creatinine from the model predicting death, and the exclusion of relative change in creatinine in the model predicting the combined outcome. Finally, we performed a model using AKIN stage 3 AKI as the outcome of interest (Supplemental Table 1). This model retained covariates that were very similar to the model predicting dialysis. The c-statistics for the performance of the parsimonious model examining this outcome in the validation cohorts were 0.81 (95% CI, 0.79–0.84) and 0.82 (95% CI, 0.80–0.85).
Discussion
Risk-stratifying patients with AKI is vital for appropriate allocation of resources, informed discussions with caregivers, and identifying patients most likely to benefit from therapeutic interventions. In this study, we document that a small group of easily measured clinical factors has good ability to predict mortality and the need for dialysis in severe AKI. Here we present data on a large and diverse AKI population, spanning three hospitals in a health system, which includes patients regardless of ICU, surgical, or medical status. To our knowledge, this is the first study of its kind to analyze data at the patient-day level using intention-to-treat Cox regression, allowing application of these models at any time point ranging from onset of AKI to dialysis, death, or hospital discharge.
Models based upon these data may serve as a baseline to assess the added value of novel biomarkers of early AKI future studies. The overall predictive power of our statistical models was particularly surprising, because reliable urine output data were not available for this cohort. Although oliguria and anuria are, no doubt, powerful predictors of dialysis, they are present in a minority of patients with AKI (10) and may be reflected by other measures of severity of AKI that were obtained in this cohort.
In unadjusted analyses, baseline creatinine was higher in the dialyzed group (although still within a normal range), suggesting that even mild baseline renal insufficiency predisposes to RRT in the setting of AKI. We note that adjustment for time-varying BUN and serum bicarbonate ablates this association. This suggests that those with higher baseline creatinine are more predisposed to the metabolic consequences of AKI due to lower renal reserve capacity.
Our adjusted model demonstrated several notable associations with the provision of RRT, including a “protective” effect of black race, even in the presence of multivariable adjustment. The unadjusted HR for dialysis among black patients was 0.54 (95% CI, 0.39–0.74; P<0.001), despite no difference in peak creatinine concentrations (P=0.76). Mean baseline creatinine was 0.80 mg/dl in black patients and 0.81 mg/dl in nonblack patients (P=0.59). Peak potassium was slightly lower in black patients at 5.0 (SD 0.9) mEq/L versus 5.1 (SD 0.9) mEq/L (P<0.001), as was peak BUN at 41 (SD 27) mg/dl versus 54 (SD 32) mg/dl (P<0.001). Minimum bicarbonate did not differ between the groups at 19 (SD 5) mEq/L among black patients versus 19 (SD 6) mEq/L among nonblack patients (P=0.07). One plausible explanation for these findings is that the black patients suffered less severe renal insults, despite similar increases in creatinine concentration, perhaps due to their higher muscle mass. Supporting this hypothesis, the unadjusted in-hospital mortality rate among black patients within this cohort was 21% versus 29% in nonblack patients (P<0.001). Similar findings were reported in the Nationwide Inpatient Sample by Waikar et al. (25). Nonetheless, this finding may also signal systematic differences in access to RRT across race groups, a possibility that will need to be assessed prospectively in a broader set of healthcare settings.
Thiazide diuretic usage was strongly associated with the provision of dialysis, but was protective in terms of mortality. This result is difficult to interpret without reliable urine output data. We hypothesize that the use of this class of drugs may identify patients with oliguria who are at increased risk of requiring dialysis, but may in fact be efficacious in terms of improving volume management, because volume overload has been shown to be a significant predictor of mortality in AKI (26). Presence of thiazide diuretic usage remained protective even in analyses restricted to the never-dialyzed, with a multivariable adjusted HR of 0.59 (95% CI, 0.42–0.83; P=0.003). Interestingly, loop diuretic usage was not associated with initiation of dialysis when added to the parsimonious predictive model (P=0.81), perhaps because of its broader use in the AKI population.
The presence of liver disease was a strong predictor of dialysis, death, and the combined endpoint in all of our models, and identifies a group of patients who are at particular risk for these events. Although the higher incidence of death and dialysis in patients with liver disease may be due to the metabolic disturbance inherent to that diagnosis, we suspect that decreased creatinine generation (due to muscle wasting) and increased volume of distribution of creatinine blunt the rise of serum creatinine in AKI. This would cause patients with liver disease to appear to have less severe renal injury in adjusted analyses, despite perhaps very poor true GFR.
Although many of the HRs in Tables 2 and 3 are similar in magnitude and sign, certain covariates had inverse relationships. Higher serum creatinine concentration was strongly associated with the provision of dialysis, but was negatively associated with all-cause mortality after multivariable adjustment. In fact, merely adjusting for BUN and serum bicarbonate concentration yielded a HR for death of 0.87 (95% CI, 0.79–0.95; P=0.002) per mg/dl serum creatinine concentration. Modeling the creatinine × BUN interaction reveals a reduction in the HR of creatinine on death by 3.6% (95% CI, 1.8%–5.5%; P<0.001) for each 10 mg/dl increase in serum BUN concentration. Although a faster rate of change in creatinine (associated with both dialysis and death) may be a marker of severity of renal injury, we believe that the absolute creatinine concentration (after adjustment for other GFR proxies) is reflective of higher muscle mass. This result is consistent with associations seen in chronic hemodialysis patients, where higher serum creatinine (no longer a measure of GFR but reflective of muscle mass) is protective (27,28), and with the results of the Cleveland Clinic Mortality in Renal Failure Model (4).
This study should be interpreted in light of notable limitations. It was conducted within a single health system where nephrologic care may differ from other settings. However, the study did include three separate hospitals, each with an independent nephrology practice, and the predictive models generally performed well in the validation settings. The model predicting death is only generalizable to patients with AKI before the initiation of dialysis, because patient-days after the initiation of dialysis were excluded from model-building. This cohort, by design, excluded patients with abnormal baseline creatinine, in an attempt to limit patients with preexisting CKD or AKI. This was done to allow accurate assessment of AKI onset, the crucial attribute that was used to assemble our study cohort. Therefore, these findings are not necessarily generalizable to the AKI population with preexisting CKD. That said, the baseline eGFR was <60 ml/min per 1.73 m2 in 304 patients (7.7%), likely due to inclusion of elderly patients within the creatinine cut-off range. These patients had a higher rate of death and dialysis than the overall cohort, and our predictive models were not as potent within this subgroup but still performed well. Our cohort also only includes patients who suffered a “severe” kidney injury of AKIN stage 2 or greater (18). This may have reduced generalizability and introduced a survival bias, because patients were required to develop a serum creatinine twice their baseline to be included in analysis. Finally, reliable measurements of urine output were not available in this cohort. Low urine output is likely a strong predictor of the provision of dialysis and may predict death as well. The strong performance of our predictive models in the absence of these data may suggest that other covariates in the models are a proxy for oliguria.
Although external validation of these results is necessary, scoring systems based upon these factors may help clinicians identify those patients at highest risk for progressive AKI, leading to early intervention and potentially improved management of this highly morbid condition.
Disclosures
H.I.F. is an expert consultant to GlaxoSmithKline, is a consultant to the National Institutes of Health, and has had various speaking engagements.
Acknowledgments
We thank the Penn Data Store for their assistance in assembling the laboratory and medication databases used in this study.
This research was conducted under Grant 1F32DK093223-01 from the National Institute of Diabetes and Digestive and Kidney Diseases awarded to F.P.W.
These data were presented in abstract form at the Annual Meeting of the American Society of Nephrology, October 30–November 4, 2012, in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06450612/-/DCSupplemental.
References
- 1.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, Golgran-Toledano D, Descorps-Declere A, Chemouni F, Hamidfar-Roy R, Azoulay E, Timsit JF: Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: A competing risks analysis. Crit Care 15: R128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douma CE, Redekop WK, van der Meulen JH, van Olden RW, Haeck J, Struijk DG, Krediet RT: Predicting mortality in intensive care patients with acute renal failure treated with dialysis. J Am Soc Nephrol 8: 111–117, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Paganini EP, Halstenberg WK, Goormastic M: Risk modeling in acute renal failure requiring dialysis: The introduction of a new model. Clin Nephrol 46: 206–211, 1996 [PubMed] [Google Scholar]
- 5.Sasaki S, Gando S, Kobayashi S, Nanzaki S, Ushitani T, Morimoto Y, Demmotsu O: Predictors of mortality in patients treated with continuous hemodiafiltration for acute renal failure in an intensive care setting. ASAIO J 47: 86–91, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Tsai CW, Lin YF, Wu VC, Chu TS, Chen YM, Hu FC, Wu KD, Ko WJ, NSARF Study Group : SAPS 3 at dialysis commencement is predictive of hospital mortality in patients supported by extracorporeal membrane oxygenation and acute dialysis. Eur J Cardiothorac Surg 34: 1158–1164, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Maccariello E, Valente C, Nogueira L, Bonomo H, Ismael M, Machado JE, Baldotto F, Godinho M, Valença R, Rocha E, Soares M: SAPS 3 scores at the start of renal replacement therapy predict mortality in critically ill patients with acute kidney injury. Kidney Int 77: 51–56, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Brar H, Olivier J, Lebrun C, Gabbard W, Fulop T, Schmidt D: Predictors of mortality in a cohort of intensive care unit patients with acute renal failure receiving continuous renal replacement therapy. Am J Med Sci 335: 342–347, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Franzen D, Rupprecht C, Hauri D, Bleisch JA, Staubli M, Puhan MA: Predicting outcomes in critically ill patients with acute kidney injury undergoing intermittent hemodialysis—a retrospective cohort analysis. Int J Artif Organs 33: 15–21, 2010 [PubMed] [Google Scholar]
- 10.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM, Program to Improve Care in Acute Renal Disease : Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Leacche M, Winkelmayer WC, Paul S, Lin J, Unic D, Rawn JD, Cohn LH, Byrne JG: Predicting survival in patients requiring renal replacement therapy after cardiac surgery. Ann Thorac Surg 81: 1385–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, Masson JB, Gutiérrez MJ, Clavel MA, Bertrand OF, Pibarot P, Rodés-Cabau J: Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 31: 865–874, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle JM, Moualla S, Arrigain S, Worley S, Bakri MH, Starling RC, Heyka R, Thakar CV: Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am J Kidney Dis 48: 787–796, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Candela-Toha A, Elías-Martín E, Abraira V, Tenorio MT, Parise D, de Pablo A, Centella T, Liaño F: Predicting acute renal failure after cardiac surgery: External validation of two new clinical scores. Clin J Am Soc Nephrol 3: 1260–1265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James MT, Wald R, Bell CM, Tonelli M, Hemmelgarn BR, Waikar SS, Chertow GM: Weekend hospital admission, acute kidney injury, and mortality. J Am Soc Nephrol 21: 845–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez WG, Hung O, Bruno GR, Galea S, Chiang WK: Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am J Emerg Med 23: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH, The Auriculin Anaritide Acute Renal Failure Study Group : Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. J Am Soc Nephrol 9: 692–698, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y-F, Ko W-J, Wu V-C, Chen Y-S, Chen Y-M, Hu F-C, Shiao CC, Wu MS, Chen YW, Li WY, Huang TM, Wu KD, Chu TS, NSARF Study Group : A modified sequential organ failure assessment score to predict hospital mortality of postoperative acute renal failure patients requiring renal replacement therapy. Blood Purif 26: 547–554, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM: Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 19: 766–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newson R: Confidence intervals for rank statistics: Somers' D and extensions. Stata J 6: 309–334, 2006 [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Waikar SS, Curhan GC, Ayanian JZ, Chertow GM: Race and mortality after acute renal failure. J Am Soc Nephrol 18: 2740–2748, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network : Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 6: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink JC, Burdick RA, Kurth SJ, Blahut SA, Armistead NC, Turner MS, Shickle LM, Light PD: Significance of serum creatinine values in new end-stage renal disease patients. Am J Kidney Dis 34: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482, 1990 [DOI] [PubMed] [Google Scholar]