Summary
Background and objectives
Atherosclerotic renal artery stenosis (ARAS) reduces renal blood flow and is a potential cause of chronic kidney injury, yet little is known regarding inflammatory pathways in this disorder in human participants. This study aimed to examine the hypothesis that reduced renal blood flow (RBF) in ARAS would be associated with tissue TGF-β activation and inflammatory cell accumulation.
Design, setting, participants, & measurements
This cross-sectional study of ARAS of varying severity compared transjugular biopsy specimens in patients with ARAS (n=12, recruited between 2008 and 2012) with tissue from healthy kidney donors (n=15) and nephrectomy specimens from individuals with total vascular occlusion (n=65). ARAS patients were studied under controlled conditions to measure RBF by multidetector computed tomography and tissue oxygenation by blood oxygen level–dependent magnetic resonance imaging.
Results
Compared with the nonstenotic contralateral kidneys, RBF was reduced in poststenotic kidneys (242±149 versus 365+174 ml/min; P<0.01) as was single-kidney GFR (28±17 versus 41±19 ml/min; P<0.01), whereas cortical and medullary oxygenation were relatively preserved. Tissue TGF-β immunoreactivity was higher in ARAS patients compared with those with both normal kidneys and those with total occlusion (mean score 2.4±0.7 versus 1.5+1.1 in the nephrectomy group and versus 0±0 in donors; P<0.01). By contrast, the number of CD68+ macrophages was higher with greater disease severity (from 2.2±2.7 in normal to 22.4±18 cells/high-power field in nephrectomy samples; P<0.001).
Conclusions
The results of this study indicate robust stimulation of TGF-β associated with macrophage infiltration within the human kidney with vascular occlusive disease.
Introduction
Vascular occlusion in patients with atherosclerotic renal artery stenosis (ARAS) reduces blood flow and GFR, impairs BP control, and can ultimately lead to irreversible tissue damage (1). Although hemodynamically significant ARAS commonly reduces kidney volume, some patients tolerate these reductions without evident further loss of kidney function during antihypertensive drug therapy, sometimes for many years (2). However, a subgroup of these patients gradually develops progressive renal injury, in some cases leading to ESRD (3) even after technically successful revascularization. Some authors suggest that between 5% and 16% of patients reaching ESRD have ARAS as a primary etiologic factor (4,5). Experimental models of the renin-angiotensin system (RAS) demonstrate a major rise in gene expression of inflammatory and fibrogenic cytokines, including TGF-β, particularly early in the development of renovascular disease (6–8). Kidneys removed after total arterial occlusion typically demonstrate widespread glomerular collapse with interstitial inflammation and fibrosis (9).
The roles of cytokine signaling including TGF-β are complex and appear to participate in both repair and injury pathways in experimental models.
We undertook the following cross-sectional studies of patients with ARAS of variable severity to test the hypothesis that subcritical vascular occlusion would be associated with both TGF-β activation and recruitment of inflammatory cells.
Materials and Methods
Patient Enrollment and Selection
Patients were identified as part of a clinical investigation of tissue oxygenation in human renovascular disease between 2008 and 2012. Twelve patients underwent transvenous biopsy of the right-sided stenotic kidney via the jugular vein. Inclusion criteria were the presence of unilateral right-sided ARAS >70% obstruction, as previously described (10), and systolic hypertension >155 mmHg and/or the use at least of two antihypertensive medications. Diabetic patients were excluded as well as patients with serum creatinine >2.0 mg/dl. Informed, written consent was obtained after receiving approval from the Institutional Review Board (IRB) of the Mayo Clinic in adherence with the Declaration of Helsinki. All patients received either an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) for hypertension. A 3-day inpatient protocol was performed in the Clinical Research Unit of St. Mary’s Hospital, Rochester, Minnesota. Daily isocaloric sodium intake was maintained at 150 mEq.
Supplementary IRB approval was obtained for review of clinical data and histopathological analysis of two comparison groups. Implantation biopsies obtained from 15 living kidney donors, chosen to have a similar distribution of age and sex, were identified from the Mayo Kidney transplant program as previously described (11). Tissue samples from 65 additional patients subjected to therapeutic nephrectomy for uncontrolled hypertension associated with total renal artery occlusion between 1990 and 2000 (12). Values for BP, serum creatinine, body mass index (BMI), sex, and lipid profiles at the time of nephrectomy were extracted from electronic medical records of the Mayo Clinic.
Histopathological Data
Detailed analysis of interstitial, glomerular, and vascular compartments for each sample was performed by a senior renal pathologist blinded to the sample source. Hematoxylin and eosin, periodic acid–Schiff, and Masson’s trichrome stains were employed. Banff ’97 grading systems (13) were used to assign scores for interstitial, glomerular, and vascular lesions. Each sample was graded for interstitial fibrosis, inflammation, and vascular changes.
Degree of Tubulointerstitial Fibrosis.
Quantitative analysis of interstitial compartment occupied by fibrotic tissue was performed using computer-assisted analysis of tissue sections stained with Masson’s trichrome. Images were acquired using a Nikon DXM1200 digital camera and analysis using MetaImaging Series 4.6, MetaView V4.6.8 software (Fryer Company Inc). The cortical region was analyzed in a stepwise fashion as a series of consecutive fields. For each field, a region of interest (ROI) was traced that included cortical tubules and interstitial space. Glomeruli, large or medium-sized blood vessels, and medullary tissue were excluded from ROI. The degree of interstitial fibrosis and inflammation was scored according to the estimated fraction of affected renal parenchyma: 0 corresponded to <25%; 1, 25%–50%; 2, 51%–75%; and 3, >75% of tissue affected.
Quantification of Tissue TGF-β and Cellular Infiltrates.
Biopsies were stained for TGF-β with mAb against TGF-β1, macrophage cell marker CD68 (Dako monoclonal mouse anti-human CD68, clone KP1), lymphocyte T cell markers CD3 (Dako polyclonal rabbit anti-human CD3) and CD134 (Biolegend purified mouse anti-human CD134 [OX40], clone Ber-ACT35), as well as lymphocyte B cell marker CD20 (Dako monoclonal mouse anti-human CD20cy, B cell, clone L26). TGF-β tissue immunostaining was graded on the following scale: 0, 0%; 1, <25%; 2, 25%–50%; and 3, >50%. Automated counts of stained cells were achieved after setting a colorimetric threshold.
Hemodynamic Data for ARAS Patients
Patients with unilateral ARAS were studied during a 3-day inpatient protocol as reported previously (10). In brief, the first study day included measurement of GFR by iothalamate clearance. BP was measured by automated oscillometric recordings at 4-hour intervals.
On the second day, blood oxygen level–dependent (BOLD) magnetic resonance imaging (MRI) was performed on a GE Twin Speed Signa EXCITE 3.0T system (GE Medical Systems, Waukesha, WI) (14). On the third day of the protocol, the right internal jugular vein was cannulated with a 6F sheath and blood samples were drawn from the right and left renal veins and infrarenal inferior vena cava with a 5F pigtail Cobra catheter (Cook Inc, Bloomington, IN) for venous oxygen and plasma renin activity. The catheter was then exchanged for 5F pigtail, which was placed into the superior vena cava for central venous injection of contrast for transit time studies using a multidetector computed tomography (MDCT) (15). Additional images were reconstructed at 0.6-mm slice thickness for quantitative vascular stenosis evaluation (16). Image analysis was performed using ANALYZE (Biomedical Imaging Resource Center, Mayo Clinic, Rochester, MN). Analysis of MDCT flow studies were undertaken by selecting a ROI in cross-sectional images from the aorta, individual kidney cortex, and medulla (15). Single-kidney blood flows were determined as the sum of medullary and cortical blood flows, defined by medullary and cortical perfusion per cubic centimeter of renal tissue and volumes calculated using the stereology module within ANALYZE. Quantitation of vessel stenosis was undertaken by comparing the area of maximally narrowed cross-sections of the artery to disease-free proximal segments (17).
After completion of MDCT, the jugular vein access sheath was upsized to 9F and patients underwent a venous biopsy of the right kidney using a transjugular biopsy set (Cook Inc).
Biochemical Assays
Renal venous plasma samples for TGF-β1 from ARAS study patients were aliquoted into microtubes and acid activated to immuno-reactive TGF-β detectable by ELISA using the Quantikine TGF-β1 ELISA kit (R&D Systems, Minneapolis, MN). Plasma renin activity (PRA) was measured using RIA (Diasorin, Stillwater, MN), and plasma 8-epi-PGF2α-isoprostanes using enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) as previously described (18).
Statistical Analyses
Results were expressed using means and SDs for normally distributed parameters and analyzed using JMP version 8.1. statistical software. Comparisons between healthy donors, ARAS, and nephrectomy groups were performed with ANOVA as appropriate. A numerical value for renal artery stenosis was assigned as 0% in healthy kidney donors and 100% in the nephrectomy group. Comparisons between stenotic and contralateral kidneys within the same patients were performed using paired t tests. A P value <0.05 was considered statistically significant. Multivariate analysis was performed to study the correlations of TGF-β score with other histopathologic factors, estimated GFR (eGFR), and renal artery stenosis estimates.
Results
Demographics
Clinical characteristics of the three groups are summarized in Table 1. Patients were selected to have a similar distribution of age and sex. Similar proportions of ARAS and nephrectomy groups were taking statins. There was no statistical difference in mean BMI between these groups. Despite antihypertensive drug therapy, BP levels were higher in the ARAS and nephrectomy groups than in healthy kidney donors. Mean serum creatinine levels were lower in kidney donors compared with ARAS patients, and were elevated in individuals undergoing nephrectomy. Values for eGFR were lower in ARAS patients as compared with living kidney donors.
Table 1.
Clinical characteristics of human participants from whom kidney tissue was examined
| Kidney Donors (n=15 Kidneys) | P | Moderate ARAS (n=12 Kidneys) | P | Nephrectomy (n=65 Kidneys) | |
|---|---|---|---|---|---|
| Age (yr) | 61.4±8.4 | 0.12 | 65.4±9.9 | 0.17 | 63.9±10.8 |
| Sex (female/male) | 7/8 | 0.48 | 5/7 | 0.38 | 36/29 |
| BMI | 27±4.2 | 0.54 | 26±4.2 | 0.56 | 27.5±2.9 |
| Creatinine (mg/dl) | 1.0±0.2 | 0.03 | 1.2±0.4 | 0.04 | 1.9±0.7 |
| eGFR (ml/min per 1.73 m2) | 86.1±10.3 | <0.001 | 58.2±23.9 | 0.09 | 45.1±15.4 |
| ACEI or ARB prescription (% of patients) | 0 | <0.001 | 100 | 0.02 | 64.3 |
| Statin prescription (% of patients) | 13.3 | 0.11 | 40 | 0.59 | 30 |
| SBP (mmHg) | 124.6±13.8 | 0.004 | 141.2±16.4 | <0.001 | 177.9±26.4 |
| DBP (mmHg) | 73.5±9.2 | 0.46 | 71.2±7.9 | <0.001 | 92.6±11.3 |
| MAP (mmHg) | 90.5±10.1 | 0.14 | 94.5±8.8 | <0.001 | 121±13.9 |
| Number of antihypertensive drugs | 0.4±0.8 | <0.001 | 2.8±1.1 | 0.74 | 3.2±0.9 |
ARAS, atherosclerotic renal artery stenosis; BMI, body mass index; eGFR, estimated GFR using the Chronic Kidney Disease Epidemiology Collaboration equation; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure.
Kidney Volumes and Blood Flows in the Poststenotic ARAS Kidney
Measurements of kidney volumes, hemodynamics, and tissue oxygenation in ARAS participants are summarized in Table 2. Compared with the contralateral kidney, renal blood flow (RBF), kidney volume, and single-kidney GFR were reduced in the affected poststenotic kidneys. Renal vein measurements indicated higher levels of venous PO2 in kidneys with reduced GFR (P<0.01 versus the contralateral kidney), whereas levels of renal vein renin (PRA) tended to be elevated. Renal vein renin was substantially higher from the stenotic kidney compared with the nonaffected side (median values, 16 versus 5.4 ng/ml per hour; P=0.01) in a nephrectomy subgroup in whom these measurements were made (n=11). In the ARAS cohort, serum levels of TGF-β in the renal veins did not differ between stenotic and contralateral kidneys. Urinary and venous measurements of TGF-β did not correlate with blood flow, TGF-β staining, or kidney function in these studies.
Table 2.
Comparison between stenotic and contralateral kidney in patients with unilateral ARAS (n=12 patients)
| Measured Variable | Stenotic Kidney | Contralateral Kidney | P |
|---|---|---|---|
| Renal blood flow (ml/min) | 241.9±149.1 | 365.6±173.7 | 0.004 |
| Kidney volume (cm3) | 111±46.4 | 154.1±53.4 | 0.01 |
| Single-kidney GFR (ml/min) | 27.8±17.1 | 41±19.4 | 0.01 |
| Renal vein PO2 (mmHg) | 68.9±8.4 | 63.9±8.2 | 0.01 |
| Renal vein TGF-β (ng/ml) | 1659.9±1514.6 | 2126.5±3015.5 | 0.25 |
| Renal vein PRA (ng Ang 1/ml/h) | 20.6±21.5 | 15.3±17.6 | 0.07 |
| Kidney size (long axis, cm) | 10.1±1.5 | 11.1±1.2 | 0.04 |
| Cortical R2* sec−1 | 18.4±3.1 | 17.6±2.3 | 0.08 |
| Medullary R2* sec−1 | 39.3±6 | 40.6±4.1 | 0.34 |
ARAS, atherosclerotic renal artery stenosis; PRA, plasma renin activity; R2*, coefficient of magnetic-field “relaxivity” proportional to the deoxyhemoglobin level.
Remarkably, oxygenation in both cortical and medullary regions measured using BOLD MRI remained within normal ranges in the ARAS group as reported previously (10).
Histopathology
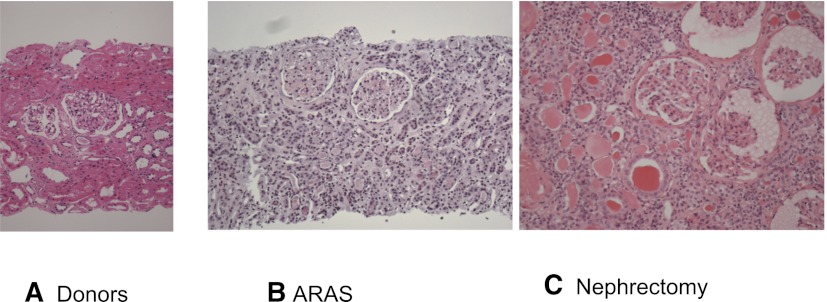
Representative hematoxylin and eosin–stained sections from biopsy and tissue samples are shown in Figure 1. Transjugular biopsies obtained from moderately stenotic kidneys in ARAS patients showed preserved glomerular and tubular structures, but demonstrated a range of interstitial cellular infiltrates. By contrast, samples from nephrectomy participants demonstrated major obliteration and collapse of glomerular and tubular structures with marked diffuse interstitial inflammation and fibrosis.
Figure 1.
Tissue histology in vascular occlusive disease. Tissue sections (H&E stain) from a normal kidney donor at implantation (A), patients with ARAS undergoing transjugular biopsy (B), and a nephrectomy specimen removed for total vascular occlusion of a “pressor” kidney (C). Overall architecture of glomeruli and tubular structures were variable but relatively preserved in ARAS with some degrees of inflammation, as compared with total occlusion where tubular structures are collapsed and largely replaced with fibrosis and inflammatory infiltrates. Original magnification, ×20. H&E, hematoxylin and eosin; ARAS, atherosclerotic renal artery stenosis.
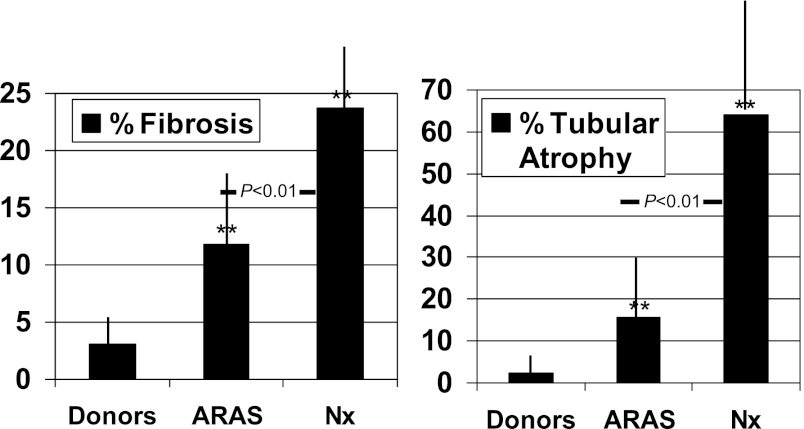
Quantitative estimates for tubular atrophy and fibrosis are illustrated in Figure 2. For each of these measures, the percentage of affected tissue was higher when the renal artery stenosis was more severe, reaching >60% for tubular atrophy in nephrectomy specimens (Figure 2).
Figure 2.
Scores of fibrosis and tubular atrophy. Semi-quantitative scoring (see text) of tissue specimens for the degree of fibrosis and tubular atrophy indicated more extensive changes with the more severe vascular occlusion. **P<0.01 versus donors. ARAS, atherosclerotic renal artery stenosis; Nx, nephrectomy.
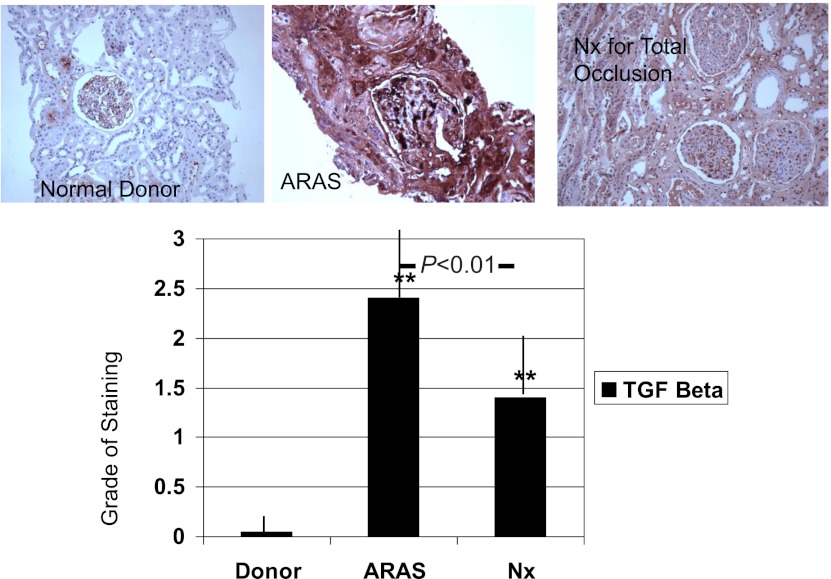
Although ARAS patients had substantially less tissue fibrosis by trichrome on biopsy (ranging from 4% to 26%) compared with nephrectomy samples, semi-quantitative estimates of TGF-β staining were higher in ARAS than those observed in nephrectomy specimens, as illustrated in Figure 3. The correlations between TGF-β staining scores and another histologic finding are summarized in Table 3. For ARAS specimens, the percentage of total RBF delivered to the poststenotic kidney (RBF fraction and absolute RBF on the affected side) was inversely related to the trichrome estimates of fibrosis (R=−0.58; P<0.01) and interstitial inflammation (R=−0.62; P=0.01). No relationship was evident between RBF reduction and tissue TGF-β expression.
Figure 3.
TGF-β staining. Tissue staining for TGF-β demonstrated widespread expression in ARAS that was not evident in normal kidneys and was much less evident in samples from nephrectomy tissue with total occlusion. TGF-β was identified within parenchymal and glomerular regions independent of the degree of tubular atrophy or evident fibrosis. **P<0.01 versus donors. ARAS, atherosclerotic renal artery stenosis; Nx, nephrectomy.
Table 3.
Multivariate analysis for TGF-β scores correlations with histologic findings, percentage of renal artery stenosis, and eGFR
| Histologic Factors and eGFR | Spearman Coefficient | P Value |
|---|---|---|
| Interstitial inflammation (%) | 0.46 | 0.01 |
| Interstitial fibrosis (%) | 0.38 | 0.05 |
| Fibrosis by trichrome (%) | 0.61 | 0.001 |
| CD68+cells (n) | 0.54 | 0.003 |
| Renal artery stenosis (%) | 0.71 | <0.001 |
| eGFR (per 1.73 m2) | −0.56 | 0.001 |
eGFR, estimated GFR.
Inflammatory Cell Types.
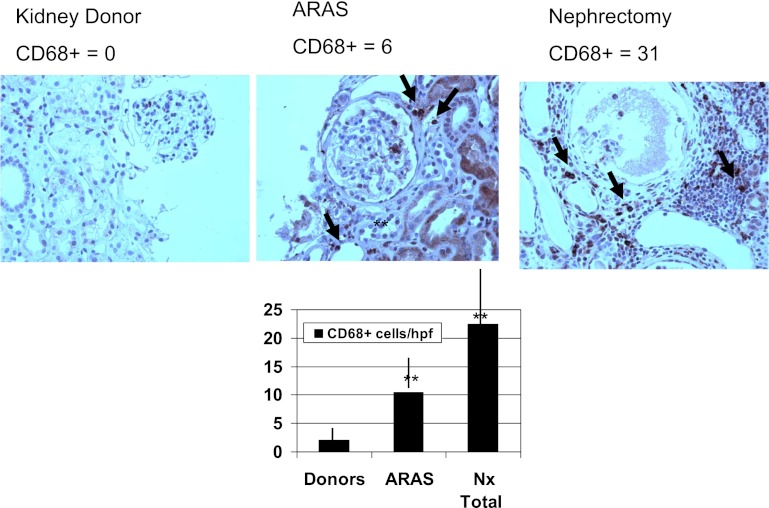
Numbers of inflammatory T cells (CD3+) and macrophages (CD68+) identified by immunohistochemical staining are summarized in Figure 4 and Table 4. CD68+ cell counts were higher in patients with more severe occlusive vascular disease, from negligible levels in donor kidneys to moderate in ARAS and substantially higher levels in nephrectomy samples (Figure 4). In the latter case, CD68+ cells were often interspersed within clusters of other inflammatory cell types that did not stain for these surface markers. In addition, number of CD68+ cells correlated positively with a percent of renal artery stenosis in the ARAS group (Spearman coefficient = 0.69; P<0.001). Tissue TGF-β score appeared positively related with the number of CD68+ cells (R=0.53; P=0.003) on histologic sections. CD3+ cells were occasionally present in both ARAS and nephrectomy samples, but were not significantly higher, although their presence also was conspicuous in clusters of inflammatory cells in nephrectomy tissue. CD20+ cells were infrequently identified in any group, yet were more abundant in nephrectomy samples than in healthy donors.
Figure 4.
CD68+ macrophages in vascular occlusive disease. Counts for CD68+ cells within tissue samples demonstrated more macrophages in ARAS and nephrectomy samples, in addition to CD3+ cells. CD68+ cells were evident in interstitial compartments and interspersed among clusters of inflammatory cells in advanced disease and were positively related to TGF-β expression (see text). **P<0.01 versus donors. ARAS, atherosclerotic renal artery stenosis; Nx, nephrectomy; hpf, high-power field.
Table 4.
Histologic features of kidney biopsies from normal human kidney donors compared with vascular occlusive disease
| Kidney Donors (n=15 Kidneys) | P | Moderate ARAS (n=12 Kidneys) | P | Nephrectomy (n=65 Kidneys) | |
|---|---|---|---|---|---|
| Glomerular diameter (µm) | 176.4±22 | 0.002 | 138.1±27.9 | NA | NAa |
| 174.47 (158.3–196.1) | 126.9 (117.7–164) | ||||
| Average glomerular area (µm2) | 21,236±4683 | 0.002 | 13,508±4932 | NA | NAa |
| 20,200 (18,680–26,491) | 12,024 (10,081–18235) | ||||
| Vessel sclerosis score ≥2 (% of patients) | 9.1 | 0.95 | 16.7 | 0.001 | 75 |
| 1 (0–1) | 0.5 (0–1) | 2 (1.25–3) | |||
| Arteriolar sclerosis score ≥2 (% of patients) | 9.1 | 0.75 | 8.3 | <0.001 | 100 |
| 1 (1–1) | 1 (0.25–1) | 2 (2–3) | |||
| Fibrosis (trichrome, %) | 3.1±1.6 | 0.01 | 11.8±13.6 | 0.01 | 23.7±12.6 |
| 3.2 (1.61–4.38) | 8.8 (7.2–20.7) | 22.2 (9.7–38.6) | |||
| Tubular atrophy (%) | 2.4±1.7 | 0.16 | 15.7±18.7 | <0.001 | 64.1±31.5 |
| 2 (1–4) | 7 (3–30) | 65 (45–93.8) | |||
| Inflammation (%) | 1.1±1.9 | 0.14 | 11.5±12.3 | 0.01 | 33.3±28.7 |
| 0 (0–3) | 7 (0–25) | 30 (10–60) | |||
| CD68 (cells/hpf) | 0.8 (0.6–1.6) | 0.19 | 7.2 (1.8–19.7) | 0.02 | 16.2 (10.3–31.1) |
| CD3 (cells/hpf) | 3.8 (2.6–12) | 0.23 | 9.8 (2.9–19.1) | 0.91 | 9.1 (4.8–22) |
| CD20 (cells/hpf) | 0.4 (0.2–0.8) | 0.47 | 3.3 (0.7–8.4) | 0.21 | 4.2 (1.2–11) |
Data are shown as mean ± SD or median (25th–75th percentile) unless otherwise indicated. NA, not applicable; hpf, high-power field.
Extensive glomerular collapse and sclerosis precluded meaningful estimates of diameter and area in these kidneys.
Discussion
Our results provide for the first time histologic evaluation of biopsies from the stenotic kidneys of patients with subtotal atherosclerotic vascular occlusion, demonstrating widespread tissue TGF-β staining associated with reduced blood flow. TGF-β expression was elevated despite relative preservation of tissue oxygenation in this ARAS cohort with creatinine levels <1.7 mg/dl, as reported previously (10). More severe decrements in blood flow were associated with higher severity of tissue fibrosis and tubular atrophy, although TGF-β expression was lower in kidneys distal to total occlusion. By contrast, inflammatory cellular infiltrates, particularly CD68+ macrophages, were more prominent in both subtotal and total occlusion compared with biopsies from normal kidney donors. The overall number of CD68+ cells correlated with TGF-β score.
It should be emphasized that the ARAS patients reported here had sufficient vascular occlusion to reduce blood flow, but were treated with agents blocking the RAS and statin therapy. Despite reduced flow and GFR in the poststenotic kidney, overall renal architecture remained preserved with only modest overt fibrosis in these patients with treated hypertension. Blockade of the RAS has the potential to blunt fibrogenic stimuli and tissue remodeling in experimental systems (19). Many were also treated with statins, which themselves may modify the degree of fibrosis and inflammation (12). Nonetheless, the degrees of tubular atrophy and fibrosis were higher as the proportion of blood flow fell to the affected kidney as measured by MDCT. Interestingly, tissue TGF-β levels were more elevated in ARAS than in patients with extreme vascular occlusion undergoing nephrectomy, despite less extensive fibrosis and inflammation in ARAS patients. These data argue for an early role for activation of TGF-β in poststenotic kidneys in humans, possibly as part of the response to reduced perfusion and the active injury and repair process that precedes development of advanced tissue fibrosis. We cannot exclude the hypothesis that the more extensive tubular atrophy in kidneys from patients with complete obstruction may explain the less intense TGF-β staining in the nephrectomy group. Previous experimental studies indicate progressive upregulation of TGF-β gene expression in the poststenotic kidney and transient elevation in the contralateral kidney in mice models with renal artery stenosis (8) consistent with its activation as a dynamic process. TGF-β is recognized as an important mediator of tissue repair in many kidney conditions, including glomerulopathies and diabetic renal disease (7,20–22). To our knowledge, our results are the first to show tissue TGF-β expression in human renovascular disease. Measurement of circulating TGF-β did not correlate with tissue levels in our participants. In experimental renal artery stenosis, TGF-β upregulation is thought to result from multiple stimuli, including tissue underperfusion, intrarenal hypoxia, and activation of the renin-angiotensin-aldosterone system (1,6,8). Recognized as a multifunctional cytokine, TGF-β promotes epithelial to mesenchymal cell transformation and fibrogenesis by myofibroblasts (23,24), and modulates immune cell proliferation and migration into the tissues (25). Many of these actions appear to depend upon the Smad3 signaling pathway (24). Experimental data from studies in Smad3 knockout animals indicate protection from renal inflammation and fibrosis in a renal artery stenosis model (26). Under some conditions, however, TGF-β can limit the extent of inflammatory and/or infectious reactions, and is capable of inhibiting activation of T and B lymphocytes (27,28), thus supporting a role for TGF-β in wound healing, inflammation, and tissue repair (25).
Our data are consistent with, but do not prove, an interaction between TGF-β and progressive accumulation of macrophages in human ARAS, even with preserved kidney architecture. Macrophages, effectively recruited by TGF-β and themselves secreting other cytokines, are recognized as important mediators of progressive kidney injury and/or recovery from acute phases of kidney injury (27,29,30). Recent studies support a role for diverse macrophage phenotypes that may favor either inflammation or a transition to reduced inflammation and tissue repair (31–33). Conceivably, upregulation of TGF-β in the poststenotic but viable kidney might serve to recruit macrophages involved in tissue repair, and its subsequent downregulation might indicate completion of tissue remodeling. Indeed, the relative proportion of inflammatory (M1) versus repair-oriented (M2) macrophages in human ARAS merits further study. Moreover, although not assessed directly in this study, kidneys with extremely high-grade vascular occlusion develop overt tissue hypoxia (34), which itself may attract inflammatory cells.
The accumulation of multiple inflammatory cell types, including macrophages and T cells, with ARAS and total renal occlusion underscores the potent inflammatory component that characterizes kidney injury associated with renovascular disease. Our results identify for the first time the quantitative distribution of these cell types in human participants with variable degrees of vascular occlusion. These inflammatory and cytokine pathways may partly explain the limited effectiveness of restoring vascular patency alone to recover kidney function in renovascular disease. Whereas many patients with poorly explained CKD have an element of vascular occlusion (4), results of renal revascularization to recover kidney function have been ambiguous, at best (2,35–37). When endovascular or surgical repair is achieved early after loss of renal perfusion or in kidneys for which some collateral blood flow has been preserved (38), salvage of renal function sometimes can be accomplished. However, most patients have little return of GFR, and some lose function further, leading some authors to argue that renal revascularization has little meaningful benefit (39). Our results suggest that part of the underlying renal injury represents a transition to inflammatory and fibrotic injury that does not resolve with restoring renal blood flow alone. Such injury may respond to alternative or adjunctive interventions to modify repair pathways independent of large vessel hemodynamics. As an example, recent studies using endothelial progenitor cells in experimental ARAS demonstrate restoration of both microvascular structures and GFR using cell-based therapy (40). Furthermore, delivery of adipose-derived mesenchymal stem cells in conjunction with revascularization of experimental ARAS demonstrate anti-inflammatory potential and improved efficacy of revascularization (41).
Limitations
The major limitation of our study is its cross-sectional nature. These studies were obtained in patients with complex effects of age, duration and degree of hypertension, levels of kidney function, and drug therapy. When we compared patients within the nephrectomy group, no differences were evident between TGF-β scores in those treated or not treated with ACE/ARB classes of drug. Care was taken to exclude diabetes and other conditions known to affect the inflammatory milieu. Biopsies were limited to the right kidney for technical and anatomic reasons. Individual kidney functional measurements were restricted to ARAS patients undergoing protocol studies and were unavailable in donors or nephrectomy patients. Stenotic kidney blood flows and perfusion were therefore compared with nonaffected contralateral kidneys. The decrease in cortical volume and heterogeneities in local perfusion might be confounding factors that limit the correlations between RBF and histologic changes.
Taken together, these results demonstrate higher levels of tubular atrophy, fibrosis, and inflammatory cell infiltration with more severe atherosclerotic vascular occlusion in human participants. These were correlated with reductions in blood flow to the affected kidney, but were not related directly to tissue oxygenation as measured by BOLD MRI at this level of ARAS. TGF-β levels were substantially higher in ARAS than in either donors or nephrectomy samples, consistent with early activation of this cytokine in vascular occlusive disease. Inflammatory cells were more prominent with more severe injury, and particularly CD68+ macrophages appeared to increase to a greater extent with more advanced disease. These data support a process of inflammatory injury, including macrophage recruitment to underperfused kidney tissue in human renovascular disease. Our results argue that further measures to modify the immune response to vascular occlusive injury may be a requirement for tissue repair and recovery of renal function after revascularization. Targeting specific inflammatory cellular elements may provide a means of repairing or limiting tissue injury beyond a renovascular occlusive lesion.
Disclosures
None.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
The project described was supported by Award Number PO1HL85307 from the National Heart, Lung, and Blood Institute, as well as National Institutes of Health/National Center for Research Resources Clinical and Translational Science Awards Grant Number UL1 RR024150.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Textor SC, Lerman LO: Renovascular hypertension and ischemic nephropathy. Am J Hypertens 23: 1159–1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J, ASTRAL Investigators : Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Korsakas S, Mohaupt MG, Dinkel HP, Mahler F, Do DD, Voegele J, Baumgartner I: Delay of dialysis in end-stage renal failure: Prospective study on percutaneous renal artery interventions. Kidney Int 65: 251–258, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Kalra PA, Gilbertson DT, Liu J, Chen SC, Collins AJ, Foley RN: Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation 115: 50–58, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Greco BA, Breyer JA: The natural history of renal artery stenosis: Who should be evaluated for suspected ischemic nephropathy? Semin Nephrol 16: 2–11, 1996 [PubMed] [Google Scholar]
- 6.Klahr S, Morrissey JJ: The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int Suppl 75[Suppl 75s]: S7–S14, 2000 [PubMed] [Google Scholar]
- 7.Lee SB, Kalluri R: Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl 78[119]: S22–S26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP: Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC: Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 55: 961–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rea DJ, Heimbach JK, Grande JP, Textor SC, Taler SJ, Prieto M, Larson TS, Cosio FG, Stegall MD: Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int 70: 1636–1641, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP: Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: Clinical and histopathological correlates. Nephrol Dial Transplant 25: 3615–3622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC: Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 44: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO: Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Liu PS, Platt JF: CT angiography of the renal circulation. Radiol Clin North Am 48: 347–365, viii–ix, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Waechter I, Bredno J, Weese J, Barratt DC, Hawkes DJ: Using flow information to support 3D vessel reconstruction from rotational angiography. Med Phys 35: 3302–3316, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Krier JD, Rodriguez-Porcel M, Best PJ, Romero JC, Lerman A, Lerman LO: Vascular responses in vivo to 8-epi PGF(2alpha) in normal and hypercholesterolemic pigs. Am J Physiol Regul Integr Comp Physiol 283: R303–R308, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Border WA, Noble N: Maximizing hemodynamic-independent effects of angiotensin II antagonists in fibrotic diseases. Semin Nephrol 21: 563–572, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Border WA, Noble NA: Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension 31[part 2]: 181–188, 1998 [DOI] [PubMed]
- 21.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360: 361–364, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Ziyadeh FN, Sharma K: Role of transforming growth factor-beta in diabetic glomerulosclerosis and renal hypertrophy. Kidney Int Suppl 51[suppl]: S34–S36, 1995 [PubMed] [Google Scholar]
- 23.Barnes JL, Gorin Y: Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanders KC: Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85: 47–64, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma K, Ziyadeh FN: The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol 266: F829–F842, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP: Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455–F1464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grande JP: Role of transforming growth factor-beta in tissue injury and repair. Proc Soc Exp Biol Med 214: 27–40, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Ristow HJ: BSC-1 growth inhibitor/type beta transforming growth factor is a strong inhibitor of thymocyte proliferation. Proc Natl Acad Sci U S A 83: 5531–5533, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G: Macrophage activation switching: An asset for the resolution of inflammation. Clin Exp Immunol 142: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Harris DC: Macrophages in renal disease. J Am Soc Nephrol 22: 21–27, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC: Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 58: 1066–1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Textor SC, Lerman L, McKusick M: The uncertain value of renal artery interventions: Where are we now? JACC Cardiovasc Interv 2: 175–182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimoldi SF, de Marchi SF, Windecker S, Meier B, Allemann Y: Screening renal artery angiography in hypertensive patients undergoing coronary angiography and 6-month follow-up after ad hoc percutaneous revascularization. J Hypertens 28: 842–847, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Textor SC, Wilcox CS: Renal artery stenosis: A common, treatable cause of renal failure? Annu Rev Med 52: 421–442, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Novick AC, Pohl MA, Schreiber M, Gifford RW, Jr, Vidt DG: Revascularization for preservation of renal function in patients with atherosclerotic renovascular disease. J Urol 129: 907–912, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Main J: How important is atheromatous renal artery stenosis as a cause of end-stage renal disease? Semin Dial 14: 143–145, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO: Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO: Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30: 1030–1041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]