Summary
Background and objectives
Hypothyroidism is highly prevalent among ESRD patients, but its clinical significance and the benefits of thyroid hormone replacement in this context remain unclear.
Design, setting, participants, & measurements
This study examined the association between hypothyroidism and all-cause mortality among 2715 adult dialysis patients with baseline thyrotropin levels measured between April of 2005 and April of 2011. Mortality was ascertained from Social Security Death Master Index and local registration systems. The association between hypothyroidism (thyrotropin greater than assay upper limit normal) and mortality was estimated using Cox proportional hazards models. To reduce the risk of observing reverse-causal associations, models included a 30-day lag between thyrotropin measurement and at-risk time.
Results
Among 350 (12.9%) hypothyroid and 2365 (87.1%) euthyroid (assay within referent range) patients, 917 deaths were observed during 5352 patient-years of at-risk time. Hypothyroidism was associated with higher mortality. Compared with thyrotropin in the low-normal range (0.4–2.9 mIU/L), subclinical hypothyroidism (thyrotropin >upper limit normal and ≤10.0 mIU/L) was associated with higher mortality; high-normal thyrotropin (≥3.0 mIU/L and ≤upper limit normal) and overt hypothyroidism (thyrotropin >10.0 mIU/L) were associated with numerically greater risk, but estimates were not statistically significant. Compared with spontaneously euthyroid controls, patients who were euthyroid while on exogenous thyroid replacement were not at higher mortality risk, whereas patients who were hypothyroid were at higher mortality risk. Sensitivity analyses indicated that effects on cardiovascular risk factors may mediate the observed association between hypothyroidism and death.
Conclusions
These data suggest that hypothyroidism is associated with higher mortality in dialysis patients, which may be ameliorated by thyroid hormone replacement therapy.
Introduction
Patients with CKD and ESRD are more likely to manifest hypothyroidism than patients with preserved kidney function (1–5). Third National Health and Nutrition Examination Survey data indicate that the prevalence of hypothyroidism is 5.4%, 10.9%, 20.4%, 23.0%, and 23.1% among participants with estimated GFRs of ≥90, 60–89, 45–59, 30–44, and <30 ml/min per 1.73 m2, respectively (4). In nondialysis populations, variable associations between hypothyroidism and mortality have been reported. In general, hypothyroidism seems to be associated with higher mortality in populations with high underlying cardiovascular risk (6–8) (presumably because of effects on cardiac contractility, electrical conduction, and factors influencing atherosclerotic progression) (9,10) but not populations with nonelevated risk (11,12).
Given the exceptionally high cardiovascular risk inherent to ESRD, it might be inferred that the ill effects of hypothyroidism would be particularly potent among dialysis patients. Nonetheless, supportive data are limited. Several studies among dialysis patients have shown that low triiodothyronine (T3) levels are associated with endothelial dysfunction (13,14), atherosclerosis (13), ventricular dysfunction (15), and higher cardiovascular (16,17) and all-cause mortality (16–19). However, interpretation of these findings is made difficult because of potential confounding: T3 levels are altered by nonthyroidal illness, malnutrition, inflammation, and uremia that, in and of themselves, influence mortality (2,3,20). By virtue of greater sensitivity and specificity, thyrotropin (TSH) levels are the clinical gold standard for assessing hypothyroidism (21,22). To date, no studies have examined the association between hypothyroidism—defined by TSH levels—and mortality among dialysis patients.
To better inform the field, we conducted a retrospective study of a cohort of dialysis patients, who had TSH measurements made at baseline, to assess whether hypothyroidism (defined by elevated TSH) was associated with mortality. We also examined whether thyroid hormone replacement therapy was associated with improved prognosis.
Materials and Methods
Study Cohort
We conducted a retrospective cohort study using data from the Partners Healthcare Research Patient Data Repository (RPDR), a clinical data registry aggregating >1.5 billion ambulatory and inpatient medical records from >4.5 million patients within the Partners Healthcare System, the two largest constituent organizations of which are Brigham and Women’s Hospital and Massachusetts General Hospital. The RPDR contains longitudinal data, including sociodemographics, diagnostic and procedure codes, laboratory data, medications, inpatient/ambulatory encounters, and vital status. Vital status information is updated from Partners Healthcare registration systems and through monthly linkage to the Social Security Death Master Index. This database has been used in numerous epidemiologic studies (23,24). The study protocol was approved by the Partners Healthcare Institutional Review Board.
The source cohort consisted of adult inpatients and outpatients from Brigham and Women’s Hospital and Massachusetts General Hospital who had ≥1 TSH measurement after having had an established ESRD diagnosis (International Classification of Diseases, Ninth Revision code 585.6; both occurring between April 14, 2005 and April 1, 2011). To limit consideration to dialysis patients, we excluded patients who had undergone renal transplantation (International Classification of Diseases, Ninth Revision codes 55.6 and 55.69; Current Procedural Terminology codes 50327, 50328, 50329, 50360, 50365, and S2065; data dating back to 1988), unless there was evidence of an interim return to dialysis. Finally, because the comparison of interest was between hypothyroidism and euthyroidism, we excluded patients who had TSH levels below the reference range (i.e., hyperthyroidism).
Exposure Ascertainment
In the primary analysis, we examined the association between thyroid functional status and survival. Exposure status was defined by TSH level: patients with TSH greater than assay referent range (upper limits of normal [ULNs] were 5.0 and 5.7 mIU/L for assays used) were considered to be hypothyroid; patients with TSH within assay referent range were ascribed as euthyroid. To provide greater granularity, we conducted secondary analyses in which TSH level was defined as low-normal (0.4–2.9 mIU/L), high-normal (≥3 mIU/L and ≤ULN), subclinical hypothyroidism (greater than ULN and ≤10.0 mIU/L), and overt hypothyroidism (>10.0 mIU/L). In addition, we considered TSH in the low-normal, high-normal, and subclinical hypothyroid ranges as a continuous predictor of mortality, described as a restricted cubic spline with knots corresponding to the 20th (1.1 mIU/L), 40th (1.7 mIU/L), 60th (2.4 mIU/L), and 80th (3.9 mIU/L) percentiles of observed values as well as an anchoring knot at 10.0 mIU/L. In the spline analyses, estimates for TSH>10 mIU/L became unstable owing to a paucity of observations and therefore, were not included.
To examine the influence of thyroid hormone replacement therapy on the association between hypothyroidism and mortality, we parsed euthyroid patients into patients who were and were not using exogenous thyroid hormone and compared mortality risk among the following categories: euthyroid without medication (i.e., spontaneously euthyroid), euthyroid on medication (i.e., hypothyroid treated to target), and hypothyroid with or without medication.
In instances where TSH was measured on more than one date, we considered the first value after a qualifying ESRD diagnosis. In instances where duplicate TSH measurements were made on the same date, we considered the mean of values; in all such instances, contemporaneous TSH levels were concordant (e.g., both high or both normal).
Outcome Ascertainment
The primary outcome of interest was all-cause death. Severe systemic illness affects TSH levels in the absence of true thyroid pathology (i.e., euthyroid sick syndrome) (25); it also renders patients at a greater likelihood of dying. To minimize the likelihood of observing reverse-causal associations on this basis, analyses imposed a 30-day lag period between TSH measurement and start of at-risk time (thereby excluding patients who died immediately after TSH measurement, in whom likelihood of observing a reverse-causal association is high). For completeness, we also conducted corresponding nonlagged analyses in which at-risk time began the day after TSH measurement. Patients remained at risk until death, censoring for renal transplantation, or end of study (June 1, 2011).
Statistical Analyses
Baseline characteristics between exposure groups were compared using chi-squared, Wilcoxon rank sum, and two-sample t tests as dictated by data type. Analogous methods were used to estimate exposure–mortality associations in all analyses. Unadjusted exposure–mortality associations were estimated using Kaplan–Meier plots, log-rank testing, and unadjusted Cox proportional hazards models. Adjusted associations were estimated using multivariable Cox models containing covariate terms for confounders. Candidate covariates were selected as those covariates that were plausibly associated with thyroid dysfunction and mortality based on published evidence but not thought to be on causal pathways linking hypothyroidism to death. These covariates included age, sex, race (white versus nonwhite), diabetes, and hospitalization for noncardiovascular indication in the preceding year. Effect modification of exposure–outcome associations on the basis of sex, race, and diabetes was explored through the addition of two-way interaction terms with exposure (separately) using likelihood ratio testing. The proportional hazards assumption was confirmed graphically and through Schoenfeld residual testing.
To explore whether cardiovascular pathways may mediate the association between hypothyroidism and mortality, we conducted sensitivity analyses in which we added covariate terms for potential pathway intermediates to multivariable models and observed for effect estimate attenuation. These terms included hypertension, hyperlipidemia, cerebrovascular disease (CVD), coronary artery disease (CAD), congestive heart failure (CHF), and hospitalization for cardiovascular indication in the preceding year. Analyses were performed using STATA MP 10.1 (StataCorp, College Station, TX).
Results
Cohort Description
The study cohort qualifying for the primary (lagged) analysis consisted of 2715 patients: 350 (12.9%) hypothyroid and 2365 (87.1%) euthyroid controls. Within the cohort, 45 (1.7%) and 2670 (98.3%) patients were on peritoneal dialysis and hemodialysis, respectively; 149 (5.5%) patients were previous transplant recipients who returned to dialysis. Comparison of baseline characteristics between hypothyroid patients and controls is presented in Table 1. Hypothyroid patients and controls were similar in terms of age, sex, and prevalence of diabetes, hyperlipidemia, CVD, and likelihood of noncardiovascular hospitalization in the preceding year. Hypothyroid patients were less likely to be hypertensive, and they were more likely to be white, have CAD and CHF, and have had a cardiovascular hospitalization in the preceding year.
Table 1.
Comparison of baseline characteristics between hypothyroid patients and euthyroid controls
| Euthyroid (n=2365) | Hypothyroid (n=350) | P Value | |
|---|---|---|---|
| Thyrotropin (mIU/L) | |||
| Median | 1.83 | 7.65 | N/A |
| p25, p75 | 1.19, 2.69 | 6.06, 12.3 | N/A |
| Minimum to maximum | 0.40–5.21 | 5.03–520 | N/A |
| Age (yr) | 63.1±15.7 | 64.7±14.3 | 0.08 |
| Female sex | 43.9% | 46.3% | 0.40 |
| Nonwhite race | 38.0% | 29.1% | 0.001 |
| Diabetesa | 56.1% | 57.1% | 0.70 |
| Hospitalized for noncardiovascular indication in preceding yeara | 42.0% | 42.9% | 0.70 |
| Hypertensiona | 85.1% | 77.4% | <0.001 |
| Hyperlipidemiaa | 66.1% | 62.6% | 0.20 |
| Cerebrovascular diseasea | 32.5% | 34.3% | 0.50 |
| Coronary artery diseasea | 64.3% | 70.0% | 0.04 |
| Congestive heart failurea | 64.5% | 76.3% | <0.001 |
| Hospitalized for cardiovascular indication in preceding yeara | 12.7% | 17.7% | 0.01 |
| Baseline use of exogenous thyroid hormone | 13.7% | 41.7% | <0.001 |
| Date of ESRD diagnostic code | 0.40 | ||
| 2005 | 16.0% | 17.7% | |
| 2006 | 23.4% | 21.4% | |
| 2007 | 20.3% | 20.3% | |
| 2008 | 16.9% | 14.0% | |
| 2009 | 14.5% | 14.6% | |
| 2010 | 8.3% | 11.1% | |
| 2011 | 0.6% | 0.9% |
Thyroid functional status categorizations: euthyroid (referent group; thyrotropin within reference range) and hypothyroid (thyrotropin greater than upper limit normal). Data presented as mean ± SD or percent, except where indicated. Significance testing by two-sample t tests or chi-squared tests. N/A, not applicable.
Ascertained using International Classification of Diseases, Ninth Revision diagnostic codes.
To assess the validity of our TSH-based definition of thyroid functional status with respect to the possibility of secondary/tertiary thyroid disease (i.e., pituitary/hypothalamic origin), we examined concurrent total thyroxine and free thyroxine (FT4) levels among patients in whom the levels were available. Of 136 patients classified as hypothyroid on the basis of TSH, none had high total thyroxine or FT4, providing reassurance that secondary hyperthyroidism was not misclassified as primary hypothyroidism.
Hypothyroidism and Mortality
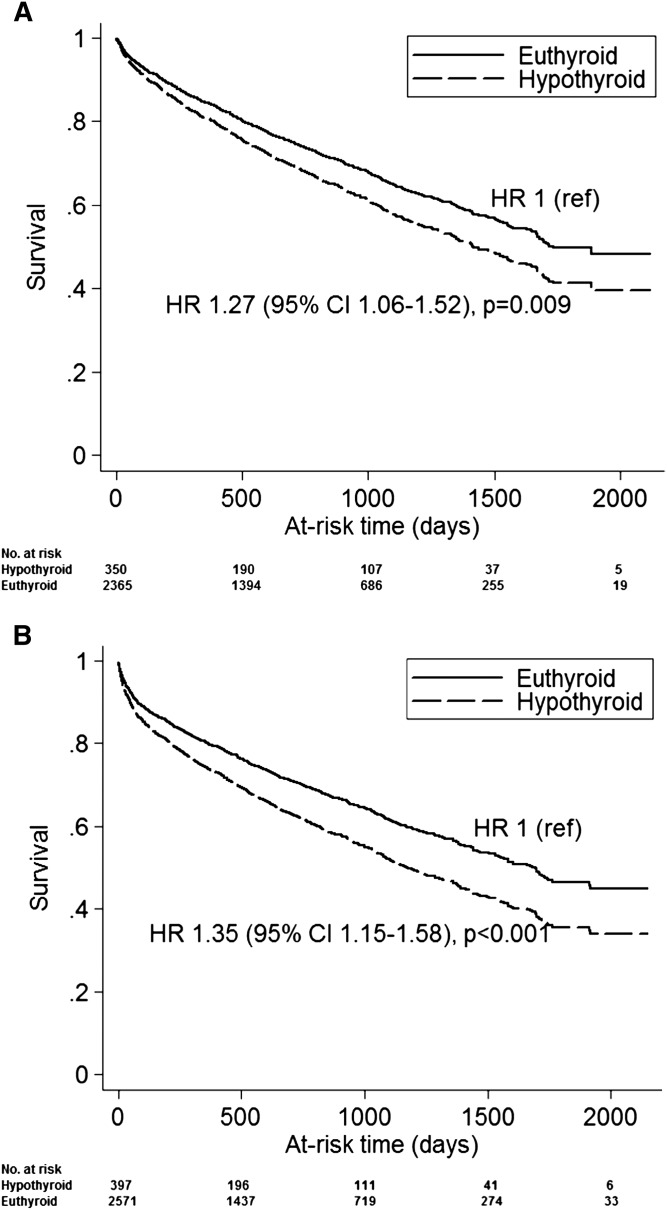
In the primary analysis, patients contributed a total of 5352 patient-years of at-risk time, during which 917 deaths were observed; median time at risk was 1.7 years. On unadjusted analysis, hypothyroidism was associated with a higher mortality risk: hazard ratio (HR)=1.34 (95% confidence interval=1.12–1.60; P=0.001). On case mix adjustment, the association was modestly attenuated but remained statistically significant (Figure 1A). When a 30-day lag period was not imposed between TSH measurement and start of at-risk time (5585 patient-years of at-risk time; 1120 deaths), the association between hypothyroidism and mortality was larger (Figure 1B). We did not detect effect modification of the hypothyroidism–mortality association on the basis of sex, race, or diabetes (interaction P values of 0.80, 0.10, and 0.30, respectively).
Figure 1.
Time to death based on baseline thyroid functional status. Survival curves are adjusted for age, sex, race, diabetes, and hospitalization for noncardiovascular indication in the preceding year. (A) shows results of the primary analysis in which the start of at-risk time began 30 days after thyrotropin (TSH) measurement. (B) shows estimates from an analogous model that did not consider the 30-day lag period. 95% CI, 95% confidence interval; HR, hazard ratio.
Secondary Analyses Based on Thyrotropin Level
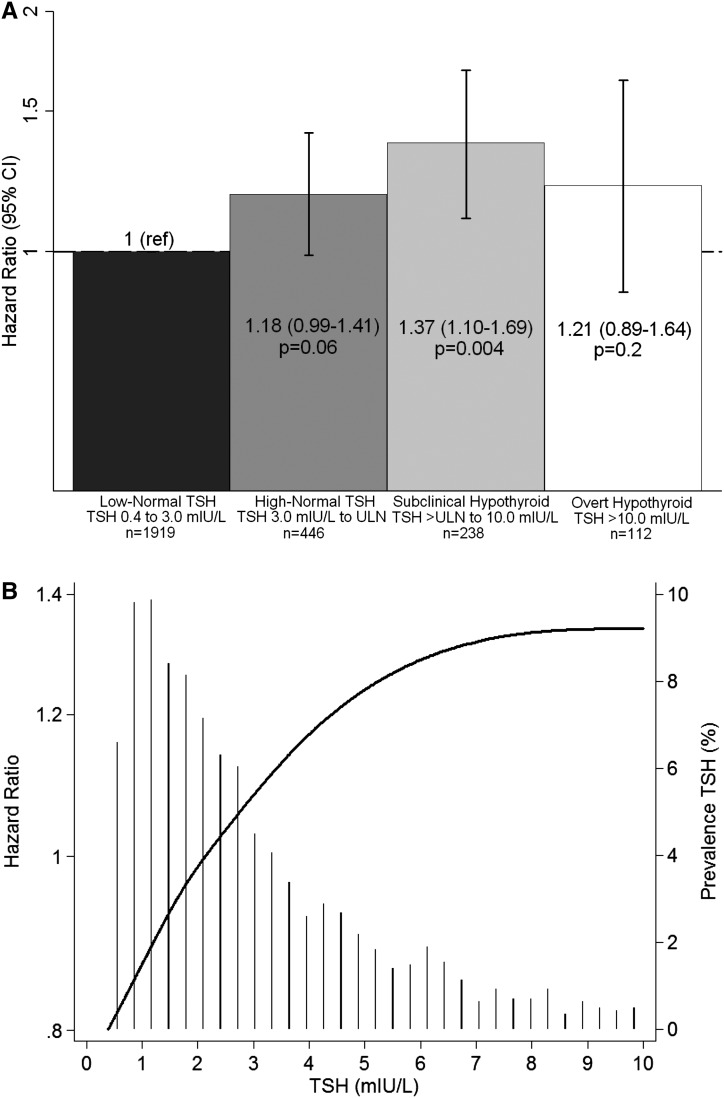
When TSH was considered in finer gradations, subclinical hypothyroidism was associated with significantly greater mortality compared with low-normal TSH (Figure 2A and Supplemental Table 1). High-normal TSH and overt hypothyroidism trended to higher risk, but associations did not achieve statistical significance. When TSH was considered as a continuous variable (represented as a restricted cubic spline), the HR for death increased monotonically up to ∼7.5 mIU/L, after which it leveled off (Figure 2B).
Figure 2.
Secondary analyses of adjusted associations between gradations in TSH and mortality. (A) presents HRs (95% CIs) for low-normal TSH, high-normal TSH, subclinical hypothyroidism, and overt hypothyroidism. (B) shows TSH analyzed as a restricted cubic spline with knots at 1.1, 1.7, 2.4, 3.9, and 10.0 mIU/L; a histogram of observed TSH values is overlaid. All analyses considered a 30-day lag between TSH assessment and the start of the at-risk time, and they were adjusted for age, sex, race, diabetes, and hospitalization for noncardiovascular indication within the preceding year. ULN, upper limit normal.
Thyroid Hormone Use and Mortality
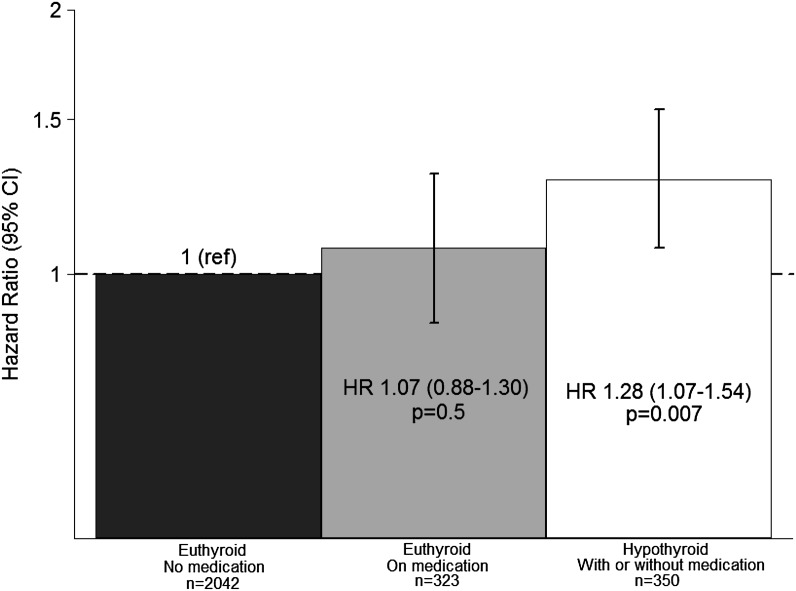
To examine the potential mitigating effects of therapy, we considered patients in terms of TSH level and exogenous thyroid hormone use/nonuse. Compared with euthyroid patients without medication, hypothyroid patients were at greater risk of death, whereas patients who were euthyroid on medication (i.e., adequately treated hypothyroidism) were not (Figure 3 and Supplemental Table 2).
Figure 3.
Adjusted HRs (95% CIs) for mortality based on baseline functional thyroid status and exogenous thyroid hormone status. Analyses considered a 30-day lag between TSH assessment and the start of the at-risk time, and they were adjusted for age, sex, race, diabetes, and hospitalization for noncardiovascular indication within the preceding year.
Exploration of Potential Pathways between Hypothyroidism and Mortality
To explore whether the hypothyroidism–mortality association might be mediated by cardiovascular pathways, we performed sensitivity analyses in which we adjusted for hypertension, hyperlipidemia, CVD, CAD, CHF, and preceding cardiovascular hospitalization (in addition to case mix factors) and observed for effect estimate attenuation. The association between hypothyroidism and mortality was greatly attenuated and no longer statistically significant: adjusted HR (95% confidence interval)=1.12 (0.94–1.34; P=0.20).
Discussion
To our knowledge, this study is the first to show an association between hypothyroidism—defined by TSH levels (the clinical gold standard)—and mortality in dialysis patients. In primary analyses, we found that hypothyroidism was significantly and independently associated with mortality and that milder subcategories of hypothyroidism (i.e., subclinical) also conferred higher mortality in secondary analyses.
In nondialysis populations with high underlying cardiovascular risk, hypothyroidism is associated with greater cardiovascular and all-cause mortality (6–8). The exaggerated cardiovascular risk inherent to ESRD suggests that dialysis patients might be particularly vulnerable to the ill effects of hypothyroidism. Existing data in CKD/ESRD patients show that hypothyroidism is associated with surrogate cardiovascular outcomes, such as ventricular dysfunction and hypertrophy (15), atherosclerosis (13,14), and endothelial dysfunction (13,14,26). Some studies have considered hard outcomes, showing that lower (total/free) T3 and/or thyroxine (T4) levels are associated with all-cause (16–19) and cardiovascular mortality (16,17) in dialysis populations.
However, T3/T4 levels are particularly prone to deviations on the basis of nonthyroidal illness and thus, may not accurately reflect thyroid functional status (2,3). For example, peripheral conversion of T4 to T3 by types I and II deiodinase (the source of 80% of T3) (27) is sensitive to health status (2,27), cytokines (28–31), cortisol (27), medications (27), and uremia (20). Additionally, uremic toxins as well as low albumin states interfere with in vitro FT4 assays, rendering observed measures lower than actual circulating levels (2,32). For these reasons, studies using T4/T3 to define thyroid function are subject to confounding, and serum TSH is considered to be a more specific thyroid functional status metric. Moreover, owing to the logarithmic T3/T4 and TSH relationship (i.e., minimal T3/T4 changes induce large reciprocal TSH changes), TSH is a more sensitive marker of hypothyroidism, and it is more robust to measurement error (22,33). Although the clearance, diurnal pulsatility, response to thyrotropin releasing hormone, and half-life of TSH may be altered in renal failure (2,20), most CKD/ESRD patients have normal TSH levels (20,34) and respond appropriately to changes in circulating levels of thyroid hormone (i.e., TSH levels decrease and rise in response to exogenous T3 and thyroid ablation, respectively) (34). Furthermore, metabolic testing in ESRD patients has shown that TSH is a more reliable indicator of thyroid functional status than T3 (35).
Several of the above studies evaluating hypothyroidism and mortality in ESRD patients have indirectly examined for—and not detected—associations between TSH levels and mortality (16,17,19). However, none contained a sufficient number of patients with actual hypothyroidism (i.e., elevated TSH), in instances caused by exclusionary restriction, to be powered in this regard. Instead, studies compared TSH deviations within the normal range. Our study is, therefore, the first to show an association between hypothyroidism per se and mortality in the ESRD population. Because of data limitations, we were unable to directly examine the association between hypothyroidism and cause-specific mortality. However, we performed sensitivity analyses in which we adjusted for cardiovascular risk factors and observed dramatic attenuation of the hypothyroidism–mortality association. There are two potential explanations for this observation. (1) Cardiovascular illness is one means by which hypothyroidism increases mortality (i.e., hypothyroidism→cardiovascular morbidity→death); under this logic, the true impact of hypothyroidism on survival is better represented by estimates that are not adjusted for cardiovascular comorbidity. (2) Cardiovascular disease is a confounder of the association between hypothyroidism and death (i.e., cardiovascular disease→hypothyroidism→death); under this logic, the true impact of hypothyroidism on survival is better represented by estimates that are adjusted for cardiovascular comorbidity. Empirical distinction between these two explanations is not possible, and interpretation must, therefore, be guided by content area logic. We favor the former interpretation, because whereas there is abundant data indicating that hypothyroidism induces cardiovascular disease (e.g., alterations in myocyte contractility/relaxation, flow-mediated vasodilation, and arterial stiffness [9,10,20] that distort ventricular geometry and function; cardiac channel expression changes that prolong the cardiac action potential and QT interval and increase Torsades risk [10]; hyperhomocysteinemia [36]; coagulation and fibrinolysis alterations [37]; and systemic vascular resistance and lipid derangements accelerating atherogenesis [10]), we are not aware of data suggesting that cardiovascular disease causes hypothyroidism. However, given data limitations, we were not able to assess the temporal sequence of TSH measurement versus comorbid cardiovascular event incidence, and therefore, we could not formally distinguish whether cardiovascular morbidities were confounders or pathway intermediates. Additional mechanistic study of the hypothyroidism–mortality association in ESRD is needed.
In the general population, there remains controversy as to whether subclinical hypothyroidism adversely impacts survival (38,39), and prior studies have yielded conflicting results (6–8,11,12,40,41). Studies in patients with high underlying cardiovascular risk have tended to show a higher mortality risk (6–8), whereas studies in patients of older age (>65 years) and average cardiovascular risk have shown a neutral (11,12) or protective effect (40). Cardiovascular disease accounts for 50% of deaths in ESRD, with many of these fatalities related to CAD, CHF, and sudden cardiac death (42,43). Given their greater cardiovascular burden, ESRD patients may represent a subpopulation in whom subclinical hypothyroidism portends greater mortality risk than in the general population.
Although there was a trend to an association between overt hypothyroidism and mortality, estimates did not achieve statistical significance. However, our cohort consisted of few patients with overt disease (n=112; 45 deaths). It is uncertain whether the absence of statistical significance indicates true absence of biologic association or a chance finding stemming from limited statistical power. Additional study is warranted to elaborate the association between overt hypothyroidism and mortality in ESRD.
In the general population, there is controversy regarding the TSH ULN. Some experts advise lowering the ULN from 4.0–5.0 to 2.5–3.0 mIU/L in the broader population, because >95% of the euthyroid population has TSH levels below the latter threshold (44–46); others recommend age-based normal ranges (47). To address this uncertainty within the dialysis population, we examined TSH in finer gradations and also considered TSH as a restricted cubic spline. The former analysis did not conclusively show a higher mortality risk associated with a TSH range of 3.0 mIU/L to the ULN, but a nonsignificant trend was detected. The latter analysis did not identify a discrete threshold above which mortality risk increases, suggesting a continuum of risk across TSH levels, including those levels in the high-normal range. Additional study is needed to clarify the prognostic significance of high-normal TSH levels.
To our knowledge, this study is also the first showing that exogenous thyroid hormone might ameliorate the hypothyroidism–mortality association in ESRD. Our findings showed that, compared with spontaneously euthyroid patients, those patients who were euthyroid on medication (i.e., adequately-treated hypothyroidism) had a similar mortality risk, whereas hypothyroid patients had greater mortality. Our findings are consistent with observational data from the general population in which subclinically hypothyroid patients receiving treatment had a lower risk of cardiac events compared with untreated patients (48). Dedicated studies with rigorous attention to longitudinal treatment are needed to confirm the benefits of thyroid hormone supplementation in hypothyroid ESRD patients.
Our study has several strengths, including its large sample size, extended follow-up, and use of analytical techniques to minimize the likelihood of observing reverse-causal associations. The cohort case mix was similar to the case mix of the broader US dialysis population (49), engendering generalizability. However, several limitations bear mention. First, the indications for which TSH levels were measured are not known. However, the requirement for TSH measurement applied equally to hypothyroid patients and controls and therefore, should not have created for differential bias. Second, thyroid functional status categorizations were based on one-time TSH measurements and therefore, were subject to misclassification. However, any residual bias is expected to be nondifferential and would not explain the observed associations. Third, we were unable to define thyroid functional status using concomitant TSH and T3/T4 measurements because of sparse T3/T4 data. However, in all instances in which T4 data were available, they confirmed that TSH abnormality was not attributable to secondary hyperthyroidism. Fourth, we were unable to determine the setting in which thyroid functional testing was conducted, and it is possible that this setting was a mixture of ambulatory and inpatient measurements. Fifth, our clinical registry had limited ability to ascertain cause of death. However, exploratory analyses of potential intermediates pointed to cardiovascular pathways, and additional mechanistic studies are needed. Finally, as with all observational studies, we cannot exclude the possibility of residual confounding.
In conclusion, our study supports an association between hypothyroidism and mortality in ESRD patients and suggests that this association may be ameliorated by thyroid hormone supplementation. Given the high prevalence of hypothyroidism in patients with renal dysfunction, hypothyroidism may be considered a viable prognostic marker as well as a target for potential intervention. Additional studies are needed to confirm findings, elaborate underlying mechanisms, and better examine the efficacy and effectiveness of thyroid hormone supplementation vis à vis effects on survival.
Disclosures
E.K.A. receives research support from Veracyte, Inc. and Asuragen, Inc. Since completing work on this study, S.M.B. has become a full-time employee of DaVita Clinical Research. None of the other authors declare any relevant conflicts of interest.
Acknowledgments
The authors would like to thank the Partners Healthcare Research Patient Data Registry Group for access to the data used in these analyses.
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK007527-26 (to C.M.R.), DK093201 (to C.M.R.), and DK079056 (to S.M.B.).
Portions of the data were presented as an abstract during the annual American Society of Nephrology (ASN) conference, October 30 to November 4, 2012, San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06920712/-/DCSupplemental.
References
- 1.Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G: Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1296–1300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaptein EM: Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev 17: 45–63, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Kaptein EM, Quion-Verde H, Chooljian CJ, Tang WW, Friedman PE, Rodriquez HJ, Massry SG: The thyroid in end-stage renal disease. Medicine (Baltimore) 67: 187–197, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Lo JC, Chertow GM, Go AS, Hsu CY: Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 67: 1047–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ng YY, Wu SC, Lin HD, Hu FH, Hou CC, Chou YY, Chiu SM, Sun YH, Cho SS, Yang WC: Prevalence of clinical and subclinical thyroid disease in a peritoneal dialysis population. Perit Dial Int 32: 86–93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, L’Abbate A, Pingitore A: Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 167: 1526–1532, 2007 [DOI] [PubMed] [Google Scholar]
- 7.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ: Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS database study. Thyroid 21: 837–843, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Molinaro S, Iervasi G, Lorenzoni V, Coceani M, Landi P, Srebot V, Mariani F, L’Abbate A, Pingitore A: Persistence of mortality risk in patients with acute cardiac diseases and mild thyroid dysfunction. Am J Med Sci 343: 65–70, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Cini G, Carpi A, Mechanick J, Cini L, Camici M, Galetta F, Giardino R, Russo MA, Iervasi G: Thyroid hormones and the cardiovascular system: Pathophysiology and interventions. Biomed Pharmacother 63: 742–753, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Klein I, Ojamaa K: Thyroid hormone and the cardiovascular system. N Engl J Med 344: 501–509, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW: Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295: 1033–1041, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waring AC, Harrison S, Samuels MH, Ensrud KE, LeBLanc ES, Hoffman AR, Orwoll E, Fink HA, Barrett-Connor E, Bauer DC, Osteoporotic Fractures in Men (MrOS) Study : Thyroid function and mortality in older men: A prospective study. J Clin Endocrinol Metab 97: 862–870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatar E, Kircelli F, Asci G, Carrero JJ, Gungor O, Demirci MS, Ozbek SS, Ceylan N, Ozkahya M, Toz H, Ok E: Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin J Am Soc Nephrol 6: 2240–2246, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatar E, Sezis Demirci M, Kircelli F, Gungor O, Yaprak M, Asci G, Basci A, Ozkahya M, Ok E: The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int Urol Nephrol 44: 601–606, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Zoccali C, Benedetto F, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P, Malatino LS, Bonanno G, Seminara G: Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. J Hypertens 24: 2039–2046, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, Bárány P, Heimbürger O, Suliman ME, Alvestrand A, Lindholm B, Stenvinkel P: Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med 262: 690–701, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Meuwese CL, Dekker FW, Lindholm B, Qureshi AR, Heimburger O, Barany P, Stenvinkel P, Carrero JJ: Baseline levels and trimestral variation of triiodothyronine and thyroxine and their association with mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol 7: 131–138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enia G, Panuccio V, Cutrupi S, Pizzini P, Tripepi G, Mallamaci F, Zoccali C: Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 22: 538–544, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P: Low triiodothyronine and survival in end-stage renal disease. Kidney Int 70: 523–528, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mariani LH, Berns JS: The renal manifestations of thyroid disease. J Am Soc Nephrol 23: 22–26, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Fatourechi V: Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin Proc 84: 65–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiersinga WM: Hypothyroidism and myxedema coma. In: Endocrinology, Vol. 2, 5th Ed., edited by DeGroot LJ, Jameson JL, Philadelphia, Elsevier Saunders, 2006, pp 2081–2099 [Google Scholar]
- 23.Bhan I, Dubey A, Wolf M: Diagnosis and management of mineral metabolism in CKD. J Gen Intern Med 25: 710–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triant VA, Brown TT, Lee H, Grinspoon SK: Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 93: 3499–3504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvatore D, Davies TF, Schlumberger MJ, Hay ID, Larsen PR: Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Williams Textbook of Endocrinology, 12th Ed., edited by Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, Philadelphia, Elsevier Saunders, 2011, pp 327–361 [Google Scholar]
- 26.Yilmaz MI, Sonmez A, Karaman M, Ay SA, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: Low triiodothyronine alters flow-mediated vasodilatation in advanced nondiabetic kidney disease. Am J Nephrol 33: 25–32, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cooper D, Ladenson PW: The thyroid gland. In: Greenspan's Basic and Clinical Endocrinology, 9th Ed., edited by Gardner DG, Shoback D, New York, McGraw Hill Medical, 2011, pp 163–226 [Google Scholar]
- 28.Corssmit EP, Heyligenberg R, Endert E, Sauerwein HP, Romijn JA: Acute effects of interferon-alpha administration on thyroid hormone metabolism in healthy men. J Clin Endocrinol Metab 80: 3140–3144, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Nagaya T, Fujieda M, Otsuka G, Yang JP, Okamoto T, Seo H: A potential role of activated NF-kappa B in the pathogenesis of euthyroid sick syndrome. J Clin Invest 106: 393–402, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stouthard JM, van der Poll T, Endert E, Bakker PJ, Veenhof CH, Sauerwein HP, Romijn JA: Effects of acute and chronic interleukin-6 administration on thyroid hormone metabolism in humans. J Clin Endocrinol Metab 79: 1342–1346, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Van der Poll T, Romijn JA, Endert E, Borm JJ, Büller HR, Sauerwein HP: Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol 261: E457–E465, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Lim VS: Thyroid function in patients with chronic renal failure. Am J Kidney Dis 38[Suppl 1]: S80–S84, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Weiss RE, Wu SY, Refetoff S: Diagnostic tests of the thyroid. In: Endocrinology, Vol. 2, 5th Ed., edited by DeGroot LJ, Jameson JL, Philadelphia, Elsevier Saunders, 2006, pp 1899–1961 [Google Scholar]
- 34.Carrero JJ, Stenvinkel P, Lindholm B: Endocrine aspects of chronic kidney disease. In: Taal: Brenner and Rector's The Kidney, 9th Ed., edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, Philadelphia, Elsevier Saunders, 2012, pp 2122–2137 [Google Scholar]
- 35.Spector DA, Davis PJ, Helderman JH, Bell B, Utiger RD: Thyroid function and metabolic state in chronic renal failure. Ann Intern Med 85: 724–730, 1976 [DOI] [PubMed] [Google Scholar]
- 36.Morris MS, Bostom AG, Jacques PF, Selhub J, Rosenberg IH: Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis 155: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G: Components of the fibrinolytic system are differently altered in moderate and severe hypothyroidism. J Clin Endocrinol Metab 86: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Cooper DS, Biondi B: Subclinical thyroid disease. Lancet 379: 1142–1154, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ: Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA 291: 228–238, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG: Thyroid status, disability and cognitive function, and survival in old age. JAMA 292: 2591–2599, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J, Thyroid Studies Collaboration : Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304: 1365–1374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler DC, Hayes R, Landray MJ, Baigent C: Cardiovascular aspects of kidney disease. In: Taal: Brenner and Rector's The Kidney, 9th Ed., edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, Philadelphia, Elsevier Saunders, 2012, pp 2060–2075 [Google Scholar]
- 43.US Renal Data System : USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005 [Google Scholar]
- 44.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR, Guidelines Committee, National Academy of Clinical Biochemistry : Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13: 3–126, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL, American Association of Clinical Endocrinologists : American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 8: 457–469, 2002 [PubMed] [Google Scholar]
- 46.Wartofsky L, Dickey RA: The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 90: 5483–5488, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Surks MI, Hollowell JG: Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: Implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92: 4575–4582, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Razvi S, Weaver JU, Butler TJ, Pearce SS: Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 172: 811–817, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 [DOI] [PubMed] [Google Scholar]