Abstract
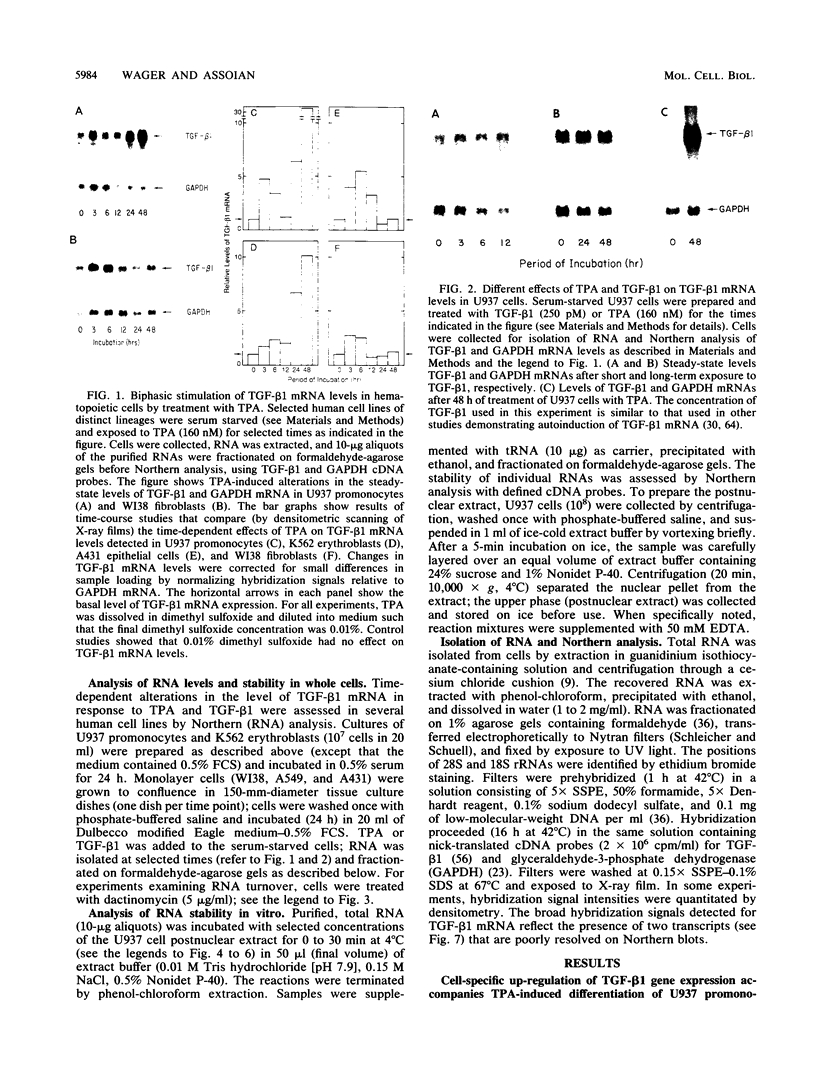
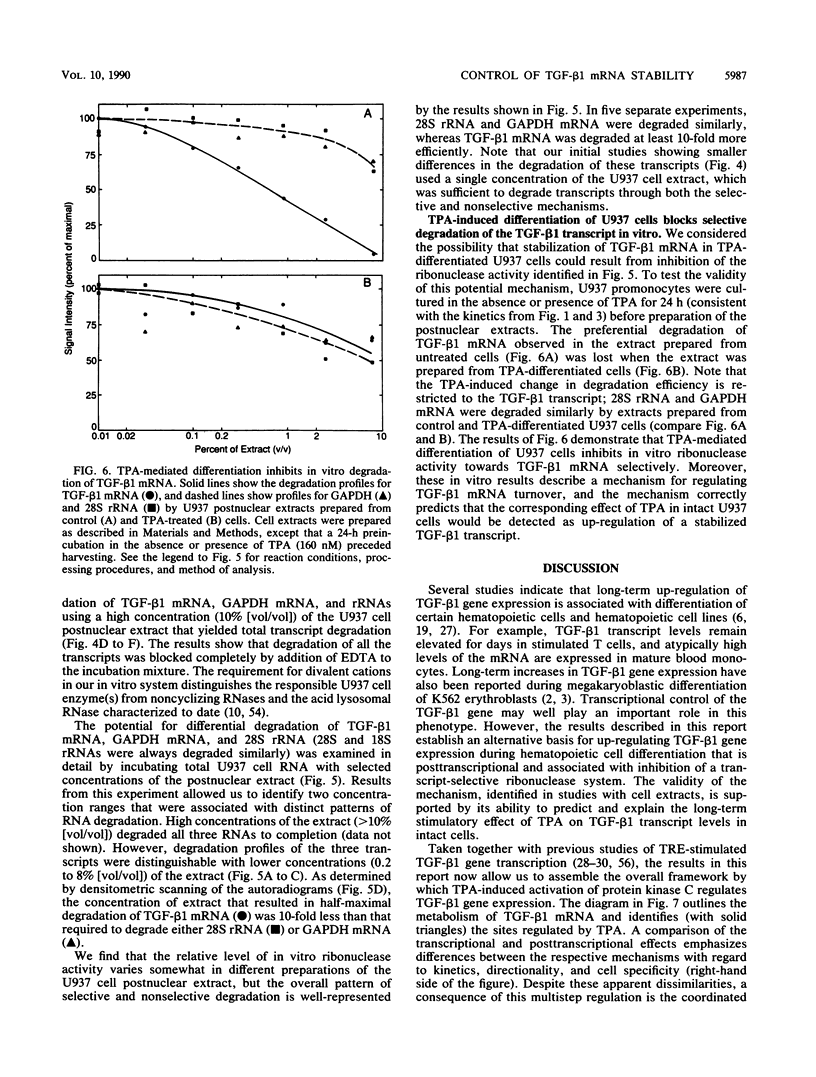
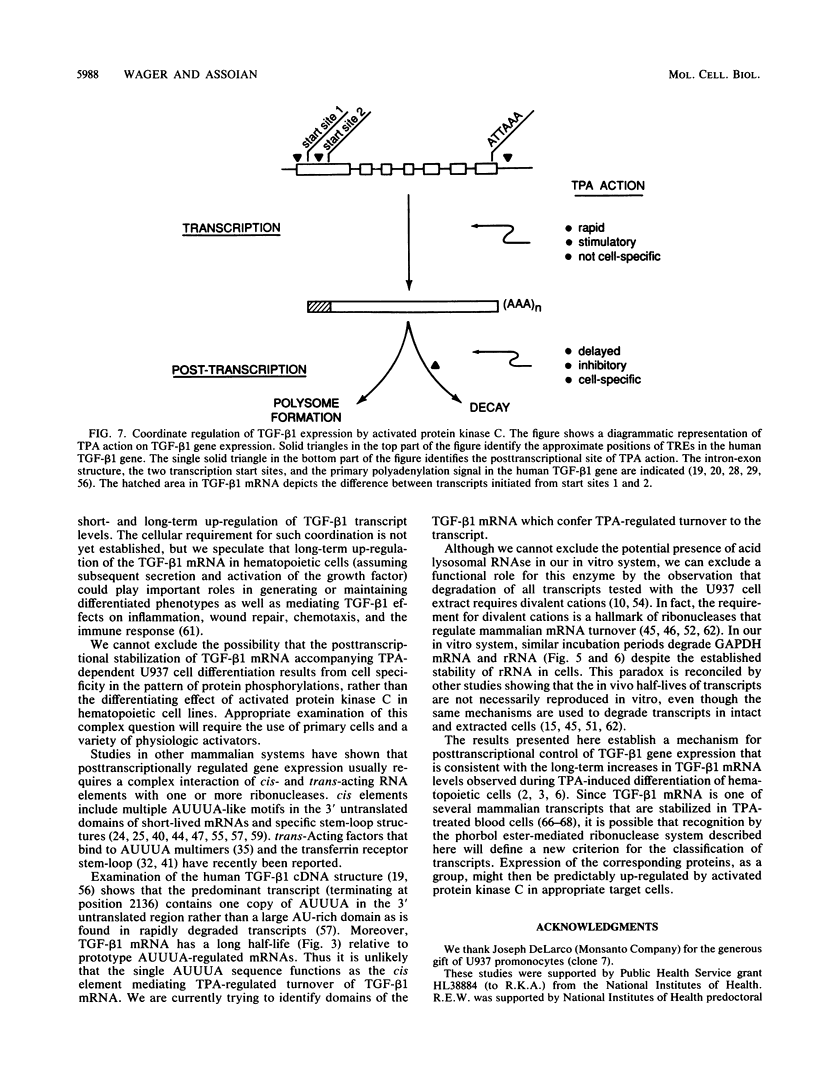
12-Tetradecanoylphorbol-13-acetate (TPA)-induced differentiation of U937 promonocytes leads to a 30-fold increase in transforming growth factor beta 1 (TGF-beta 1) gene expression, and this effect results from a stabilized mRNA. Similar up-regulation was detected in TPA-treated K562 erythroblasts but was absent from cell lines that do not differentiate in response to TPA. Related studies in vitro showed that postnuclear extracts of U937 promonocytes contain a ribonuclease system that degrades TGF-beta 1 mRNA selectively and that this system is completely blocked by prior treatment of the cells with TPA. These data identify a new mechanism for regulating TGF-beta 1 mRNA levels and allow us to establish the overall basis for control of TGF-beta 1 gene expression by activation of protein kinase C. Our results also provide a new basis for understanding the long-term up-regulation of TGF-beta 1 gene expression that can accompany hematopoietic cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Fee F., Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- Alitalo R., Andersson L. C., Betsholtz C., Nilsson K., Westermark B., Heldin C. H., Alitalo K. Induction of platelet-derived growth factor gene expression during megakaryoblastic and monocytic differentiation of human leukemia cell lines. EMBO J. 1987 May;6(5):1213–1218. doi: 10.1002/j.1460-2075.1987.tb02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo R., Mäkelä T. P., Koskinen P., Andersson L. C., Alitalo K. Enhanced expression of transforming growth factor beta during megakaryoblastic differentiation of K562 leukemia cells. Blood. 1988 Apr;71(4):899–906. [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Barnard E. A. Ribonucleases. Annu Rev Biochem. 1969;38:677–732. doi: 10.1146/annurev.bi.38.070169.003333. [DOI] [PubMed] [Google Scholar]
- Bascom C. C., Wolfshohl J. R., Coffey R. J., Jr, Madisen L., Webb N. R., Purchio A. R., Derynck R., Moses H. L. Complex regulation of transforming growth factor beta 1, beta 2, and beta 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors beta 1 and beta 2. Mol Cell Biol. 1989 Dec;9(12):5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. M., Piechaczyk M., Dani C., Chambard J. C., Franchi A., Pouyssegur J., Jeanteur P. c-myc gene is transcribed at high rate in G0-arrested fibroblasts and is post-transcriptionally regulated in response to growth factors. Nature. 1985 Oct 3;317(6036):443–445. doi: 10.1038/317443a0. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Rhee L., Chen E. Y., Van Tilburg A. Intron-exon structure of the human transforming growth factor-beta precursor gene. Nucleic Acids Res. 1987 Apr 10;15(7):3188–3189. doi: 10.1093/nar/15.7.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Piechaczyk M., Henglein B., Blanchard J. M., Traub B., Kofler E., Wiest S., Lenoir G. M., Bornkamm G. W. Aberrant c-myc RNAs of Burkitt's lymphoma cells have longer half-lives. EMBO J. 1985 Dec 30;4(13B):3717–3725. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Cole M. D. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3' untranslated sequences. Mol Cell Biol. 1987 Dec;7(12):4513–4521. doi: 10.1128/mcb.7.12.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Denhez F., Kim K. Y., Holt J. T., Sporn M. B., Roberts A. B. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989 Nov 15;264(32):19373–19378. [PubMed] [Google Scholar]
- Kim S. J., Glick A., Sporn M. B., Roberts A. B. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989 Jan 5;264(1):402–408. [PubMed] [Google Scholar]
- Kim S. J., Jeang K. T., Glick A. B., Sporn M. B., Roberts A. B. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989 Apr 25;264(12):7041–7045. [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Pachter J. S., Yen T. J., Cleveland D. W. Autoregulation of tubulin expression is achieved through specific degradation of polysomal tubulin mRNAs. Cell. 1987 Oct 23;51(2):283–292. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Calame K. Differential stability of c-myc mRNAS in a cell-free system. Mol Cell Biol. 1988 Jul;8(7):2860–2868. doi: 10.1128/mcb.8.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Brewer G., Kobs G., Ross J. Substrate specificity of the exonuclease activity that degrades H4 histone mRNA. J Biol Chem. 1987 Jul 5;262(19):9382–9388. [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Williams N., Moore M. A., Litcofsky P. B. Induction of antibody-dependent and nonspecific tumor killing in human monocytic leukemia cells by nonlymphocyte factors and phorbol ester. Cell Immunol. 1982 Aug;71(2):215–223. doi: 10.1016/0008-8749(82)90257-x. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Ross J., Kobs G., Brewer G., Peltz S. W. Properties of the exonuclease activity that degrades H4 histone mRNA. J Biol Chem. 1987 Jul 5;262(19):9374–9381. [PubMed] [Google Scholar]
- Ross J., Kobs G. H4 histone messenger RNA decay in cell-free extracts initiates at or near the 3' terminus and proceeds 3' to 5'. J Mol Biol. 1986 Apr 20;188(4):579–593. doi: 10.1016/s0022-2836(86)80008-0. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Saha B. K., Graham M. Y., Schlessinger D. Acid ribonuclease from HeLa cell lysosomes. J Biol Chem. 1979 Jul 10;254(13):5951–5957. [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Scotto L., Vaduva P. I., Wager R. E., Assoian R. K. Type beta 1 transforming growth factor gene expression. A corrected mRNA structure reveals a downstream phorbol ester responsive element in human cells. J Biol Chem. 1990 Feb 5;265(4):2203–2208. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Tucker R. F., Moses H. L. Type beta transforming growth factor/growth inhibitor stimulates entry of monolayer cultures of AKR-2B cells into S phase after a prolonged prereplicative interval. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4147–4151. doi: 10.1073/pnas.82.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
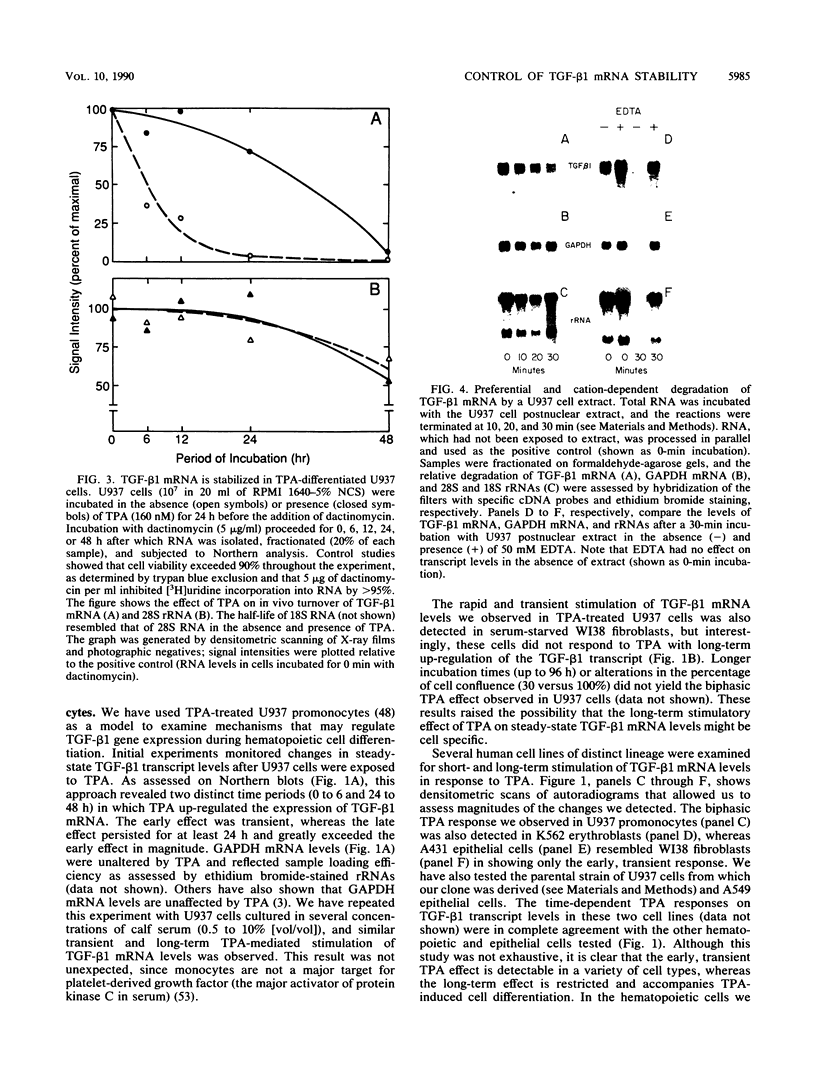
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunitha I., Slobin L. I. An in vitro system derived from Friend erythroleukemia cells to study messenger RNA stability. Biochem Biophys Res Commun. 1987 Apr 29;144(2):560–568. doi: 10.1016/s0006-291x(87)80003-7. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
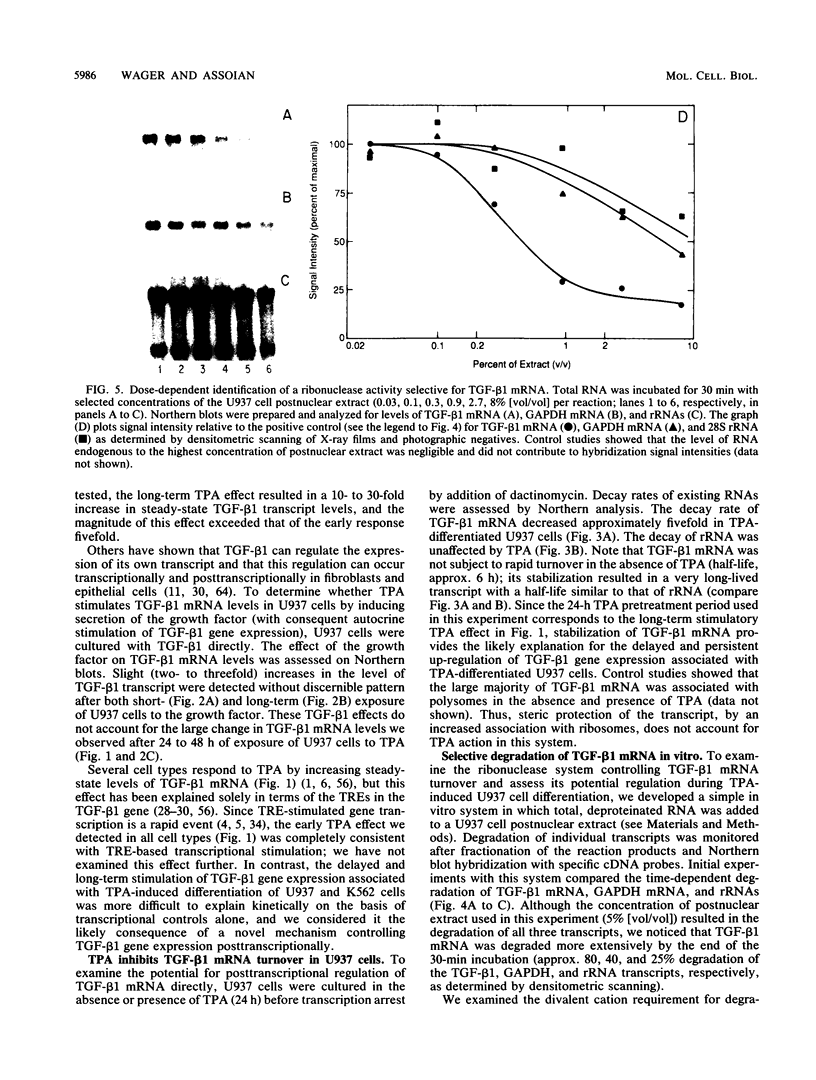
- Weber B., Horiguchi J., Luebbers R., Sherman M., Kufe D. Posttranscriptional stabilization of c-fms mRNA by a labile protein during human monocytic differentiation. Mol Cell Biol. 1989 Feb;9(2):769–775. doi: 10.1128/mcb.9.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Moroni C. Regulation of interleukin 3 mRNA expression in mast cells occurs at the posttranscriptional level and is mediated by calcium ions. Proc Natl Acad Sci U S A. 1990 Jan;87(2):777–781. doi: 10.1073/pnas.87.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato K., el-Hajjaoui Z., Koeffler H. P. Regulation of levels of IL-1 mRNA in human fibroblasts. J Cell Physiol. 1989 Jun;139(3):610–616. doi: 10.1002/jcp.1041390322. [DOI] [PubMed] [Google Scholar]
- Yen T. J., Gay D. A., Pachter J. S., Cleveland D. W. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol Cell Biol. 1988 Mar;8(3):1224–1235. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]