Abstract
Sepsis is an unusual systemic reaction to what is sometimes an otherwise ordinary infection, and it probably represents a pattern of response by the immune system to injury. A hyper-inflammatory response is followed by an immunosuppressive phase during which multiple organ dysfunction is present and the patient is susceptible to nosocomial infection. Biomarkers to diagnose sepsis may allow early intervention which, although primarily supportive, can reduce the risk of death. Although lactate is currently the most commonly used biomarker to identify sepsis, other biomarkers may help to enhance lactate’s effectiveness; these include markers of the hyper-inflammatory phase of sepsis, such as pro-inflammatory cytokines and chemokines; proteins such as C-reactive protein and procalcitonin which are synthesized in response to infection and inflammation; and markers of neutrophil and monocyte activation. Recently, markers of the immunosuppressive phase of sepsis, such as anti-inflammatory cytokines, and alterations of the cell surface markers of monocytes and lymphocytes have been examined. Combinations of pro- and anti-inflammatory biomarkers in a multi-marker panel may help identify patients who are developing severe sepsis before organ dysfunction has advanced too far. Combined with innovative approaches to treatment that target the immunosuppressive phase, these biomarkers may help to reduce the mortality rate associated with severe sepsis which, despite advances in supportive measures, remains high.
Keywords: CD64, C-reactive protein, lactate, procalcitonin, sepsis
Introduction
Sepsis is an unusual systemic reaction to what is sometimes an otherwise ordinary infection. Recognized since ancient times as a deadly menace, sepsis is still a potentially lethal complication. Over the past decade, many hospitals have begun to adopt recommendations of the Surviving Sepsis Campaign for the management of septic patients, and they have seen mortality rates decline from approximately 37% to 30%1. However, this is still unacceptably high. The incidence of sepsis in hospitalized patients has almost doubled during the same period of time2 and sepsis is now also frequently recognized in outpatients seeking attention in the Emergency Department (ED), especially for upper respiratory complaints3. Because the elderly are at increased risk, it is likely that sepsis will become an even greater problem as the population ages. We do not fully understand the pathogenesis of sepsis and there is no specific treatment. Therefore, it is important to recognize it early, so that supportive measures which have been shown to be successful may be implemented as soon as possible.
The original model for sepsis was the immune response to endotoxin, a lipopolysaccharide (LPS) found in the cell walls of Gram-negative bacteria4. Endotoxin is an excellent example of a pathogen-associated molecular pattern (PAMP). Innate immune cells such as macrophages have receptors that recognize different types of PAMPs5. Toll-like receptors (TLRs) and lectin receptors on the cell surface recognize a variety of bacterial substances in the extra-cellular space. In fact, the receptor for LPS was the first TLR found in mammals. Other types of receptors in the cytoplasm recognize bacterial peptidoglycans and/or nucleic acids. When engaged by bacterial ligands, these receptors stimulate macrophages to produce tumor necrosis factor (TNF), interleukin-1β (IL-1β) and IL-6. These three pro-inflammatory cytokines produce a systemic inflammatory response which is characteristic of early sepsis, and for many years physicians believed that sepsis essentially represented an unusually robust reaction on the part of the innate immune system to a bacterial infection.
A consensus conference in 1991 defined “sepsis” as the combination of an infection with two or more features of what was called the “systemic inflammatory response syndrome” (SIRS): altered body temperature, elevated pulse rate, elevated respiratory rate and abnormal white blood cell count6. An update to that original definition, published in 20037, expanded the criteria to include other signs and symptoms commonly seen in critical illness (Table 1). In addition, the update recommended that physicians make the diagnosis of sepsis when infection is strongly suspected, even if documentation is lacking. This change reflected the fact that it was often very difficult to identify an infection in patients based on characteristic clinical signs and symptoms, which made the use of the earlier definition problematic. It also highlighted the fact that most of the clinical features of sepsis are similar regardless of the nature of the infection. In septic patients, it appears that the immune response, not the inciting microorganism, is the problem.
Table 1.
Definitions of sepsis.
| Criteria for SIRSa |
| Two or more of the following are required: |
| • Body temperature >38 °C or <36 °C |
| • Heart rate >90 beats/min |
| • Respiratory rate >20 breaths/min (or arterial pCO2 <32 mmHg, indicating hyperventilation) |
| • White blood cell count >12.0 × 109/L or <4.0 × 109/L (or >10% immature forms) |
| Sepsis = Infection + SIRS |
| Severe sepsis = Sepsis + evidence of organ dysfunction |
The 2001 update to the Definitions stresses that documentation of infection may not be required for the diagnosis of sepsis if strong suspicion exists. Additional criteria, such as altered mental status, edema, hyperglycemia in the absence of diabetes, and elevated CRP or elevated PCT, are also included.
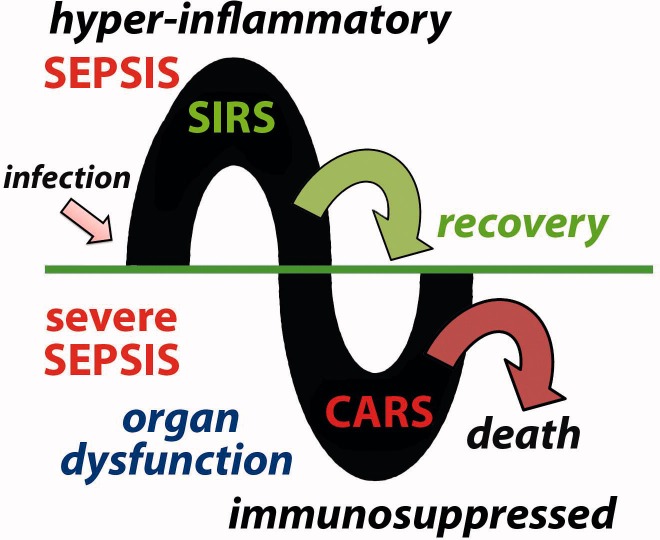
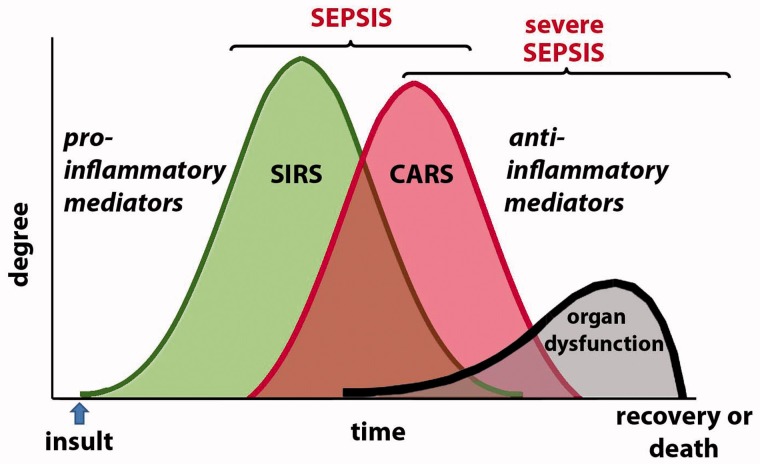
Most investigators credit Dr Roger C. Bone with the recognition that there was more to sepsis than the exuberant hyper-inflammatory SIRS8. Bone helped to stress the importance of a “compensatory anti-inflammatory response syndrome”, which he called CARS9, that often follows the hyper-inflammatory phase, especially in patients who develop what is called “severe” sepsis (Figure 1). In severe sepsis, evidence of widespread organ dysfunction is also present. This may include lung, liver and/or kidney injury, as well as cognitive impairment. So-called septic shock, in which patients suffer cardiovascular collapse and often are unresponsive to fluid resuscitation and vasopressor therapy, is often the terminal event of severe sepsis.
Figure 1.
Sepsis may be divided into two phases. Following infection, a hyper-inflammatory phase is characterized by SIRS. This may resolve or the patient may progress to what is called severe sepsis. During this phase, there is evidence of CARS with immunosuppression and multiple organ dysfunction. This may also resolve, especially with appropriate support, but it often leads to death.
The cause of the organ failure in severe sepsis is unknown, but it resembles the multiple organ dysfunction syndrome (MODS) seen in patients who survive serious traumatic injury10. Many investigators now consider both sepsis and post-traumatic MODS to represent the same stereotypical immunologic response to a severe insult. In this paradigm, the innate immune system initially generates a pro-inflammatory state in response to PAMPs, or in the case of tissue injury, in response to similar molecules called damage-associated molecular patterns (DAMPs) that are derived from damaged host cells. In most patients, this pro-inflammatory response is self-limited, even in the absence of effective treatment. But, in patients who develop sepsis, the response is exaggerated (or “hyper-inflammatory”) and leads to a compensatory down-regulation of the immune system. It is not clear why this happens in some patients and not others. A major risk factor appears to be some degree of pre-existing immune dysfunction. For instance, elderly patients (who usually have some degree of immunodeficiency) and immunosuppressed patients both have a higher incidence of sepsis, as well as a higher mortality rate. Some other underlying factor or genetic predisposition may also be involved. This subject has been recently reviewed by Chung and Waterer11.
As the paradigm of sepsis pathogenesis has evolved over time and as different therapeutic approaches to sepsis have been tried, different biomarkers have been used for diagnosis of sepsis and monitoring of treatment. The initial focus in the 1980s was on the early hyper-inflammatory phase, and high-dose corticosteroids were an important component of sepsis treatment12. TNF, IL-1β and IL-6, the three pro-inflammatory cytokines that produce SIRS, as well as C-reactive protein (CRP), a well-established member of the group of proteins whose synthesis in the liver is up-regulated by IL-6, were all investigated as potential biomarkers. In the 1990s, investigators discovered that the levels of procalcitonin (PCT), the precursor of the hormone calcitonin, were elevated in patients with bacterial infection, and it emerged as another potential biomarker13. Elevations of both CRP and PCT were added to the updated definition of sepsis in 2003. Then, in the early part of the past decade, studies of intensive “goal-directed” treatment of severe sepsis and septic shock used elevated lactate levels to guide therapy14, and obtaining a lactate level when monitoring patients at risk of developing sepsis became standard practice. Recently, as therapies targeting the anti-inflammatory phase of sepsis have begun to enter into clinical trials15, novel biomarkers that attempt to detect changes associated with the down-regulation of the immune system have also been studied.
No single biomarker of sepsis may be ideal, but many are helpful in terms of at least identifying critically ill patients who need more careful monitoring so that the condition may be diagnosed and treated as soon as possible. This review will discuss all the major types of biomarkers of sepsis which have been proposed, and will try to place them within the context of both the different stages of sepsis and the targeted therapeutic approaches.
Pro-inflammatory cytokines as markers of the hyper-inflammatory phase of sepsis
TNF, IL-1β and IL-6 are the cytokines that mediate the initial response of the innate immune system to injury or infection. TNF and IL-1β both activate endothelial cells, attracting circulating polymorphonuclear leukocytes (PMNs) to the site. They also enter the circulation, causing fever and other systemic symptoms. IL-6 enhances the liver’s production of the so-called acute phase reactants, including CRP, and also stimulates a shift in the production of cells in the bone marrow so that more PMNs are produced. Therefore, these three cytokines are essentially responsible for the features of SIRS and could be potentially useful as biomarkers of sepsis (Figure 2).
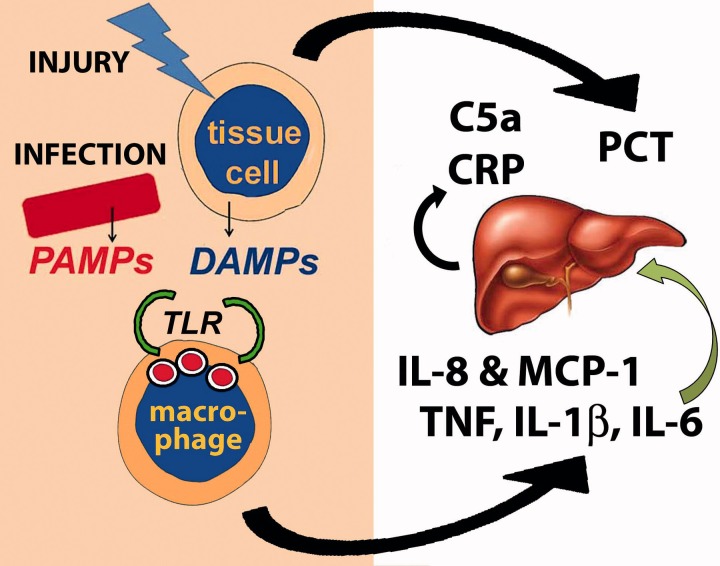
Figure 2.
Sepsis begins with either infection or tissue injury. PAMPs from invading organisms or DAMPs from injured tissue cells (or both) are recognized by macrophage receptors such as the TLRs. This results in the production of pro-inflammatory cytokines such as TNF, IL-1β and IL-6 and chemokines such as IL-8 and MCP-1. IL-6 stimulates the liver to produce CRP and complement proteins. Many cells in the body also produce PCT in response to both infection and injury.
TNF and IL-1β levels are both elevated in endotoxin-related Gram-negative sepsis. Indeed, administration of TNF (or IL-1β) to experimental animals is as effective as endotoxin itself in terms of inducing septic shock16. However, the pre-treatment TNF level does not appear to affect outcome in clinical trials that use anti-TNF antibody therapy17 , 18. Levels of IL-1β are not elevated to the same degree as TNF19, and the role of IL-1β, the related IL-1α, and the naturally occurring IL-1 receptor antagonist in the development of sepsis remains somewhat controversial20. For these reasons, neither TNF nor IL-1β has emerged as a major biomarker for sepsis.
Of the three major pro-inflammatory cytokines, IL-6 has received the most attention. It is more reliably measurable in plasma than the other two cytokines, and it also has other potential clinical uses, such as diagnosis and management of autoimmune rheumatic disorders. Unlike TNF and IL-1β, immunoassays, including some designed to be performed at the patient’s bedside, are commercially available21. However, like TNF and IL-1β, IL-6 is not specific for sepsis, and its major role as a biomarker of sepsis appears to be prognostic, not diagnostic. Numerous studies have shown that elevated levels of IL-6 in septic patients are associated with an increase in mortality22–24. This has been demonstrated even more powerfully in a mouse model of acute septic peritonitis (cecal ligation and puncture, or CLP) in which IL-6 levels not only predict survival, but also are able to target those mice that could benefit most from treatment25. Thus, IL-6 meets one of the desired attributes of an ideal biomarker of sepsis because it may be able to identify those patients with sepsis who are at increased risk of developing severe sepsis, and who therefore need supportive therapy.
Another group of pro-inflammatory cytokines which have been investigated as biomarkers of sepsis are the chemotactic cytokines called chemokines. Although the classification of chemokines is based on the arrangement of their amino-terminal cysteine residues, there are two major types, based on function26. Homing chemokines help to organize the adaptive immune system, especially in secondary lymphoid tissue, while inflammatory chemokines attract PMNs and monocytes to sites of inflammation and enhance their movement through the blood vessel wall. Therefore, many inflammatory chemokines are potential biomarkers of sepsis, and some have been shown to be superior to IL-6. These include the chemokine IL-8 for diagnosis of sepsis27 and monocyte chemoattractant protein (MCP-1) for prediction of sepsis mortality28. Although it promotes inflammation by attracting monocytes to sites of injury or infection, MCP-1 may also promote the synthesis of IL-10, an anti-inflammatory cytokine discussed below. As such, MCP-1 may represent a key element in the evolution of sepsis from the pro-inflammatory phase to the immunosuppressive phase.
PCT and CRP as biomarkers of sepsis
PCT and CRP are both proteins produced in response to infection and/or inflammation. They are probably the two most widely used clinical tests to diagnose and manage patients with sepsis, with the exception of lactate.
CRP is a well-established biomarker of infection and inflammation29. It is one of a group of acute phase reactants mentioned previously – proteins whose synthesis in the liver is up-regulated by IL-6. Some of these proteins play a supportive role and enhance inflammation (e.g. complement), while others appear to protect the host from inflammatory tissue injury (e.g. protease inhibitors). CRP’s role during acute inflammation is not entirely clear. It may bind the phospholipid components of microorganisms (and damaged host cells), facilitating their removal by macrophages. Because the levels of CRP rise much more significantly during acute inflammation than the levels of the other acute phase reactants, the test has been used for decades to indicate the presence of significant inflammatory or infectious disease, especially in pediatrics30 and, more recently, as a biomarker of the inflammation that accompanies atherosclerosis and cardiovascular disease31. Although its low specificity may be its primary drawback as a biomarker of sepsis in adults, it is commonly used to screen for early onset sepsis (occurring during the first 24 h of life) because its sensitivity is generally considered to be very high in this setting32. CRP is also often used to monitor patients after surgery; levels are typically elevated compared to pre-operative levels, but they fall quickly unless post-operative infection is present33.
Pentraxin 3 (PTX3) is another protein with structural similarity to CRP, which may be produced primarily by inflammatory cells rather than the liver. Like CRP, elevated levels of PTX3 have been shown to correlate with the severity of sepsis34. However, it is also elevated in non-infectious inflammatory disorders and therefore offers no advantage over CRP.
The widespread availability of PCT immunoassays in the past several years may have somewhat lessened the importance of CRP as a biomarker of sepsis. PCT is the precursor of mature calcitonin, a hormone with no significant physiological effect in humans, but capable of reducing plasma calcium levels when administered pharmacologically. In the early 1990s, investigators discovered elevated PCT levels in patients with invasive bacterial infection35. Subsequent studies have shown that many tissues throughout the body, not just cells at the local site of infection, produce PCT36, and that PCT is part of the systemic response that leads to severe sepsis13. Like CRP, PCT may also have pro-inflammatory effects37. PCT has been recommended by an expert panel as a useful test in critically ill patients who develop new fever38, and most commercially available PCT assays have been approved by the US Food and Drug Administration (FDA) specifically as an aid to assess the risk, on their first day of admission to an intensive care unit, of critically ill patients progressing to severe sepsis.
Over the past decade, numerous studies have investigated the diagnostic usefulness of PCT, usually comparing it with CRP. Initially, PCT was found, not surprisingly, to be more sensitive and specific than CRP for bacterial infection39, and a number of recent studies have demonstrated that it may be helpful in predicting the results of blood cultures in critically ill patients40–42. Whether PCT is more sensitive and specific than CRP for the diagnosis of sepsis, however, is still being debated. Although there have been scores of reports comparing the two markers, differences in patient populations and cut-offs used, and other factors, have prevented any clear consensus from being reached.
The only large meta-analysis of published investigations comparing PCT and CRP for the diagnosis of sepsis, as opposed to the diagnosis of bacterial infection, was reported by Uzzan et al.43. These authors collected 49 studies, of which 15 assessed both PCT and CRP simultaneously. Their conclusion was that both tests performed effectively, although the global odds ratio for PCT (14.69) was significantly higher than that for CRP (5.43). The Q value was also higher for PCT than for CRP (0.78 versus 0.71). In 2010, as part of a smaller meta-analysis, Yu et al. 44 identified nine trials which compared PCT and CRP, all for the diagnosis of late-onset neonatal sepsis. Four of the studies required documentation of infection and, in these, pooled sensitivity for PCT was higher than that for CRP (72% versus 55%, p < 0.05); the authors commented that this might be attributed to the fact that PCT levels probably rise earlier than CRP in neonatal infection. Pooled specificity, odds ratio and Q value were also higher for PCT, but without statistical significance. In five trials evaluating the two biomarkers that did not require evidence of infection, overall accuracy for PCT was higher but, again, without statistical significance. In 2011, a meta-analysis of a small number of reports comparing the two biomarkers in burn patients could not show superiority of one over the other45.
A meta-analysis of studies looking at the ability of PCT to diagnose sepsis without comparison to CRP was published in 2007 by Tang et al46. These investigators collected 672 reports, of which 18 were considered suitable for analysis. The high rate of rejection was primarily due to the fact that they eliminated all studies that did not provide evidence of infection in the septic patients. Because it is now generally accepted that detection of bacteremia is not a prerequisite for making the clinical diagnosis of sepsis, the rejection of such studies has been raised as a major criticism of their conclusion that PCT cannot accurately distinguish sepsis from SIRS in critically ill patients47. Nonetheless, despite many favorable clinical studies and a specific indication approved by the FDA, several issues regarding the use of PCT in the stratification of patients at risk of developing severe sepsis remain to be resolved.
Although much less likely than CRP to be elevated in patients with systemic inflammation but without sepsis, elevations of PCT are not as specific for infection as was once believed. This biomarker may be elevated in a number of disorders in the absence of infection, especially following trauma48 , 49. Therefore, one common cut-off for the diagnosis of sepsis (or risk of sepsis) is probably not feasible. For instance, the PCT cut-off used to determine the risk of sepsis appears to be higher in critically ill patients admitted to intensive care units from the surgical service than in those admitted from the medical service50. Such observations bolster the view that the condition we call sepsis may represent a stereotypical response on the part of the immune system to injury of any kind, whether infectious or not.
Another problem with most clinical studies of PCT’s accuracy for the diagnosis of sepsis may be the fact that most correlate PCT levels on admission to the intensive care unit either with the subsequent diagnosis of sepsis or with overall mortality. PCT levels may vary early during the development of sepsis and the test’s predictive power is probably only significant later in the patient’s course51 , 52. Consequently, although low levels may be helpful in ruling out the risk of sepsis because of a high negative predictive value, initially elevated levels in critically ill patients may be misleading. Several studies have tried to address this problem by monitoring PCT levels over time, looking for trends that may be more predictive than the single initial level on admission. The most comprehensive of these was a large randomized trial, called the Procalcitonin and Survival Study (PASS).
This trial tested whether knowledge of PCT levels in patients being monitored in a critical care setting resulted in earlier detection of sepsis as well as more effective treatment. Unfortunately, the results did not support the use of PCT results in this manner, and, ironically, the patients in the PCT group had a longer hospital stay53. The authors of this study initially speculated that the greater exposure to broad-spectrum antibiotics observed in patients with elevated PCT levels may have been harmful, and they have recently reported that renal function was, indeed, compromised to a greater degree in this group54. PCT elevations alone should probably not lead to aggressive treatment of critically ill patients, especially if the treatment consists primarily of adding broad-spectrum antibiotics. This observation is interesting because decreasing PCT levels are currently being investigated as a tool to determine whether antibiotics may be discontinued in hospitalized patients being treated for specific infections. A meta-analysis reviewing the large number of clinical trials that have been conducted to validate PCT’s role in antibiotic stewardship programs was recently published by Scheutz et al.55. In the future, this may turn out to be the major utility of PCT as a laboratory test.
Biomarkers of complement proteins in sepsis
Complement proteins enhance phagocytosis of microorganisms by opsonizing their surfaces with a fragment of complement protein 3 (C3) called C3b. Activation of the complement cascade also produces pro-inflammatory peptides such as C5a, a cleavage product of complement protein 5. There is considerable evidence that complement has a role in promoting the inflammatory state in sepsis, and the focus has been on C5a as a potentially useful biomarker. In the CLP mouse model of sepsis, all three complement pathways (classical, lectin and alternative) are activated with downstream elevations in the level of C5a56. Elevated levels of C5a have also been shown to be present in patients with severe sepsis57.
Although most clinical laboratories measure overall complement activity (CH50) as well as the levels of major complement proteins such as C3 and C4, these are not terribly helpful in most situations in which complement activation occurs because these complement proteins are acute phase reactants. Low levels of these proteins will be observed only when the degree of complement consumption overwhelms the liver’s ability to produce them. There are commercially available assays for C5a, which have been utilized in the diagnosis of autoimmune inflammatory disorders58, but these are not widely used and, at the current time, there does not appear to be a major role for monitoring C5a as a biomarker of sepsis.
The role of C5a in sepsis is also complicated because, like the chemokine MCP-1 described above, C5a may have both pro-inflammatory and anti-inflammatory effects during the development of sepsis. For instance, C5a binding by activated PMNs appears to suppress important innate immune functions such as phagocytosis and the respiratory burst59. This effect appears to be mediated by the internalization of surface C5a receptors60.
Biomarkers of activated neutrophils and monocytes in sepsis
As previously mentioned, one of the effects of the elevated levels of the pro-inflammatory cytokine IL-6 is increased production of PMNs by the bone marrow. Depending on the level on inflammation, this stimulation may also cause PMN precursors to leave the bone marrow before they have completely matured. Either an increase in the total number of circulating PMNs or an increase in the percentage of immature forms is one of the criteria for SIRS. Circulating PMNs in patients with sepsis are also already activated by cytokines, and this activation results in changes in their appearance. Toxic granulations represent increased concentrations of antimicrobial compounds in the primary granules, and Döhle bodies are aggregates of endoplasmic reticulum; both are markers of PMN activation in bacterial infection61. Activation of PMNs may be detected even earlier by analyzing the levels of certain cell differentiation molecules on the PMN cell surface, using quantitative flow cytometry (Figure 3). The focus has primarily been on CD64, a high-affinity receptor for the Fc portion of the immunoglobulin molecule, although other activation markers have also been examined as biomarkers of sepsis.
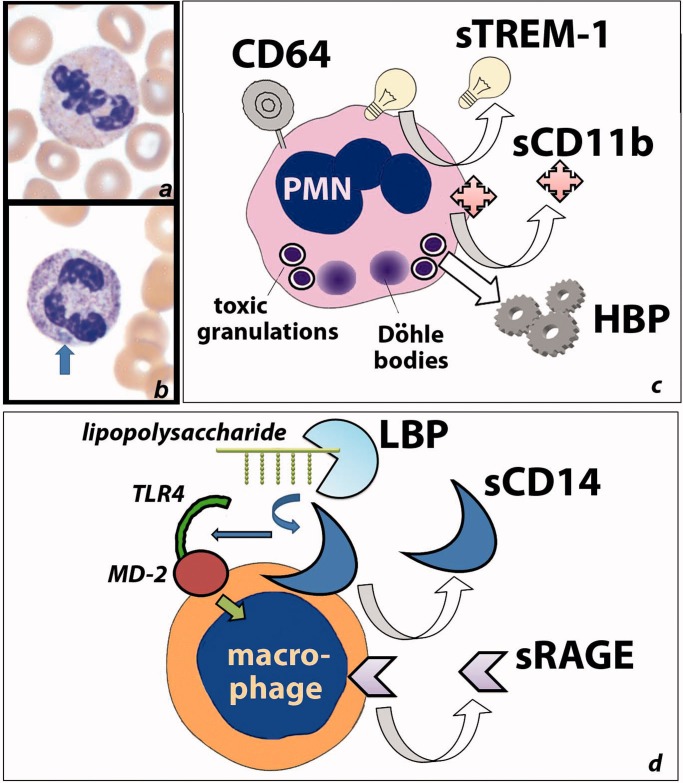
Figure 3.
Activated inflammatory cells up-regulate a number of proteins which may be detected as biomarkers of sepsis, either on the cell surface or as soluble forms in plasma. (a) An unstimulated PMN; (b) a stimulated PMN with darker (“toxic”) granules and a Dohle body (arrow); (c) frequently utilized biomarkers of sepsis related to PMNs include CD64, the soluble forms of TREM-1 and CD11b, and HBP. (d) Frequently utilized biomarkers of sepsis related to macrophages or monocytes include the soluble forms of CD14 (which facilitates recognition of bacterial lipopolysaccharides) and the receptor for RAGE.
In the 1990s, investigators observed that cell-surface expression of CD64, which is negligible in resting PMNs, was increased in infection62. Over the past several years, there have been a number of studies looking at the ability of PMN CD64 expression to detect the presence of infection and/or the presence of sepsis. Elevated CD64 identified sepsis in a small study of critically ill adult patients with a sensitivity comparable to PCT but with better specificity63. Also, quantitative CD64 expression was shown to correlate with the progression of sepsis to severe sepsis in another small study of critically ill adult patients64. One recent study found CD64 to be somewhat less sensitive for sepsis than had been previously reported65, but this study has been criticized for delaying the measurement by flow cytometry for up to 36 h66. CD64 does appear to be stable in anti-coagulated blood specimens67, so the reason for this discrepancy is still not clear.
A recently reported large prospective study of CD64 in a neonatal intensive care unit provides significant support for the use of this marker in this setting. Over a thousand sepsis evaluations were performed on over 700 infants with a low prevalence of sepsis (5%). Most of the cases were late-onset sepsis and, in this population, elevated CD64 expression demonstrated a sensitivity of 75% and a specificity of 77%, using the optimal cut-off68. It is likely that this test will receive more widespread acceptance, especially because it is available on some of the automated hematology analyzers used to measure the total WBC count, a commonly ordered test in sepsis evaluations.
The expression of the integrin CD11b, which enhances the ability of neutrophils to adhere to the endothelium in sites of inflammation, is also increased in bacterial infection, and some have proposed the use of both CD64 and CD11b together to diagnose sepsis69. Several other neutrophil activation markers have been investigated as potential biomarkers of sepsis but they have been measured in plasma by immunoassay, either as soluble versions of the cell-surface proteins or because the proteins are released during degranulation. Most of these have been investigated in experimental models of sepsis and reports of their usefulness in clinical settings are limited.
The best studied is probably the triggering receptor expressed on myeloid cells-1 (TREM-1). TREM-1 is a member of the immunoglobulin superfamily which, like CD64, is up-regulated when PMNs are exposed to bacteria70. However, clinical studies of the ability of the soluble form of TREM-1 to reliably identify patients with sepsis have not been promising71. A recent report in which soluble TREM-1 predicted poor survival in ED patients more accurately than either PCT or CRP may renew interest in this biomarker72.
Heparin-binding protein (HBP, also known as azurocidin) is released from PMN granules when the surface integrins of the PMNs engage selectins on the endothelial cell surface73. It alters endothelial cytoskeletal structure and induces disassembly of the intercellular junctions, enhancing the ability of the PMNs to pass through the endothelial cell barrier. One study showed it to be an excellent predictor of the severe edema and vascular collapse seen in severe sepsis74. This would make HBP an excellent biomarker for severe sepsis, but there has not been any significant follow-up to these initial reports.
Activation markers such as CD64, CD11b and TREM-1 are also expressed on monocytes. However, investigation of monocyte activation markers as potential biomarkers of sepsis has focused on the soluble form of the receptor for advanced glycation end-products (RAGE). Just as the receptor for endotoxin is an excellent example of a PAMP receptor, RAGE may be considered the prototype of a DAMP receptor. Although originally believed to be specific for oxidized cross-linked glycated protein (hence its name), it appears to also be able to bind a large variety of DAMPs. These include high-mobility group box 1 (HMGB1), a non-histone DNA-binding protein and other proteins released by necrotic, but not apoptotic, cells75. In 2008, detection of elevated circulating soluble RAGE (sRAGE), produced by either alternative splicing or proteolytic cleavage of the extracellular domain of the membrane receptor, was able to predict survival in severe sepsis76. Recently, elevated sRAGE levels were similarly predictive of poor survival in patients with community-acquired pneumonia77. There is controversy about the potential use of sRAGE as a biomarker of sepsis in patients with pneumonia, however. Lung alveolar type 1 cells normally express high levels of RAGE and, therefore, sRAGE levels may be elevated in pulmonary infection in the absence of sepsis78.
The TLR on the surface of macrophages and monocytes that recognizes endotoxin requires the assistance of another membrane-bound protein, CD14, as well as an acute phase reactant (lipopolysaccharide-binding protein or LBP) which facilitates endotoxin binding to CD14. Although several studies have shown that elevated levels of LBP can identify patients with infection, at very elevated concentrations this protein effectively neutralizes LPS, and may even be anti-inflammatory79. Therefore, LBP may be less discriminating than other biomarkers with regard to risk of developing severe sepsis80. Recently, however, there has been interest in measuring a soluble form of CD14 as a biomarker of sepsis. Soluble CD14 levels were shown to be comparable to PCT for diagnosis of bacterial infection81 and correlated with the degree of severity in septic patients82.
Detection of infectious organisms and their products in sepsis
If sepsis is defined as SIRS in a patient with infection, then the ultimate biomarker would be the identification of the microorganism responsible. This would not only confirm the diagnosis; it would also provide a specific target for therapy. Despite the fact that sepsis may represent an unusual response to infection which might not be successfully treated by eradicating the microorganism, many have searched for ways to better detect the presence of pathogens in critically ill patients at risk of developing sepsis.
Blood cultures to detect bacteremia are the mainstay of such attempts when patients do not display localizing signs or symptoms. The presence of SIRS has been shown to increase the likelihood that the blood culture will be positive83 but, as has been noted above, blood cultures are often negative in patients with clinical sepsis. Many approaches to the detection of bacteremia by amplifying specific target nucleic acid sequences using polymerase chain reaction (PCR) or other techniques have been applied to both blood culture bottles and patient blood samples84. These include real time multiplexed PCR systems designed to detect the most frequently observed bacteria (and fungi) in patients with sepsis, as well as amplification of universal 16S and 18S ribosomal ribonucleic acid (RNA) followed by sequencing of the amplification target. In a prospective study of severe sepsis in a surgical intensive care setting, PCR techniques identified approximately twice the number of positive specimens compared with conventional blood culture85. This approach has promise but it will be important to document that PCR-positive, blood culture-negative specimens are not false positives by correlating the results with other clinical data86.
PAMPs produced by microorganisms and DAMPs released during tissue injury have themselves been investigated as biomarkers of sepsis. During the past decade, endotoxin, the classic PAMP, has been studied in patients with critical illness and sepsis using a unique immunoassay approach, which is easier to perform than the traditional test that relies on coagulation of the hemolymph of the horseshoe crab. An antibody to a conserved lipid moiety forms immune complexes in the patient’s whole blood with any endotoxin present, and these interact with the patient’s neutrophils to produce an oxidative burst response that is measured by chemiluminescence87.
In 2004, a large observational study showed that endotoxin was present in more than one-half of all patients admitted to intensive care units on the day of their admission, despite the fact that only a small number had documented bacterial infection. Approximately, 10% of the patients in this study developed severe sepsis, and the level of endotoxin was a significant risk factor88. This observation has been confirmed by several subsequent studies, although the utility of the endotoxin assay in patients who do not have documented gram-negative bacterial infection or who have only intermediate levels of endotoxin is unclear. The addition of other biomarkers, such as PCT, may be necessary to reliably identify risk in such patients89.
HMGB1 is elevated in most patients with severe sepsis90. However, there have been discrepancies in several reports which have correlated levels with organ dysfunction using Sequential Organ Failure Assessment (SOFA) scores, and there is a consensus that HMGB1 levels do not offer any helpful prognostic information with regard to survival91. Another important category of DAMP is a group of S100 proteins, called calgranulins or myeloid related proteins, that are expressed on myeloid cells and form heterodimers when released from damaged neutrophils during inflammation. Despite the fact that mice deficient in these proteins show enhanced survival in an experimental model of abdominal sepsis, and the observation that blood levels of these proteins are elevated in patients with sepsis92, there is not yet any significant evidence that they are clinically useful as biomarkers of sepsis.
Biomarkers of the immunosuppressive phase of sepsis
Bone recognized the importance of CARS, which follows the hyper-inflammatory state in septic patients, more than 15 years ago12. Recently, several biomarkers of the immunosuppressive phase of sepsis have received considerable attention (Figure 4).
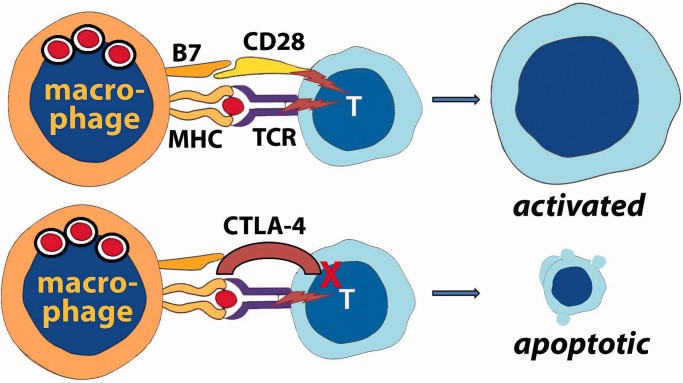
Figure 4.
There is significant evidence that patients with severe sepsis have defective adaptive immunity. Macrophages (or monocytes) may lose expression of the Class II MHC proteins which display foreign peptide to the TCR. However, more importantly, T-cells upregulate expression of CTLA-4, an alternative ligand for the co-stimulator B7 on the antigen-presenting cell. Instead of providing co-stimulation and activation of the T-cell, which would occur if B7 interacted with CD28, interaction with CTLA-4 results in T-cell unresponsiveness and, eventually, death by apoptosis.
The earliest sign of dampening of the immune response, in both patients with sepsis as well as patients who survive severe traumatic injury, is a reduction in the expression of the Class II major histocompatibility complex (MHC) proteins (HLA-DR) (HLA, human leukocyte antigen) on the surface of macrophages and other antigen-presenting cells. These proteins display peptides derived from phagocytized protein to T-cells. If recognized by the T-cell’s unique antigen receptor, and the appropriate “second signal” is also transmitted via co-stimulatory molecules, T-cell activation occurs. Clinical studies have focused on monocyte HLA-DR expression, which is markedly suppressed in most patients with sepsis at onset but recovers within ten days in survivors93. As mentioned, it may also be suppressed after major trauma, and the failure of levels to return during the first week of hospital stay is an accurate predictor of the development of sepsis in these patients94. Low levels of HLA-DR expression predict poor survival95 as well as increased risk of nosocomial infection96.
Autopsy studies by Hotchkiss et al.97 demonstrated that patients who died of severe sepsis had significant depletion of T-cells and B-cells in the spleen, and these investigators have recently extended these studies using spleen tissue harvested immediately after death from patients with active severe sepsis98. Compared with control spleens, the number of T-cells was markedly reduced and the T-cells of the patients who died of sepsis also produced significantly lower levels of cytokines when stimulated. The exact cause for the loss of T-cells in severe sepsis through apoptosis is not known. However, just as HLA-DR expression on monocytes is decreased, clinical studies of T-cells clearly demonstrate that patients with sepsis have increased expression of the negative co-stimulatory molecule CTLA-4 (cytotoxic T lymphocyte-associated antigen-4)99 as well as another molecule associated with T-cell apoptosis, PD-1 (programmed death-1)100.
Normally, T-cells express a positive co-stimulatory molecule called CD28. When the T-cell antigen receptor (TCR) recognizes antigen in the context of the antigen-presenting cell’s Class II MHC, simultaneous engagement of CD28 by a molecule called B6 on the antigen-presenting cell delivers the “second signal” that activates the T-cell. Decreased expression of CD28 and enhanced expression of the alternative ligand CTLA-4 (also called CD152) tends to promote apoptosis rather than activation99. This effect may be mediated by T-regulatory cells101.
The clinical usefulness of measuring the anti-inflammatory cytokine IL-10, which inhibits the expression of both Class II MHC and co-stimulator molecules, and TGF-β (TGF, transforming growth factor), which suppresses T-cell proliferation, has been examined. Elevated levels of IL-10 predict mortality in severe sepsis and have also been shown to correlate with the suppression of monocyte HLA-DR expression102 as well as expression of PD-1 and its ligand on T-cell and monocytes, respectively103. IL-10 has also been reported to be an accurate biomarker for neonatal sepsis104 but, perhaps in keeping with the studies discussed below showing IL-10 elevation soon after onset, there was no difference in IL-10 levels when early onset and late-onset sepsis were compared105. TGF-β, whose anti-inflammatory activity may be less relevant than its ability to promote tissue repair, has not been shown to be as useful as IL-10 in terms of identifying sepsis patients who are unlikely to survive, but it has been shown to predict the development of acute respiratory distress syndrome in septic patients106.
It may be too early to predict which of these new biomarkers of the immunosuppressive phase of sepsis will be useful clinically. There is little information in the literature regarding the time course of these changes, their ability to predict survival, and how effectively they may be utilized in support of novel therapies targeting negative co-stimulatory molecules.
Biomarkers of organ dysfunction in sepsis
A variety of well-established routine laboratory tests help physicians assess whether end-organ dysfunction has advanced the patient’s clinical status from sepsis to severe sepsis. Some of these are included in physiological scoring systems, such as APACHE (acute physiology and chronic health evaluation) and SOFA, used to gauge the degree of critical illness in hospitalized patients. Elevated bilirubin and creatinine levels indicate liver and kidney dysfunction, respectively. However, the most widely utilized biomarker indicating organ dysfunction is the blood lactate level.
Glucose is metabolized to pyruvate anaerobically and, in most tissues, pyruvate is further oxidized in the mitochondria. In the absence of adequate oxygen, however, mitochondrial metabolism is compromised. When this occurs, cells form lactate from pyruvate in order to regenerate the co-factor nicotinamide adenine dinucleotide (NAD) required for upstream anaerobic glycolysis to continue. It is commonly assumed that lactate levels rise in patients with sepsis because decreased tissue perfusion produces hypoxic organs, which then resort to anaerobic glycolysis. There is considerable evidence for vascular compromise, either due to endothelial injury, disseminated intravascular coagulation or hypotension. However, there are other explanations for the lactate elevations seen in sepsis.
Lactate is constantly being produced by red blood cells (which lack mitochondria) and by some tissues with high rates of glycolysis, even when tissue perfusion is not compromised. The liver converts much of this lactate back into glucose and oxidizes the rest. Therefore, liver dysfunction associated with sepsis may result in impaired lactate clearance107. Systemic inflammation also induces increased anaerobic glycolysis because the increased rate of glucose metabolism in the injured tissue often exceeds the oxidative capacity of mitochondria. Finally, the tissues of patients with sepsis appear to acquire mitochondrial dysfunction, due to some as yet unknown mechanism108. A recent comparison of patients with septic shock who either did or did not have elevated lactate levels seems to support the idea that some other factors may be responsible for lactate production in sepsis109.
Although most hospitals that utilize lactate as a screen for sepsis use a cut-off of 4.0 mmol/L (with a reference range of approximately <2.0 mmol/L), recent studies have indicated that this may be too high. In a retrospective cohort study of ED patients with severe sepsis, investigators showed that hemodynamically stable patients with intermediate lactate levels (2.0–4.0 mmol/L) were also at significant risk, compared to patients with levels less than 2.0 mmol/L110. This observation has been confirmed in another retrospective cohort study which found that even patients with “high normal” (1.5–2.3 mmol/L) lactate levels had mortality comparable to patients with intermediate lactate levels (2.3–4.0 mmol/L). These findings may have implications for future stratification of patients at the risk of severe sepsis111.
Shortly after the introduction of intensive goal-directed resuscitation of septic patients based on their initial lactate level, monitoring lactate clearance, a tool originally developed for use in trauma patients, was also utilized in sepsis patients. A low lactate clearance, based on serial measurement over the course of several hours, was shown to correlate with elevations of PCT and IL-6 as predictors of the development of sepsis in trauma patients49, and to be useful as a predictor of mortality in patients with severe sepsis112 , 113. There is controversy, however, regarding the use of serial lactate measurements, rather than more conventional parameters such as central venous oxygen saturation, to determine the success of resuscitation114 , 115.
Endothelial dysfunction, whether due to systemic inflammation or some other as yet unknown mediator, is probably a major contributor to the organ dysfunction observed in severe sepsis and is obviously the cause of septic shock. A large number of biomarkers of endothelial activation, including angiopoietins, soluble adhesion molecules and endocan, have been shown to be elevated in sepsis116. However, it is unlikely that any of these endothelial cell biomarkers will be widely utilized until we can show that they support related novel therapies.
One major area of vascular pathology which clearly influences mortality involves the coagulation system. When the innate immune system is stimulated, regardless of the cause, the coagulation cascade is also initiated as a stereotypical response to injury117. Consumption of coagulation factors and platelets, coupled with inhibition of the fibrinolytic system, results in microvascular fibrin deposition which is believed to contribute to organ dysfunction due to the resultant hypoxia. A variety of clinical tests related to coagulation and fibrinolysis have been used to monitor hemostatic abnormalities associated with sepsis. Disseminated intravascular coagulation (DIC), with the deposition of thrombi throughout the microvasculature, occurs in a significant percentage of patients with sepsis and, if present, increases mortality118. DIC is diagnosed using a scoring system proposed by the International Society on Thrombosis and Hemostasis, which includes platelet count, prothrombin time, fibrinogen level and a marker of fibrin formation. The most commonly utilized fibrin-related marker has been the assay for D-dimer. This immunoassay uses antibodies that recognize the fibrin fragment containing cross-linked ends of the fibrin monomers (called D-dimer because the two globular ends of the monomer are termed the “D” domains). DIC scores using D-dimer predict sepsis severity and survival119.
Although the reason for the high prevalence of DIC in patients with sepsis is not well-understood, attention has focused on the down-regulation of a natural inhibitor of the coagulation cascade, thrombomodulin, on the vascular endothelial surface120. In the absence of this regulatory protein, which binds thrombin and activates protein C, the procoagulant factors Va and VIIIa are not inhibited. This failure to impede coagulation led to the development of the only specific pharmacologic agent for the treatment of sepsis that was approved by the FDA: recombinant human activated protein C (drotrecogin alfa, Xigris® Eli Lilly, Indianapolis, IN). The drug’s approval in 2001 for patients with severe sepsis and a high risk of death was controversial. It was based primarily on a single randomized, controlled trial and studies conducted following its release raised concerns about its efficacy and the risk of bleeding. In 2012, the results of a large, double-blind, placebo-controlled, multicenter trial that showed no reduction of mortality in any of the subgroups tested were published121, and the drug was withdrawn from the market.
A relatively new area of investigation in sepsis is the study of microparticles. Different from exosomes, which are intracellular vesicles shed via exocytosis, the term microparticles usually refers to vesicles shed directly from the cell surface via blebbing. These may break down and release their contents into the immediate microenvironment or circulate and interact with target cells distant from their site of origin. Originally discovered as a platform for the interaction of tissue factor with the coagulation system, they may also play a significant role in inflammation122. Microparticles may mediate both systemic inflammation and DIC in sepsis. Because the endothelium may be a primary target of circulating microparticles, they may also play a role in the significant and widespread increased vascular permeability that contributes to septic shock123.
Multi-marker approach to the diagnosis of sepsis
No one biomarker is likely to adequately reflect the rapidly evolving nature of a potentially septic patient’s status, even if monitored frequently during the course of the patient’s hospital stay. This is the important lesson from the failure of PCT to provide helpful information in the PASS study, when used as a single biomarker. Several investigators have reported attempts to use a panel of biomarkers in order to better identify patients at risk.
In 2007, Kofoed et al. 124 reported that the combination of three or six pro-inflammatory biomarkers more accurately identified patients with bacterial infection than any one biomarker alone. In 2009, Shapiro et al. applied this approach to the diagnosis of severe sepsis125. Samples from approximately 1000 patients on presentation in the ED were used to try to predict outcome 72 h later. The rate of development of severe sepsis was 52%, while the mortality rate of septic patients was 12%, compared to 0.9% for patients who did not develop sepsis. Using multivariate logistic regression, the investigators narrowed an initial list of over 150 different biomarkers to a panel of nine, and then found three which, combined into a “sepsis score”, best predicted the onset of severe sepsis.
Surprisingly, this panel of three biomarkers did not include any of the traditional ones previously utilized for the diagnosis of sepsis (or, for that matter, discussed in this review). The three best predictors were the antagonist of the IL-1 receptor (IL-1ra), protein C and neutrophil gelatinase-associated lipocalin (NGAL). Each is plausible as a potential biomarker of sepsis, either as an anti-inflammatory protein (IL-1ra), an important component of the coagulation scheme (protein C), or a marker of organ injury (NGAL). However, it would have been difficult to predict that levels of these three biomarkers would perform better than traditional biomarkers. A similar bioscore, utilizing the results of three more traditional biomarkers (PCT, CD64 and sTREM-1) has also been proposed66.
Perhaps the best panel of biomarkers for the diagnosis of sepsis, or for estimation of the risk of developing severe sepsis, will include both pro-inflammatory and anti-inflammatory markers. The immunosuppressive state that follows the hyper-inflammatory state in sepsis certainly explains why many patients develop nosocomial infections with opportunistic bacteria and/or reactivation of latent viral infection. But does the immunosuppression also cause the multiple organ dysfunction and, if so, how? Is the immunosuppressive state truly compensatory, meaning that somehow the immune system is trying to correct for the earlier exaggerated response? It is likely that novel therapies aimed at enhancing restoration of immunocompetency in patients with sepsis will require a better understanding of this sequence of events. Although it is usually described as the roller coaster of SIRS followed by CARS (illustrated in Figure 1), there is experimental and clinical evidence that the seeds of the down-regulation of both innate and adaptive immunity are sown relatively early while the pro-inflammatory phase is ascendant.
Analysis of cytokine production in the CLP model of sepsis shows that both pro- and anti-inflammatory cytokines are elevated early on and that both may help predict outcome126. This observation has been confirmed in studies of human patients with sepsis as well. For instance, in a cohort study of almost 2000 patients with community acquired pneumonia in whom 30% developed severe sepsis and 26% died, elevated cytokine levels were present in the majority of the patients (82%) at presentation to the ED. Cytokine levels were highest in those patients who died of severe sepsis and the pattern associated with the highest risk of death was marked elevation of both the pro-inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10127. A similar significant association between early elevation of IL-10 and development of severe sepsis was found in critically ill hospitalized patients128. We also know that the loss of cell-surface HLA-DR on circulating monocytes occurs very early and that the transition to severe sepsis is characterized, not by the loss of HLA-DR expression, but by its failure to return to normal129.
Gene expression profiling of patients, using RNA extracted from circulating PMNs and oligonucleotide microarrays, tends to support a more sequential process. Although there is an enhanced expression of pro-inflammatory cytokine genes in both early and severe sepsis, enhanced expression of anti-inflammatory cytokine genes, such as IL-10 and TGF-β, was seen only in severe sepsis130. Also, pathway analysis of the transcriptome studies confirms a pattern of immunosuppression of the adaptive immune system primarily in patients with severe sepsis. Expression of nuclear factor-kB, a transcription factor important for the activation of T-cells and B-cells, was diminished and expression of its inhibitor was enhanced in patients with sepsis, but not SIRS131. This discrepancy between the sequential model originally proposed by Bone and what might be called the concurrent model (Figure 5) suggested by van der Poll and van Deventer132 needs to be addressed.
Figure 5.
An alternative model for the progression of sepsis to severe sepsis proposes that the CARS begins while the pro-inflammatory SIRS is still present. Understanding the interplay of these opposing features may help investigators discover the pathogenesis of the organ dysfunction that occurs in patients who develop severe sepsis (and die).
Finally, the model usually ascribes survival to some event during the immunosuppressive phase that allows immune function to return to normal133. What is this event, and what factors contribute to it? A marker or, more likely, a panel of markers which could identify those patients who are moving from the hyper-inflammatory state to the anti-inflammatory state of sepsis could help identify patients who would benefit from novel therapies designed to restore immune function.
Recently, at least two studies have attempted to combine pro-inflammatory and anti-inflammatory markers. Andaluz-Ojeda et al. 134 utilized an automated multiplexed immunoassay approach to simultaneously measure almost 20 different cytokines in approximately 30 patients with severe sepsis. Levels of IL-6 and IL-8 (both of which can be considered pro-inflammatory), as well as IL-10 and MCP-1 (both of which can be considered anti-inflammatory) were all higher in patients who died (mortality rate was 59%), and a combined score was more predictive than any one cytokine, whether or not the hazard ratio was adjusted for the APACHE score. Gouel-Cheron et al. 135 combined monocyte HLA-DR expression using flow cytometry with IL-6 and IL-10 levels by immunoassay in 100 trauma patients admitted to the intensive care unit; 37% developed sepsis, but mortality was low (and probably not very different from patients who did not develop sepsis). In this study, plasma IL-10 was not measurable but the combination of a lack of increase in monocyte HLA-DR expression and elevated IL-6, after adjustment for the degree of trauma, was a powerful predictor of the development of sepsis, more than doubling the odds ratio of either biomarker alone.
This approach, in which markers of the hyper-inflammatory state are combined with markers of the anti-inflammatory state, is the one most likely to succeed in predicting the onset of severe sepsis in future studies.
Conclusion
Rory Staunton was a 12-year-old boy who grew up in Queens in New York City. During gym class on Wednesday, March 28, 2012, he dove for a basketball and scraped his arm. He did not think much of it but, later that night, his leg began to hurt. Waking up on Thursday, March 29, he felt weak and nauseous. His mother took his temperature and, when she saw that it was 104 °F, she called the family’s pediatrician who saw Rory in her office that afternoon. The doctor was concerned because Rory had vomited in the pediatrician’s waiting room and his temperature was still very elevated. The pediatrician recommended that his mother take him to a nearby medical center’s ED.
Rory and his parents arrived at the ED at approximately 7 pm that evening. Convinced that he probably had a viral infection of some kind, he was treated with an anti-emetic and hydrated. Blood was drawn for laboratory testing but he was discharged before the results were reported. His white blood cell count was elevated with an increase in immature band forms, but, apparently, no one made any note of these results. On the morning of Friday, March 30, Rory had a bout of diarrhea which actually reassured his parents because this had been predicted by the ED physician as part of the likely resolution of his gastrointestinal virus. However, Rory remained very weak and could not get out of bed on his own. Later that day, part of his body appeared mottled and dark. His parents called the pediatrician, who urged them to bring him back to the medical center. He was admitted to the intensive care unit that evening with signs and symptoms of severe sepsis, almost certainly in response to the introduction of bacteria when he scraped his arm on the gymnasium floor two days earlier. On Sunday, April 1, four days after this apparently minor trauma, he died of cardiac arrest.
In the hope that telling this story would help prevent other young children from experiencing the same fate, Rory’s parents shared his medical records with a reporter for the New York Times who was a family friend. The newspaper published the story in July 20121 3 6. Subsequently, the medical center developed a new checklist to ensure that a doctor and nurse conducted a final review of all relevant vital signs and laboratory results before a patient was discharged from the ED. News coverage of Rory’s case has stimulated discussion at many hospitals across the U.S.A., and Rory’s parents have started the Rory Staunton Foundation to increase awareness of sepsis.
Ultimately, the best way to recognize sepsis early is to increase awareness on the part of the physicians and nurses who initially examine patients. It is clear that, had the physician in the ED known about the elevated white blood cell count in addition to the fever, elevated pulse and respiratory rate, he or she would have recognized that Rory had SIRS. However, the focus was on his gastrointestinal complaints and the leading diagnosis was a viral infection. We do not know whether serum lactate was ordered. It is unlikely that any of the other potential biomarkers discussed in this review were ordered. Could any of these tests have helped to at least raise suspicion that Rory’s illness was probably more serious than his caretakers thought? The overwhelming evidence from the literature discussed above suggests strongly that it would have.
Especially given the high negative predictive value of many of the proposed biomarkers of sepsis, we can hope that they may soon guide triage in the ED for infectious disease in a manner similar to the use of troponin for patients suspected of having acute coronary syndrome. Some markers may help physicians recognize patients with sepsis who might otherwise be overlooked. Other markers may allow physicians to admit patients at high risk of developing severe sepsis, and discharge those whose infections may be safely treated as out-patients. In one study, researchers at the University of Pittsburgh found, when evaluating community-acquired pneumonia, that patients in the lowest PCT tier (<0.1 μg/L) were at low risk of septic complications, even if clinical assessment showed otherwise; this could potentially justify conservative management137.
Biomarkers of sepsis used in this way should probably be implemented as multi-marker panels that include both pro-inflammatory and anti-inflammatory biomarkers. Significant work remains to identify the right combination. Although they have been effective at reducing mortality, existing supportive measures alone will probably not be enough to finally bring sepsis under control. Since most of the new innovative approaches to treating sepsis target specific biomarkers, more robust ways to measure them will help support the success of these new modes of treatment.
Acknowledgements
The author would like to thank Rory Staunton’s parents for sharing their son’s story and, hopefully, changing the way we manage this disease.
Glossary
Abbreviations
- APACHE:
acute physiology and chronic health evaluation
- CARS:
compensatory anti-inflammatory response syndrome
- CLP:
cecal ligation and puncture
- CRP:
C-reactive protein
- CTLA-4:
cytotoxic T lymphocyte-associated antigen-4
- DAMP:
damage-associated molecular pattern
- DIC:
disseminated intravascular coagulation
- ED:
Emergency Department
- FDA:
US Food and Drug Administration
- HBP:
heparin-binding protein, azurocidin
- HLA:
human leukocyte antigen
- HMGB1:
high-mobility group box 1
- IL:
interleukin
- IL-1ra:
antagonist of the interleukin-1 receptor
- LBP:
lipopolysaccharide-binding protein
- LPS:
lipopolysaccharide
- MCP-1:
monocyte chemoattractant protein-1
- MHC:
major histocompatibility complex
- MODS:
multiple organ dysfunction syndrome
- MRP:
myeloid related protein
- NAD:
nicotinamide adenine dinucleotide
- NGAL:
neutrophil gelatinase-associated lipocalin
- PAMP:
pathogen-associated molecular pattern
- PASS:
Procalcitonin and Survival Study
- PCR:
polymerase chain reaction
- PCT:
procalcitonin
- PD-1:
programmed death-1
- PMN:
polymorphonuclear leukocyte
- PTX3:
pentraxin 3
- RAGE:
receptor for advanced glycation end-products
- RNA:
ribonucleic acid
- SIRS:
systemic inflammatory response syndrome
- SOFA:
Sequential Organ Failure Assessment
- TGF:
transforming growth factor
- TLR:
toll-like receptors
- TNF:
tumor necrosis factor
- TREM:
triggering receptor expressed on myeloid cells
Declaration of interest
The author alone is responsible for the content and writing of this article. The author has received honoraria and payment of travel expenses from BioMerieux, Inc.
References
- 1.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–31. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MJ, Williams SN, DeFrances CJ, Golosinsky A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. National Center for Health Statistics Data Brief No. 62. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Jun. 2011. http://www.cdc.gov/nchs/data/databriefs/db62.htm Available from: [last accessed 2 Jan 2013]
- 3.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 5.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 6.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 7.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 8.Balk R, Roger C. Bone, MD and the evolving paradigms of sepsis. Contrib Microbiol. 2011;17:1–11. doi: 10.1159/000323970. [DOI] [PubMed] [Google Scholar]
- 9.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 10.Choileain NN, Redmond HP. The immunological consequences of injury. Surgeon. 2006;4:23–31. doi: 10.1016/s1479-666x(06)80018-1. [DOI] [PubMed] [Google Scholar]
- 11.Chung LP, Waterer GW. Genetic predisposition to respiratory infection and sepsis. Crit Rev Clin Lab Sci. 2011;48:250–68. doi: 10.3109/10408363.2011.641517. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Fisher JC Jr, Clemmer TP, et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Eng J Med. 1987;317:653–8. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 13.Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K. Procalcitonin: a new indicator of the systemic response to severe infections. Infection. 1997;25:329–34. doi: 10.1007/BF01740811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Eng J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther. 2011;9:1013–33. doi: 10.1586/eri.11.122. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P, Cerami A. Tumor necrosis factor as a pharmacological target. Methods Mol Med. 2004;98:1–8. doi: 10.1385/1-59259-771-8:001. [DOI] [PubMed] [Google Scholar]
- 17.Qui P, Cui X, Barochia A, Li Y, et al. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Investig Drugs. 2011;20:1555–64. doi: 10.1517/13543784.2011.623125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice TW, Wheeler AP, Morris PE, et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection CytoFab in severe sepsis. Crit Care Med. 2006;34:2271–81. doi: 10.1097/01.CCM.0000230385.82679.34. [DOI] [PubMed] [Google Scholar]
- 19.Cannon JG, Tompkins RG, Gelfand JA, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Jooster LA, van de Veerdonk FL, Vonk AG, et al. Differential susceptibility to lethal endtoxinaemia in mice deficient in IL-1α, IL-1β, or IL-1 receptor type I. APMIS. 2010;118:1000–7. doi: 10.1111/j.1600-0463.2010.02684.x. [DOI] [PubMed] [Google Scholar]
- 21.Batfalsky A, Lohr A, Heussen N, et al. Diagnostic value of an interleukin-6 bedside test in term and preterm neonates at the time of clinical suspicion of early- and late-onset bacterial infection. Neonatology. 2012;102:37–44. doi: 10.1159/000336632. [DOI] [PubMed] [Google Scholar]
- 22.Patel RT, Deen KI, Korings D, Warwick J, Keighley MR. Interleukin-6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81:1306–8. doi: 10.1002/bjs.1800810914. [DOI] [PubMed] [Google Scholar]
- 23.Petilla V, Hynninen M, Takkunen O, et al. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220–5. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 24.Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody Fab’2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–82. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 25.Osuchowski MF, Connett J, Welch K, et al. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med. 2009;37:1567–73. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliasson M, Egesten A. Antibacterial chemokines: actors in both innate and adaptive immunity. In: Herwald H, Egesten A, editors. Trends in innate immunity. Vol. 15. Basel: Karger; Contrib Microbiol; 2008. pp. 101–17. [DOI] [PubMed] [Google Scholar]
- 27.Stryjewski GR, Nylen ES, Bell MJ, et al. Interleukin-6, interleukin-8, and a rapid and sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr Crit Care Med. 2005;6:129–35. doi: 10.1097/01.PCC.0000149317.15274.48. [DOI] [PubMed] [Google Scholar]
- 28.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Eng J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 30.Jaye DL, Waites KB. Clinical applications of c-reactive protein in pediatrics. Ped Infect Dis. 1997;16:735–8. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Benzaquen LR, Yu H, Rifai N. High-sensitivity C-reactive protein: an emerging role in cardiovascular risk assessment. Crit Rev Clin Lab Sci. 2002;39:459–97. doi: 10.1080/10408360290795556. [DOI] [PubMed] [Google Scholar]
- 32.Hofer N, Zacharias E, Muller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102:25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 33.Welsch T, Frommhold K, Hinz U, et al. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery. 2008;143:20–28. doi: 10.1016/j.surg.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–29. doi: 10.1007/s00134-010-1752-5. [DOI] [PubMed] [Google Scholar]
- 35.Assicot M, Gendrel D. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller B, White JC, Nylen ES, et al. Ubiquitous expression of the calcitonin-I gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 37.Tavares E, Minano FJ. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemias: neuroendocrine and systemic studies. Clin Sci. 2010;119:519–34. doi: 10.1042/CS20100007. [DOI] [PubMed] [Google Scholar]
- 38.O’Grady NP, Barie PS, Bartlett JG, et al. American College of Critical Care Medicine; Infectious Diseases Society of America. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–40. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 39.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 40.Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis. 2002;35:156–61. doi: 10.1086/341023. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura A, Wada H, Ikejiri M, et al. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock. 2009;31:586–91. doi: 10.1097/SHK.0b013e31819716fa. [DOI] [PubMed] [Google Scholar]
- 42.Riedel S, Melendez JH, An AT, et al. Procalcitonin as a marker for the detection of bacteremia and sepsis in the Emergency Department. Am J Clin Pathol. 2011;135:190–9. doi: 10.1309/AJCP1MFYINQLECV2. [DOI] [PubMed] [Google Scholar]
- 43.Uzzan B, Cohen R, Nicolas P, et al. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 44.Yu Z, Liu J, Sun Q, et al. The accuracy of the procalcitonin test for the diagnosis of neonatal sepsis: a meta-analysis. Scand J Infect Dis. 2010;42:723–33. doi: 10.3109/00365548.2010.489906. [DOI] [PubMed] [Google Scholar]
- 45.Mann EA, Wood GI, Wade CE. Use of procalcitonin for the detection of sepsis in the critically ill burn patient: a systemic review of the literature. Burns. 2011;37:549–58. doi: 10.1016/j.burns.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Tang BMP, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systemic review and meta-analysis. Lancet Infect Dis. 2007;7:210–17. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 47.Reinhart K, Brunkhorst FM. Meta-analysis of procalcitonin for sepsis detection. Lancet Infect Dis. 2007;7:500–2. doi: 10.1016/S1473-3099(07)70165-2. [DOI] [PubMed] [Google Scholar]
- 48.Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–52. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 49.Billeter A, Turina M, Seifert B, et al. Early serum procalcitonin, interleukin-6, and 24-hour lactate clearance: useful indicators of septic infections in severely traumatized patients. World J Surg. 2009;33:558–66. doi: 10.1007/s00268-008-9896-y. [DOI] [PubMed] [Google Scholar]
- 50.Clec’h C, Fosse JP, Karoubi P, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006;34:102–7. doi: 10.1097/01.ccm.0000195012.54682.f3. [DOI] [PubMed] [Google Scholar]
- 51.Brunkhorst FM, Al-Nawas B, Krummenauer F, et al. Procalcitonin, c-reactive protein and APACHE II score for risk evaluation in patients with severe pneumonia. Clin Microbiol Infect. 2002;8:93–100. doi: 10.1046/j.1469-0691.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- 52.Bele N, Darmon M, Coquet I, et al. Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients. BMC Infect Dis. 2011;11:224. doi: 10.1186/1471-2334-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen JU, Hein L, Lundgren B, et al. Procalcitonin and Survival Study PASS Group. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39:2048–58. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]
- 54.Jensen JU, Hein L, Lundgren B, et al. Procalcitonin and Survival Study PASS Group. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomized trial. BMJ Open. 2012;2:e000635. doi: 10.1136/bmjopen-2011-000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheutz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions. Arch Intern Med. 2011;171:1322–31. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber H, Rittirsch D, Flieri M, et al. Complement activation during sepsis in humans. Adv Exp Med Biol. 2006;586:217–26. doi: 10.1007/0-387-34134-X_15. [DOI] [PubMed] [Google Scholar]
- 57.Flierl MA, Rittirsch D, Nadeau BA, et al. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22:3483–90. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan J, Gou SJ, Huang J, et al. C5a and its receptors in human anti-neutrophil cytoplasmic antibody ANCA-associated vasculitis. Arthritis Res Ther. 2012;14:R140. doi: 10.1186/ar3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward PA. The harmful role of C5a on innate immunity in sepsis. J Innate Immun. 2010;2:439–45. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan C, Gao H. New insights for C5a and C5a receptors in sepsis. Front Immunol. 2012;3:368. doi: 10.3389/fimmu.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zipursky A, Palko J, Milner R, Akenzua GI. The hematology of bacterial infection in premature infants. Pediatrics. 1976;57:839–53. [PubMed] [Google Scholar]
- 62.Leino L, Sorvajarvi L, Katajisto M, et al. Febrile infection changes the expression of IgG Fc receptors in human neutrophils in vivo. Clin Exp Immunol. 1997;107:37–43. doi: 10.1046/j.1365-2249.1997.d01-899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardelli P, Ferraironi M, Amodeo R, et al. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol. 2008;21:43–49. doi: 10.1177/039463200802100106. [DOI] [PubMed] [Google Scholar]
- 64.Livaditi O, Kotanidou A, Psarra A, et al. Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine. 2006;36:283–90. doi: 10.1016/j.cyto.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Gros A, Roussel M, Sauvadet E, et al. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 2012;38:445–52. doi: 10.1007/s00134-012-2483-6. [DOI] [PubMed] [Google Scholar]
- 66.Gibot S, Bene MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186:65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 67.Elghetany M, Davis B. Impact of preanalytical variables on granulocytic surface antigen expression: a review. Cytometry. 2005;65:1–5. doi: 10.1002/cyto.b.20051. [DOI] [PubMed] [Google Scholar]
- 68.Streimish I, Bizzarro M, Northrup V, et al. Neutrophil CD64 as a diagnostic marker in neonatal sepsis. Pediatr Infect Dis J. 2012;31:777–81. doi: 10.1097/INF.0b013e318256fb07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genel F, Atlihan F, Gulez N, et al. Evaluation of adhesion molecules CD64, CD11b and CD62L in neutrophils and monocytes of peripheral blood for early diagnosis of neonatal infection. World J Pediatr. 2012;8:72–5. doi: 10.1007/s12519-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 70.Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 71.Bopp C, Hofer S, Bouchon A, et al. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur J Anaesthesiol. 2009;26:504–7. doi: 10.1097/EJA.0b013e328329afca. [DOI] [PubMed] [Google Scholar]
- 72.Jeong SJ, Song YG, Kim CO, et al. Measurement of plasma sTREM-1 in patients with severe sepsis receiving early goal-directed therapy and evaluation of its usefulness. Shock. 2012;37:574–8. doi: 10.1097/SHK.0b013e318250da40. [DOI] [PubMed] [Google Scholar]
- 73.Gautam N, Olofsson AM, Herwald H, et al. Heparin-binding protein HBP/CAP37: a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–27. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 74.Linder A, Christensson B, Herwald H, et al. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009;49:1044–50. doi: 10.1086/605563. [DOI] [PubMed] [Google Scholar]
- 75.van Zoelen MAD, Achouti A, van der Poll T. The role of receptor for advanced glycation endproducts RAGE in infection. Crit Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bopp C, Hofer S, Weitz J, et al. sRAGE is elevated in septic patients and associated with patient outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 77.Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, Rosas-Taraco AG. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis. 2012;12:15. doi: 10.1186/1471-2334-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–8. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 79.Gutsmann T, Mueller M, Carroll SF, et al. Dual role of lipopolysaccharide LPS-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942–50. doi: 10.1128/IAI.69.11.6942-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakr Y, Burgett U, Nacul FE, et al. Lipopolysaccharide-binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008;36:2014–22. doi: 10.1097/CCM.0b013e31817b86e3. [DOI] [PubMed] [Google Scholar]
- 81.Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18:891–7. doi: 10.1007/s10156-012-0435-2. [DOI] [PubMed] [Google Scholar]
- 82.Shozushima T, Takahashi G, Matsumoto M, et al. Usefulness of presepsin sCD14-ST measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria for systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764–9. doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- 83.Jones GR, Lowes JA. The systemic inflammatory response syndrome as a predictor of bacteremia and outcome from sepsis. QJM. 1996;89:515–22. doi: 10.1093/qjmed/89.7.515. [DOI] [PubMed] [Google Scholar]
- 84.Dark PM, Dean P, Warhurst G. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care. 2009;13:217. doi: 10.1186/cc7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloos F, Hinder F, Becker K, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36:241–7. doi: 10.1007/s00134-009-1705-z. [DOI] [PubMed] [Google Scholar]
- 86.Pletz MW, Wellinghausen N, Welte T. Will polymerase chain reaction PCR-based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med. 2011;37:1069–76. doi: 10.1007/s00134-011-2245-x. [DOI] [PubMed] [Google Scholar]
- 87.Romaschin AD, Harris DM, Ribeiro MB, et al. A rapid assay of endotoxin in whole blood using autologous neutrophil-dependent chemiluminescence. J Immunol Methods. 1998;212:169–85. doi: 10.1016/s0022-1759(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 88.Marshall JC, Foster D, Vincent JL, et al. MEDIC study. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis. 2004;190:527–34. doi: 10.1086/422254. [DOI] [PubMed] [Google Scholar]
- 89.Yaguchi A, Yuzawa J, Klein DJ, et al. Combining intermediate levels of the Endotoxin Activity Assay EAA with other biomarkers in the assessment of patients with sepsis: results of an observational study. Crit Care. 2012;16:R88. doi: 10.1186/cc11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein HMGB1 in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–73. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 91.Karlsson S, Pettila V, Tenhunen J, et al. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046–53. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- 92.van Zoelen MAD, Vogl T, Foell D, et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med. 2009;180:1098–106. doi: 10.1164/rccm.200810-1552OC. [DOI] [PubMed] [Google Scholar]
- 93.Tschaikowsky K, Hedwig-Geissing M, Schiele A, et al. Coincidence of pro- and anti-inflammatory responses in the early phase of sepsis: longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med. 2002;30:1015–23. doi: 10.1097/00003246-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Cheron A, Floccard B, Allaouchiche B, et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after trauma. Crit Care. 2010;14:R208. doi: 10.1186/cc9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–83. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 96.Landelle C, Lepape A, Voirin N, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1810–2. doi: 10.1007/s00134-010-1962-x. [DOI] [PubMed] [Google Scholar]
- 97.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 98.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roger PM, Hyvernat H, Breittmayer JP, et al. Enhanced T-cell apoptosis in human septic shock is associated with alteration of the costimulatory pathway. Eur J Clin Microbiol Infect Dis. 2009;28:575–84. doi: 10.1007/s10096-008-0673-5. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Li J, Zhou Y, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessel A, Bamberger E, Masalha M, Toubi E. The role of regulatory T cells in human sepsis. J Autoimmun. 2009;32:211–15. doi: 10.1016/j.jaut.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Monneret G, Finck ME, Venet F, et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95:193–8. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 103.Guignant C, Lepape A, Huang X, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urbonas V, Eidukaite A, Tamuliene I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine. 2012;57:313–5. doi: 10.1016/j.cyto.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Zeitoun AAH, Gad SS, Attia FM, et al. Evaluation of neutrophilic CD64, interleukin 10 and procalcitonin as diagnostic markers of early- and late-onset neonatal sepsis. Scand J Infect Dis. 2010;42:299–305. doi: 10.3109/00365540903449832. [DOI] [PubMed] [Google Scholar]
- 106.de Pablo R, Monserrat J, Reyes E, et al. Sepsis-induced acute respiratory distress syndrome with fatal outcome is associated to increased serum transforming growth factor beta-1 levels. Eur J Intern Med. 2011;23:358–62. doi: 10.1016/j.ejim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Toffaletti JG. Blood lactate: biochemistry, laboratory methods, and clinical interpretation. Crit Rev Clin Lab Sci. 1991;28:253–68. doi: 10.3109/10408369109106865. [DOI] [PubMed] [Google Scholar]
- 108.Garrabou G, Moren C, Lopez S, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 109.Hernandez G, Bruhn A, Castro R, et al. Persistent sepsis-induced hypotension without hyperlactatemia: a distinct clinical and physiological profile within the spectrum of septic shock. Crit Care Res Pract. 2012;preprint:536852. doi: 10.1155/2012/536852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure or shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 111.Wacharasint P, Nakada TA, Boyd JH, et al. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38:4–10. doi: 10.1097/SHK.0b013e318254d41a. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 113.Arnold RC, Shapiro NI, Jones AE, et al. Emergency Medicine Shock Research Network EMShockNet Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32:35–9. doi: 10.1097/shk.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 114.Jones AE, Shapiro NI, Trzeciak S, et al. Emergency Medicine Shock Research Network EMShockNet Investigators. Lactate clearance vs. central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puskarich MA, Trzeciak S, Shapiro MI, et al. Emergency Medicine Shock Research Network EMSHOCKNET. Prognostic value and agreement of achieving lactate clearance or central venous oxygen saturation goals during early sepsis resuscitation. Acad Emerg Med. 2012;19:252–8. doi: 10.1111/j.1553-2712.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16:S11–21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 117.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25:536–42. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hook KM, Abrams CS. The loss of homeostasis in hemostasis: new approaches in treating and understanding acute disseminated intravascular coagulation in critically ill patients. Clin Transl Sci. 2012;5:85–92. doi: 10.1111/j.1752-8062.2011.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Hemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis. 2006;17:445–51. doi: 10.1097/01.mbc.0000240916.63521.2e. [DOI] [PubMed] [Google Scholar]
- 120.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, et al. Pathogenesis of sepsis. Ann Rev Pathol Mech Dis. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ranieri VM, Thompson BT, Barie PS, et al. PROWESS-SHOCK Study Group. Drotrecogin alfa activated in adults with septic shock. N Eng J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 122.Cocucci E, Racchetti G, Meldolesi J. Shedding vesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: circulating microparticles – a new player in sepsis? Crit Care. 2010;14:R236. doi: 10.1186/cc9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kofoed K, Andersen O, Kronborg G, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37:96–104. doi: 10.1097/CCM.0b013e318192fd9d. [DOI] [PubMed] [Google Scholar]
- 126.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 127.Kellum JA, Kong L, Fink MP, et al. GenIMS Investigators. Understanding the inflammatory cytokine response in pneumonia and sepsis. Arch Intern Med. 2007;167:1655–63. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamayo E, Fernandez A, Almansa R, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22:82–7. doi: 10.1684/ecn.2011.0281. [DOI] [PubMed] [Google Scholar]
- 129.Wu JF, Ma J, Chen J, et al. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit Care. 2011;15:R220. doi: 10.1186/cc10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang BMP, McLean AS, Dawes IW, et al. The use of gene-expression profiling to identify candidate genes in human sepsis. Am J Respir Care Med. 2007;176:676–84. doi: 10.1164/rccm.200612-1819OC. [DOI] [PubMed] [Google Scholar]
- 131.Wong HR, Cvijanovich N, Allen GL, et al. Genomics of Pediatric SIRS/Septic Shock Investigators. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;27:1558–66. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van der Poll T, van Deventer SJH. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413–26. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 133.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Eng J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 134.Andaluz-Ojeda D, Bobillo F, Iglesias V, et al. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57:332–6. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Gouel-Cheron A, Allaouchiche B, Guignant C, et al. AzuRea Group. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS ONE. 2012;7:e33095. doi: 10.1371/journal.pone.0033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dwyer J. An infection, unnoticed, turns unstoppable. New York Times. 2012. http://www.nytimes.com/2012/07/12/nyregion/in-rory-stauntons-fight-for-his-life-signs-that-went-unheeded.html?pagewanted=all July 12:A15–6. Available from: [last accessed 26 Feb 2013]
- 137.Huang DT, Weissfeld LA, Kellum JA, et al. GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–52. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]