Abstract
Over the last decade, it has been proved that mesenchymal stem cells (MSCs) elicit anti-inflammatory effects. MSCs from adipose tissue (hASCs) differentiate into cells of the mesodermal lineage and transdifferentiate into ectodermal-origin cells. Although there are various etiologies to chronic pain, one common feature is that painful states are associated with increased inflammation. We believe in hASCs as a therapeutic tool also in pathologies involving neuroinflammation and neuronal tissue damage. We have investigated the effect of hASCs injected in a model of neuropathic pain [(mouse sciatic nerve chronic constriction injury (CCI)]. hASCs from 5 donors were characterized, and no major differences were depicted. hASCs were cryopreserved and grown on demand. About 1×106, 3×106, and 6×106 hASCs were intravenously injected into normal immunocompetent mice. No mouse died, and no macroscopic toxicity or behavioral changes were observed, confirming the safety of hASCs. hASCs, intravenously (i.v.) injected into C57BL/6 mice when the neuropathic pain was already established, induced a significant reduction in mechanical allodynia and a complete reversion of thermal hyperalgesia in a dose–response fashion, already 1 day after administration. Moreover, the hASCs effect can be boosted by repeated administrations, allowing a prolonged therapeutic effect. Treatment decreased the level of the CCI-induced proinflammatory cytokine interleukin (IL)-1β and activated the anti-inflammatory cytokine IL-10 in the lesioned nerve. hASCs treatment also restored normal inducible nitric oxide synthase expression in the spinal cord of CCI animals. Our data suggest that hASCs are worthy of further studies as an anti-inflammatory therapy in the treatment of neuropathic pain or chronic inflammatory diseases.
Introduction
Mesenchymal stem cells (MSCs) are a heterogeneous population that can be isolated from several tissues, expanded in vitro, and purified by plastic adherence.
Adipose tissue is an attractive abundant source of MSCs (ASCs, adipose-derived stromal/stem cells), which are able to differentiate into cells of the mesodermal lineage such as osteoblasts, adipocytes, chondrocytes, and myocytes [1–3], and also to transdifferentiate into cells of the ectodermal lineage [4–6]. In the last 10 years, the knowledge about their features and their application potential in life science have increased rapidly, and the interest in cell-based therapies involving ASCs is also exponentially growing. Until recently, ASCs have been applied in the regenerative medicine field, since they are able to promote the regeneration of damaged soft and hard tissues. We previously showed that ASCs are not significantly affected by the donor's age [7], and that the stromal vascular fraction (SVF) from subcutaneous fat tissue contains NGF+ ASCs, precursor/progenitor cells that efficiently differentiate toward osteogenic- and chondrogenic-like cells [8], confirming this tissue as a source of agents prone to regenerate various tissues.
Furthermore, ASCs, as other MSCs, are known to be immunomodulatory through the regulation of immune cells [9] by mechanisms that include both direct cellular contact and release of soluble factors such as trasforming growth factor-β, interleukin (IL)-10, leukemia inhibitory factor (LIF), and others [10]. It has been shown that ASCs reduce allogeneic lymphocyte response by displaying potent immunosuppressive effects that could be mediated by LIF [11], and that MSCs suppressed both effector T-cell and inflammatory responses. Gonzalez-Rey et al. [12] showed that human ASCs produced IL-10 interacting with monocytes, and this cell–cell contact also committed monocytes to produce high levels of IL-10, suggesting that human ASCs (hASCs) indirectly suppress T-cell activation by increasing the production of IL-10 by antigen-presenting cells. Confirming their features, ASCs have been introduced into clinical therapies for prevention or reduction of graft-versus-host disease associated to allogenic transplantation and for the treatment of autoimmune diseases, despite an incomplete understanding of their mechanism of action [13]. Animal studies clearly indicate the therapeutic role of MSCs both in autoimmune diseases such as autoimmune encephalomyelitis [14] and systemic lupus erythematosus [15] and in diabetic polyneuropathy [16]; furthermore, some clinical trials have produced promising results [17].
Hovewer, the safety and efficacy of allogeneic MSCs in long-term studies remain still to be clarified although in short-term experiments, both in animals and in humans, the use of MSCs has been shown to be safe [18–20].
Neuropathic pain, consequent to iatrogenic (amputation, cholecystectomy, etc), or traumatic injuries, tumors compressing peripheral nerves, drugs, metabolic (diabetes) or viral (Herpes Zoster, HIV) diseases, affects 1% of the population, and it most frequently involves the peripheral nervous system. Its treatment is still a complex issue, and whatever the cause, neuropathic pain is treated with antidepressant or anticonvulsant drugs, transcutaneous electrical nerve stimulation, and psychological/cognitive help. However, all these treatments provide relief in a limited percentage of patients (30%, ie, comparable to placebo) [21], before pain inevitably reappears. A nonphysiological repair of the lesioned nerve leading to the formation of neurinomas, altered nerve conduction, and spontaneous firing is considered one of the main causes of the events underlying neuropathic pain. We and others also demonstrated that a peripheral nerve lesion starts a cascade of neuroinflammation-related events that may maintain and worsen the original injury, participating in pain generation and chronicization [22–24].
In this view, the use of MSCs, due to their immunomodulatory effects on the neuroinflammatory cascade, could be a valid approach to treat neuropathic pain. Musolino and Siniscalco directly injected into the nervous system, autologous bone marrow stem cells (BMSCs) in rats, and human BMSCs in mice, respectively [25,26]; however, although promising, these approaches present several important drawbacks. In the first instance, the isolation of MSCs from bone marrow is not easy, and, even more important, the quantity of cells that can be obtained is limited. In the second instance, the intraventricular or intraganglional routes of administration are both troublesome and difficult to apply safely. We previously showed that intravenous administration of murine neural stem cells (NSCs) leads to a reduction of neuropathic pain symptoms such as hyperalgesia and allodynia in a well-established murine model of pain [sciatic nerve chronic constriction injury (CCI)] [27], and that NSCs could modulate pro- and anti-inflammatory cytokines in the sciatic nerve of CCI mice even when the pathology was already established [27]. Moreover, the NSCs effect on pain symptoms and cytokines preceded nerve repair and was maintained after cells had disappeared from the lesion site, suggesting that NSCs effects are largely due to microenvironmental changes they might induce at the lesion site.
On these bases, we thought that hASCs could offer a more suitable alternative, in consideration of their relevant immunomodulatory effects on the neuroinflammatory cascade that plays an important role in the etiopathogenesis and maintenance of this type of pain [22,28]. Moreover, they are available in great amount, and if necessary, autologous cells could be easily withdrawn.
Here, we describe the sustained and prolonged antihyperalgesic effect of intravenously injected human ASCs in a CCI preclinical model of neuropathic pain in immunocompetent C57BL/6 mice.
Materials and Methods
In vitro characterization
Isolation and culture of hASCs
Subcutaneous adipose tissues were obtained from 5 healthy female donors (age range 26–58 years/old) undergoing plastic surgery and after written consent and Institutional Review Board (IBR) authorization from Galeazzi Orthopaedic Institute, Milano, Italy. hASCs were isolated as previously described [7]. Briefly, raw lipoaspirates (20–180 mL) were enzymatically digested with 0.075% type I collagenase at 37°C, for 30 min. The SVF was obtained by centrifugation (1,200 g×10 min), filtered through a sterile medication lint, and plated (105 cells/cm2) in a control medium (CTRL) consisting of the Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, Milan, Italy), supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM l-glutamine (Sigma-Aldrich) (cDMEM). Cells were cultured at a density of 104 cells/cm2 [7]. Fibroblast colony-forming unit (CFU-F) assay was performed as follows: hASCs were plated in the DMEM supplemented with 20% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM l-glutamine, in 6-well plates starting from 1,000 cells/well to 125 cells/well. Six days later, the medium was replaced, and, at day 14, cells were fixed with 100% methanol and stained with 0.5% crystal violet (Fluka, Buchs, Switzerland). The frequency of the CFU-F was established by scoring individual colonies (consisting of at least 25 cells) with respect to the number of seeded cells. hASCs at passages either 3 or 4 were frozen in a 10:1 ratio of FBS/dimethyl sulfoxide (Sigma-Aldrich) and cryopreserved at −80°C or in liquid nitrogen until required for the in vivo studies.
To assess the genetic stability, thawed hASCs were cultured for additional 10 passages, and karyotyping was performed (TOMA Advanced Biomedical, Busto Arsizio, Italy). Cells were treated by 0.05 mg/mL colcemid (Invitrogen, Monza, Italy) for 1–2 h to block cell division in the metaphase, then harvested, and 50 metaphases in each sample were analyzed. The chromosomes were then observed by Q-banding by quinacrine [29].
Assesment of multidifferentiative potential of hASCs
At passage 3, 104 cells/cm2 were induced to differentiate into the adipogenic (cDMEM supplemented with 1 μM dexamethasone, 10 μg/mL insulin, 500 μM 3-isobutyl-1-methyl-xanthine, and 200 μM indomethacin; Sigma-Aldrich [7]), osteogenic (cDMEM with 10 nM dexamethasone, 10 mM glycerol-2-phosphate, 150 μM l-ascorbic acid-2-phosphate, and 10 nM cholecalciferol; Sigma-Aldrich [7]), myogenic (cDMEM plus 5% horse serum donor herd, 0.1 μM dexamethasone, and 50 μM hydrocortisone; Sigma-Aldrich [30]), and vascular endothelial (DMEM, 2% insulin, transferrin, and selenium, 10 ng/mL fibroblast growth factor-2 (FGF-2), 20 ng/mL insulin-like growth factor-I (IGF-I), 0.2 μg/mL hydrocortisone, 1 μg/mL ascorbic acid, 0.5 ng/mL vascular endothelial growth factor (VEGF), 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM l-glutamine; Sigma-Aldrich [31]) lineages.
Fourteen days later, adipogenic differentiation was evaluated in hASCs fixed in 10% neutral buffered formalin for 1 h and stained with a fresh Oil Red O solution (2% weight/volume Oil Red O in 60% isopropanol) for 15 min [7].
Osteogenic markers such as alkaline phosphatase (ALP), collagen production, and calcified extracellular matrix deposition were analyzed after 14 and 21 days in culture, respectively. To evaluate ALP enzymatic activity in differentiated hASCs, both undifferentiated and differentiated cells were lysed in 0.1% Triton X-100 and incubated at 37°C in the presence of 10 mM p-nitrophenylphosphate in 100 mM diethanolamine and 0.5 mM MgCl2, pH 10.5 [32]. Samples were read at 405 nm with the Wallac Victor II plate reader, and ALP activity was normalized with respect to the protein concentration for each sample determined by the BCA Protein Assay (Pierce Biotechnology, Rockford, IL). Total collagen production was determined in hASCs fixed for 1 h with Bouin's solution (Sigma-Aldrich) and then stained for 1 h with 0.1% Sirius Red F3BA in picric acid [33]. To evaluate the calcified extracellular matrix deposition, hASCs were fixed 1 h in ice-cold 70% ethanol, and then stained for 15 min with 40 mM Alizarin Red-S (pH 4.1) [34]. Washed and dried samples were then incubated 20 min at room temperature with 10% w/v cetylpyridinium chloride in 0.1 M phosphate buffer (pH 7.0); the extracted matrix was read at 550 nm.
Animals and experimental protocol
All performed experiments were in accordance with the Italian State and European regulations governing the care and treatment of laboratory animals (permission No. 94/2000A) and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain [35]. The experimental work was previously reviewed by the Animal Care and Use Committee of the University of Milan. Animals were kept on a 12-h light–dark cycle with water and food ad libitum.
CCI model
Painful neuropathy was induced on C57BL/6J male mice (20–25 g; Harlan, Italy). The CCI model originally described by Bennett and Xie [36] for rats was adapted for mice as described in our previous studies [22,23,27]. Briefly, animals were anesthetized with sodium pentobarbital [60 mg/kg, intraperitoneal (i.p.), 0.1 mL/10 g], and under a dissecting microscope, the right common sciatic nerve was exposed at the level of the mid-thigh proximal to the trifurcation of the nerve; 3 ligatures (4/0 chromic silk; Ethicon) were loosely tied around it, at about 0.5-mm spacing, until they elicited a brief twitch in the respective hind, taking care to preserve epineural circulation. Sham-operated animals (sciatic exposure without ligation) were used as controls.
Thermal hyperalgesia and mechanical allodynia evaluation
Thermal hyperalgesia was tested according to the Hargreaves procedure [37], slightly modified by us for mouse, using a plantar test apparatus (Ugo Basile, Comerio, Italy). Briefly, mice were placed in smaller clear plexiglass cubicles and allowed to acclimatize. A constant-intensity radiant heat source (beam diameter 0.5 cm and intensity 20 I.R.) was aimed at the midplantar area of the hind paw. The time, in seconds (s), from initial heat source activation until paw withdrawal was recorded. Mechanical allodynia was assessed using the dynamic plantar esthesiometer (Ugo Basile, Comerio, Italy). Animals were placed in a test cage with a wire mesh floor, and the rigid tip of a von Frey filament (punctate stimulus) was applied to the skin of the midplantar area of the hind paw. The filament exerted an increasing force, ranging up to 5 g in 20 s, starting below the threshold of detection and increasing until the animal removed its paw. Withdrawal threshold was expressed in grams. Withdrawal threshold of ipsilateral and contralateral paws was measured 4 times, and the value was the mean of the 4 evaluations. Measurements were performed on both the ipsilateral and contralateral hind paws of all mice by researchers blind to treatments.
Injection protocol
Cell administration was always performed 7 days after CCI, when the pain hypersensitivity was maximal. hASCs were mechanically dissociated to a single-cell suspension in phosphate-buffered saline (PBS) with 2.5% heparin and injected intravenously in the caudal vein to mice. The cells were administered in the amount of 1×106 or 5×105/200 μL to CCI and sham mice. In one group of animals, 1×106 cells were injected for a second time 21 days after the first 1×106 cell treatment, while a group of mice that had received 5×105 cells was reinjected with the same amount of cells 14 days later.
Thermal hyperalgesia and mechanical allodynia were evaluated in all mouse groups immediately before surgery and at day 7 after surgery, before cell injection (time 0). The subsequent evaluations were performed 24 h and at days 3, 7, 14, 21, and 28 after the first hASCs administration.
Cytokine evaluation
Twenty-four hours, 3, and 7 days after administration of 1×106 and 5×105 hASCs, mice were anaesthetized with sodium pentobarbital (60 mg/kg, i.p., 0.1 mL/10g), and under a dissecting microscope, the ipsilateral sciatic nerve, proximal to the trifurcation (about 1 cm), before the 3 ligatures in the CCI animals, was removed and immediately frozen in liquid nitrogen and stored at −80°C until the cytokine assay. Whole blood was collected 24 h and 7 days after hASCs administration by puncture of the tail vein, centrifuged, and sera were stored at −80°C until mouse and human IL-1β and IL-10 were tested.
The nerve samples were homogenized in 0.4 mL of ice-cold PBS containing a protease inhibitor cocktail (Sigma-Aldrich) and centrifuged at 10,000 g for 15 min, as previously described [23,27]. The supernatant was used to measure mouse IL-1β and mouse and human IL-10 levels and total protein content (Lowry's method). IL-1β and IL-10 protein contents were determined by enzyme-linked immunosorbent assay (ELISA) using ultrasensitive ELISA kits according to the manufacturer's instruction (mouse IL-1: R&D system Minneapolis, MN; mouse IL-10: Becton Dickinson, San Diego, CA; human IL-10 and IL-1β: Ebioscience, Prodotti Gianni, Milano Italy). Cytokine concentrations were determined by interpolation with standard curves assayed on individual plates and normalized to the protein content in each sample.
Determination of spinal cord-induced nitric oxide synthase by western blot analysis
Seven days after hASC treatment, mice were anaesthetized with sodium pentobarbital (60 mg/kg, i.p., 0.1 mL/10 g), and under a dissecting microscope, the lumbar dorsal spinal cord at the L4–L6 level was removed and immediately frozen.
To assess the level of induced nitric oxide synthase (iNOS) dosage, tissues were defrosted at room temperature, weighed, diluted (1:20 w/v) in a lysis buffer [50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.02% sodium azide, 1 mM phenylmethylsulfonyl fluoride, and 10 mM leupeptin], homogenized using an ultrasonic processor homogenizer UP 50H (Dr. Hielscher, Gmbh, Berlin, Germany) and centrifuged at 1,500 g at 4°C for 10 min. After protein dosage, the supernatant was diluted in the Laemmli buffer (0.3 M Tris–HCl, pH 6.8, containing 10% SDS, 50% glycerol, 5% dithiothreitol, and 0.05% bromophenol blue) to obtain 40 μg of proteins. The proteins were loaded onto a 7.5% SDS–polyacrylamide gel and then transferred onto a nitrocellulose membrane (Schleicher and Schull, BAS 85) with the semidry method for 90 min at room temperature. The membrane was blocked with 5% nonfat dry milk (Bio-Rad, Milan, Italy) in Tris-buffered saline tween (TBST) (20 mM Tris base, pH 7.6, 137 mM NaCl, and 0.1% Tween 20) at room temperature for 2 h and then incubated with a primary polyclonal antibody directed against mouse iNOS (Cayman Chemical, Ann Arbor, MI) diluted 1:1,000 in a blocking solution, at 4°C overnight. The nitrocellulose membrane was also probed with a polyclonal anti-β actin antibody (1:3,000; Cytoskeleton, Inc., Denver, CO) as a loading control. Immunoreactive bands were analyzed using a computer-based densitometry NIH (National Institute of Health) image program. After washing in the TBST buffer, the blot was incubated with a secondary antibody (anti-rabbit IgG, peroxidase-linked F(ab′)2 fragment, from Amersham Pharmacia Biotech, Milan, Italy, 1:1,500 in 3% blocking solution) for 1 h at room temperature. After washing in the TBST buffer, the blot was detected with an enhanced chemiluminescence detection kit (Roche Diagnostics, Monza, Italy). Gray levels, obtained by densitometric analysis of immunoreactive bands, were normalized on β-actin and expressed as the percentage of the sham-operated mouse levels.
Statistical analysis
The data are expressed as mean±SEM. Statistical analyses were performed using Student t-test or one-way ANOVA followed by Tukey's test. Differences were considered significant at P<0.05. All the statistical analyses were performed using GraphPad 4 software (San Diego, CA).
Results
Features of hASCs populations
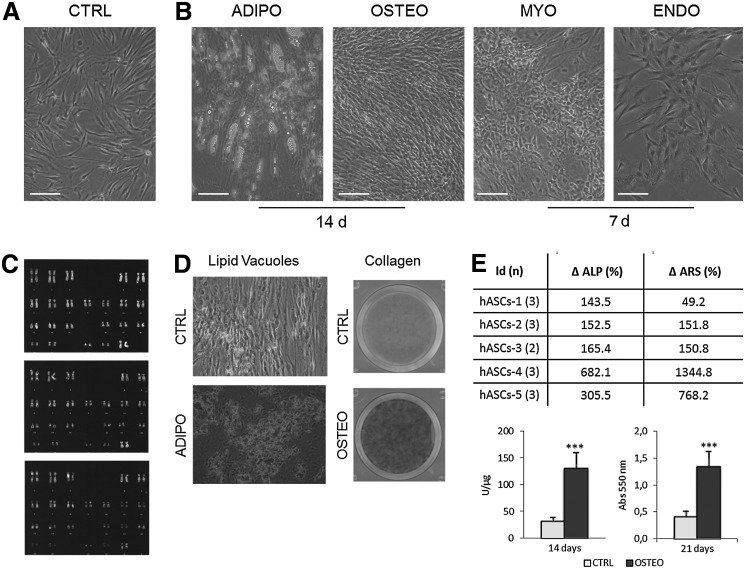
All the experiments were performed with 5 cellular populations derived from female donors, between 26 and 58 years of age (mean±SEM: 37.6±5.5), and from different anatomical regions (Table 1). Despite the mild variability, all the populations form colonies (range 0.6%–4.3% of cells endowed with clonogenic capacity); the average doubling time during early passages was also not homogeneous, and indeed spans between 69.0 and 120.2 h. All the hASCs populations showed the peculiar human morphology (Fig. 1A) both before and after freezing. For the in vivo experiments, we used cryopreserved cells; to achieve the established number of cells for the injections cells were usually split for 3 or 4 passages: 3 hASCs batches were cultured until passage 8 and the others till passage 7 (Table 1). The same populations were then maintained until passage 10, and their genetic stability during proliferation was analyzed by karyotyping. An average of 50 metaphasic plates was analyzed for each sample, and no numerical and structural chromosomal abnormalities were observed in any sample. Three representative karyotype images are shown in Fig. 1C.
Table 1.
Features of Human Adipose-Derived Stem Cell Populations Used for the CCI Model Treatment
| |
|
|
|
|
|
|
Differentiation |
|
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Id | Gender | Age | Body area | CFU-F (%) | DT hours | Immuno-phenotype | Adipo | Osteo | Myo | Endo | Cryopreservation (passage) | Injection (passage) |
| hASCs-1 | F | 26 | Abdomen | 1.6 | 71.1 | + | √ | √ | √ | √ | 3 | 7 |
| hASCs-2 | F | 58 | Breast | 1.4 | 69.0 | + | √ | √ | √ | √ | 4 | 8 |
| hASCs-3 | F | 38 | Abdomen | 4.3 | 71.4 | + | √ | √ | √ | √ | 3 | 7 |
| hASCs-4 | F | 31 | Thigh/hip | 0.6 | 120.2 | + | √ | √ | √ | √ | 4 | 8 |
| hASCs-5 | F | 35 | Abdomen | 2.5 | 100.6 | + | √ | √ | √ | √ | 4 | 8 |
Clonogenic potential (CFU-F); proliferation rate expressed as doubling time (DT) calculated as t×ln(2)/ln(N/N0), where t is the duplication time (h); N is the number of collected cells; and N0 is the number of plated cells. Immunephenotype characterizations performed by FACS analysis and differentiation ability by functional test. Time spanning between cell thawing and hASCs injection for each population.
hASCs, human adipose-derived stem cells; CCI, chronic constriction injury.
FIG. 1.
Human adipose-derived stem cell (hASCs) morphology, differentiation, and karyotype. Phase-contrast microphotographs of hASCs cultured in control medium (CTRL) (A) and adipogenic, osteogenic, myogenic, and endothelial media (B) (100× magnification, bar represents 100 μm); the differentiation time is indicated. (C) hASCs karyotyping by Q banding. (D) hASCs cultured for 14 days in CTRL or adipogenic differentiation (ADIPO) media; phase-contrast images of cells fixed and then stained by Oil Red O (200× magnification; upper panel); hASCs maintained for 14 days in CTRL or osteogenic differentiation (OSTEO) media and stained by Sirius Red (lower panel). (E) The % of increase of alkaline phosphatase (ALP) activity and calcified extracellular matrix deposition for each osteodifferentiated hASCs population compared to CTRL cells are indicated in the table. Values of ALP activity (left panel) and calcified extracellular matrix deposition (right panel) of undifferentiated (CTRL, white bars) and osteogenic-differentiated (OSTEO, filled bars) hASCs for 14 and 21 days, respectively, are shown in the lower graphs. Data are expressed as mean±SEM; ***P<0.001 (n=5).
To assess the hASCs multidifferentiative potentiality, cells were induced for 2 weeks in the presence of stimuli for osteogenic, adipogenic, myogenic, and endothelial differentiation: the morphological changes peculiar of each differentiation could be depicted already after 14 or 7 days of culture, for adipogenic and osteogenic or myogenic and endothelial differentiation, respectively, as shown in Fig. 1B. hASCs were efficiently induced toward cells of the adipogenic lineage, as revealed by lipid vacuole formation (Table 1 and Fig 1B, D). Furthermore, osteogenic stimuli promoted the secretion of collagen as shown in Fig. 1D, where differentiated hASCs-4 have been shown, and an increase in both ALP activity and calcified extracellular matrix deposition of about 312.4% and 227.3%, respectively, compared to undifferentiated cells (Fig. 1E, table and graph).
Conditions and survival rate of animals injected with hASCs
To exclude a possible toxic effect of hASCs, increasing doses of cells from 1×106 to 6×106 of human cells corresponding to 40,000 and 240,000 cells/g of animal's body weight, were intravenously injected in not-immune-compromised mice. The injected volume was always 200 μL, independent on the number of cells. Single dose of either 1×106 or 3×106 cells was injected in 1 shot, whereas the highest dose (6×106) was administered in 2 identical shots. The animals were observed twice a day. Independently on the cell dose, no mouse died either immediately after the injection or during the period of follow-up (Supplementary Table S1 Supplementary data; Supplementary Data are available online at www.liebertpub.com/scd). Moreover, they did not show any signs of macroscopic toxicity or behavioral change, including food and water intake, so that the body weight was stable in each group, and no significant differences were observed between the weights registered at day 28 with respect to the preadministration values in all the animal groups (Supplementary Table S1 Supplementary data). Despite the high number of mice we have injected (n>60), death or macroscopic toxicity was never observed, confirming that MSCs derived from adipose tissue are neither toxic nor immunogenic.
Effect of hASCs on thermal hyperalgesia and tactile allodynia in the sciatic nerve CCI, a murine model of pain
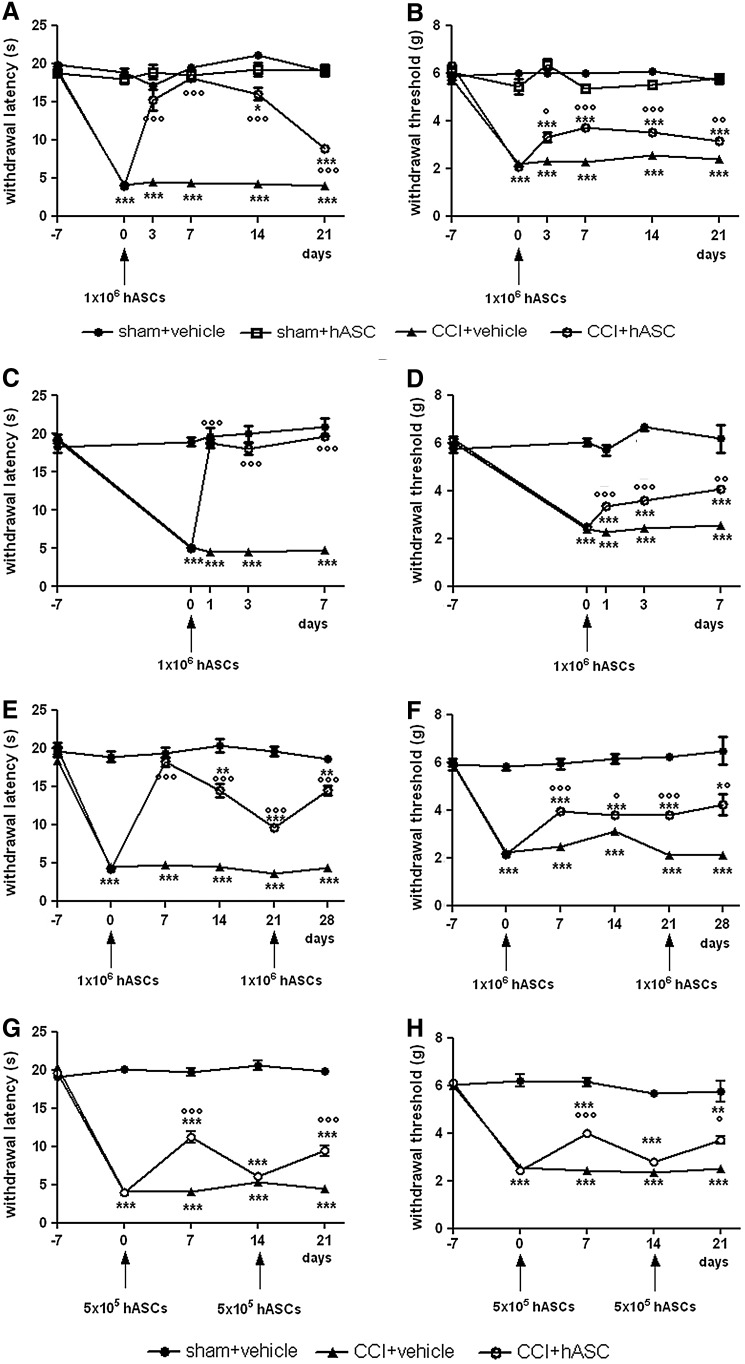
A dose of hASCs, corresponding to 1×106 cells, was administered to mice intravenously 7 days after the CCI procedures, to allow pain chronicization. As shown in Fig. 2A, B, the sciatic nerve constrictions provoked a significant reduction of the thermal withdrawal latency and mechanical threshold, for hyperalgesia and allodynia, respectively, in the CCI groups. hASCs treatment did not modify the pain threshold in the sham group, whereas in the CCI group, hASCs allowed mice to recover hyperalgesia scores comparable to those of sham mice already after 3 days, and the effect was fully maintained at least until 7 days from injection. Allodynia was also improved by hASCs injection, even if significant differences between the CCI+hASCs and sham groups persisted. From day 7, the pain reduction effect started to progressively decrease, although scores remained significantly better with respect to the CCI+vehicle group until day 21 from hASCs administration both for hyperalgesia and allodynia (Fig. 2A, B). To more deeply assess the short-term effect of 1×106 hASCs in CCI mice and to confirm the previous observations, we evaluated pain reduction in other mice treated by another hASCs population (hASCs-5) focusing on the antihyperalgesic and antiallodynic effect at 1, 3, and 7 days after injection (Fig. 2C, D): cell administration was able to significantly increase hyperalgesia scores already 1 day after treatment, and, as already observed in the previous experiment, the effect lasted at least until day 7 (Fig. 2C). Also, allodynia scores of the CCI+hASCs group were progressively and significantly improved starting from day 1, although they did not reach the same level of the sham mice (Fig. 2D).
FIG. 2.
Time course of the effect of hASCs administered intravenously (i.v.) 7 days after injury to chronic constriction injury (CCI) mice. Thermal hyperalgesia (A, C, E, G) was measured by plantar test and mechanical allodynia (B, D, F, H) by dynamic plantar esthesiometer, and the values are expressed as seconds and grams, respectively. CCI group, sham group, CCI+hASCs group, and sham+hASCs group were formed by 8–12 mice. A and B: Long-lasting effect. Seven days after surgery (time 0), mice received a single dose of 1×106 hASCs, and thresholds were measured every week for 3 weeks after treatment. C and D: Short-term effect of hASCs treatment. Seven days after surgery (time 0), mice received a single dose of 1×106 hASCs, and thresholds were measured 24 h, 3 days, and 7 days after cell administration. (E, F) Seven days after surgery (time 0) and 3 weeks after the first administration (time 21), mice received 2 different doses of 1×106 hASCs/each. (G, H) Seven days after surgery (time 0) and 2 weeks after the first administration (time 14), mice received 2 different doses of 5×105 hASCs/each. Data represent means±SEM of 8–12 mice. *P<0.05, **P<0.01, ***P<0.001 versus sham+vehicle mice; °P<0.05, °°P<0.01, °°°P<0.001 versus CCI+vehicle mice.
Since we observed, during time, a progressive decrease of the hASCs effect on pain reduction, we tested whether a second cell dose was able to produce again the beneficial effect. At first, 2 doses of 1×106 cells were administered: one after 7 days from CCI procedure and the other after further 21 days. The first cell injection caused a marked improvement in the hyperalgesia test response, and when mice were treated with a second dose, the analgesic effect was completely restored even if the mice were readministered when the withdrawal latency was still significantly higher than the lesioned mice (Fig. 2E). A similar trend was observed when allodynia was evaluated; indeed, the second injection, performed when the effect of the first one was not disappeared, provoked a new slight improvement of the thresholds (Fig. 2F). Furthermore, to test the hASCs dose–response effect, we treated another group of mice with a reduced number of cells (5×105). The effect was less evident than the one previously observed, due to the lower dose of hASCs; in particular, a reduced effect and a more rapid return to the preinjection level in comparison to the mice that had received 1×106 cells were noticed (Fig. 2G, H). For this reason, the second injection was administered just after 14 days from the first one instead of 21 days as for the higher dose. Nevertheless, the second treatment was comparable to the first one, suggesting a dose–response relation (Fig. 2G, H).
Cytokine modulation by hASCs treatment
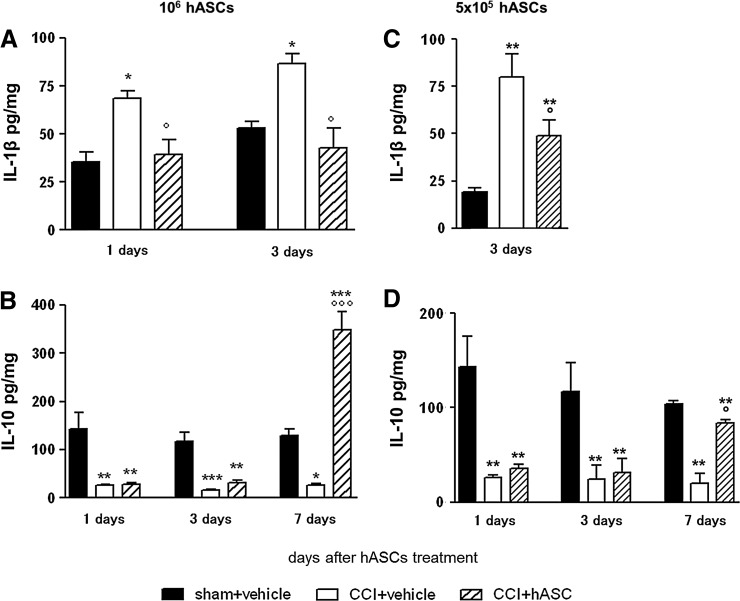
It is well known that CCI activates a neuroinflammatory cascade characterized by the increase of proinflammatory cytokines that play an important role in the etiopathogenesis and maintenance of neuropathic pain. To verify an anti-inflammatory or immunomodulatory action for hASCs-mediated antiallodynic/antihyperalgesic mechanisms, we checked inflammatory and anti-inflammatory cytokine levels in the sciatic nerve. Fig. 3A shows that, as previously reported by us and others [27,28,38], the levels of the proinflammatory cytokine IL-1β was significantly elevated in sciatic nerves obtained from CCI animals. In 1×106 hASCs-treated animals, the IL-1β increase was already reverted 1 day after administration, and the effect was complete 3 days later, as the IL-1β levels were similar to those of sham animals. Fig. 3B reports a decrease of the content of the anti-inflammatory cytokine IL-10 after the lesion. The treatment with 1×106 hASCs was able to progressively restore the IL-10 levels that 7 days after cell treatment appeared even more elevated compared to those measured in sham animals. The ability of hASCs to restore cytokine levels was dose dependent: in fact, the injection of 5×105 cells exerted a lower, although still significant, effect on cytokine production (Fig. 3C, D). No hASCs significant effects on either IL-1β or IL-10 were observed in sham animals (data not shown). To understand whether hASCs secretion may contribute to the cytokine increase, we also measured human IL-10 in the same samples at all time points. In all groups, sciatic nerve human IL-10 concentrations could not be detected in the sciatic nerve (data not shown).
FIG. 3.
Cytokine modulation by hASCs treatment. Interleukin (IL)-1β (A, C) and IL-10 (B, D) protein content in ipsilateral sciatic nerve at different days after i.v. administration of 1×106 (A, B) and 5×105 (C, D) hASCs. Seven days after surgery, hASCs were injected, as in all experiments. Cytokine protein content is normalized to sample total protein. Data are mean±SEM of 6–8 animals. *P<0.05, **P<0.01, ***P<0.001 versus sham+vehicle group at the corresponding day; °P<0.05, °°°P<0.001 versus CCI+vehicle at the corresponding day.
We evaluated murine IL-1β and IL-10 concentrations also in the sera obtained 24 h and 7 days after hASCs administration (Table 2). One day after hASCs administration, IL-1β levels were significantly elevated in the CCI group in comparison to sham animals, and the treatment with hASCs did not significantly modify IL-1β concentrations in any group. At a later time point, no effect was still present. Low concentrations of IL-10 were detected in sera of all animal groups, and no difference in CCI animals in comparison to the sham ones was observed; however, we detected a significant increase of IL-10 concentrations, 1 day after hASCs injection, in both sera of sham and CCI animals. This increase was not detectable anymore after 7 days from hASCs administration. We could not assay human cytokines in murine sera (data not shown).
Table 2.
Mouse IL-1β and IL-10 Serum Concentration Measured 1 and 7 Days After hASCs Treatment (8 and 14 Days After Lesion)
| pg/mL serum | Days | Sham | Sham+hASCs | CCI | CCI+hASCs |
|---|---|---|---|---|---|
| IL-1β | 1 | 76.0±13.0 | 96±8.2 | 167.1±12.0* | 145.1±3.0* |
| IL-1β | 7 | 46.8±16.0 | 67.3±18.7 | 74.0±24.0 | 53.0±14.4 |
| IL-10 | 1 | 52.8±2.9 | 89.3±4.0* | 53.9±1.2 | 67.2±3.5* |
| IL-10 | 7 | 54.4±3.7 | 61.3±8.4 | 54.8±5.9 | 52.9±3.0 |
Data are expressed as mean±SEM; *P<0.05 versus sham animals (n=6).
IL, interleukin.
Spinal cord iNOS modulation by hASCs treatment
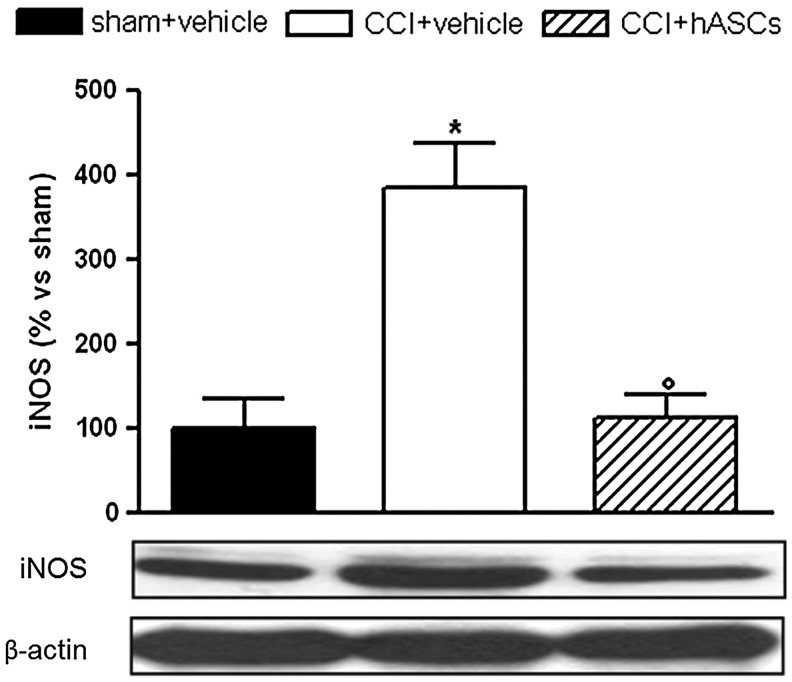
As shown in Fig. 4, 14 days after neuropathy induction, the expression of iNOS in the ligated mouse spinal cord was higher than in sham-operated animals, as expected. In the CCI-hASCs-treated group, the expression of iNOS was completely restored, indicating that the cells exert a relevant action also in the central nervous system.
FIG. 4.
Induced nitric oxide synthase (iNOS) modulation. iNOS content in the L4–L6 dorsal horn spinal cord 14 days from lesion and 7 days after injection of 1×106 hASCs. iNOS protein content was detected by western blot analysis. The density mean value of the vehicle-injected sham-operated mice within a single experiment was set to 100, and all the other values were expressed as percentage versus the levels detected in sham-operated mice. Each value represents mean±SEM of 6–8 mice. *P<0.05 versus sham+vehicle group; °P<0.05 versus CCI+vehicle.
Discussion
This study demonstrated that intravenous injection of human ASCs in a murine model of neuropathic pain (CCI model) was able to relieve the induced hyperalgesia and allodynia, in a dose-dependent manner; interestingly, repeated treatments produced a prolonged reduction of these symptoms.
Recently, there has been a considerable evolution in the comprehension of the mechanisms underlying the therapeutic effect of MSCs. The new evolving concept modifies the implicit idea that the objective of the MSCs usefulness was cell replacement therapy. Indeed, the recent literature proposes the paracrine effect of MSCs, that is, cells exert their effects by the release of factors that stimulate tissue recovery at many potential levels, including stimulation of resident stem/progenitor cells, remodeling of the extracellular matrix, and stimulation of new blood vessel formation [39,40]. In the last decade, MSCs-based therapies have been proposed as a new possible scenario for neurodegenerative diseases, since in vitro, they are able to adopt astrocytic- and neuronal-like cell features [41], acquiring their specific morphological characteristics, neural markers, and electrophysiological properties [42]. However, in animals, they also seem to limit damage or mediate repair of nervous tissue via mechanisms other than cell replacement or transdifferentiation, that is, via their paracrine function, releasing protective factors [43]. Among the neurodegenerative-related pathologies, neuropathic pain is still classified as an incurable disease [44]. A well-accepted hypothesis for the development of neuropathic pain points to a nonphysiological repair of the damaged nerve, resulting in the formation of neurinomas, deranged nerve conduction, and generation of spontaneous firing. The development of neuropathic pain involves not only neuronal pathways but also components of the peripheral immune system [45]. Inflammation, derived from the activation of innate immune cells, is also present at the site of tissue injury. These cells release immune-active substances, such as cytokines, neurotrophic factors, and chemokines, which initiate local actions and can result in a more generalized immune response [46,47] and pain sensitization. There are many studies documenting the pleiotropic effects of MSCs on the immune system by both secreting bioactive molecules and by cell–cell contact involving dendritic cells, B-, and T-cells [48,49]. MSCs are known to show strong immunosuppressive properties that can be exploited for successful autologous as well as heterologous transplantations without requiring pharmacological immunosuppression [50,51]. In our study, toxicity tests in immunocompetent mice demonstrated the safety of intravenous injection of 1×106, 3×106, and 6×106 hASCs/mouse. No animal died or changed its habits, and no side effects have been observed. The highest dose of hASCs (2.4×108/kg body weight [b.w.]) corresponds to the highest dose used in SCID mice by Ra et al. [19]; moreover, lower doses of hASCs and BMSCs (2×106/mouse, corresponding to 8×107/kg body weight) have been successfully used in previous studies in normal immunocompetent mice [52,53], confirming that human MSCs escape immune system surveillance, probably since they do not express HLA-DR [54], and so they are poorly recognized by T-cells. It is known that adipose tissue represents an abundant source of MSCs, and, for this reason, we used hASCs, believing that for the therapeutical purposes, large quantities of cells must be produced by ex vivo expansion within a small number of generations. All the hASCs populations which have been tested, despite an expected physiological variability, shared common features in vitro and produced very comparable effects in our preclinical model of neuropathic pain. Considering future clinical applications, and knowing that it would be quite convenient to use cells on demand starting from frozen stocks, here we utilized cryopreserved cells, at early passages, cultured for about 2/3 weeks before i.v. injection. A normal karyotype was maintained for at least 10 cell generations after thawing, as it was previously shown by Ra et al. [19] on fresh MSCs from adipose tissue, confirming the potential safety of this approach.
hASCs systemic injection showed a very potent dose-dependent antihyperalgesic effect. Low doses of hASCs (5×105) partially reduced the pain symptoms for 14 days in CCI mice, never reaching the level of sham animals. Comparable withdrawal latency and threshold values were observed in our previous study when 2×106 murine NSCs were injected i.v. in the same CCI model [27], thus suggesting a more potent protective effect exerted by hASCs with respect to murine NSCs. When CCI mice were treated with 1×106 hASCs i.v., a complete, rapid, and long-lasting response was observed; indeed, hASCs effects on allodynia and hyperalgesia were already fully achieved 24 h after their administration: the hyperalgesia was completely controlled, and allodynia was significantly reduced compared to the CCI+vehicle-treated group. Moreover, the protective effect was maintained for 21 days. Although not complete, the entity of the effect on mechanical allodynia is of great relevance, in consideration of the fact that this painful symptom is particularly resistant to most analgesic treatments and strategies [55,56].
Meantime, in a model of sciatic nerve-crush temporary injury in mice, it has been recently shown that 2×106 hASCs accelerated the functional recovery in the treated mice with respect to the placebo group [52]. Despite the fact that the authors just tested 1 dose of hASCs, our data showed that already low doses of hASCs may revert symptoms of neuropathic pain, and that the hASCs effect can be boosted again by repeating the administration of cell doses. We observed that the period between the 2 administrations varied in function of the number of injected cells: 5×105 cells required a second administration after 14 days, whereas 1×106 cells showed a beneficial effect lasting for 21 days. Unfortunately, we did not further prolong the experiment. For the future, chronic treatments need to be planned to evaluate the protective effect at a longer follow-up.
Our data indicate that hASCs injected intravenously possess a rapid homing ability, since the peak of the antihyperalgesic effect appears already in one day; this could be due to the MSCs full repertoire of molecules that enable their migration toward damaged tissues, allowing the researchers to move from a local administration to a systemic one [52,53].
The presence of MSCs at the lesion site has been extensively studied in several preclinical models; the results are not always consistent, maybe due to the different methods of detection with variable sensitivity. The number of traceable cells seems to decrease over time, however. Marconi et al. injected i.v. hASCs in a mouse model of sciatic crush, and they detected human cells, responsible of both the improved fiber sprouting and the reduction of the inflammatory infiltrates at the nerve site 1 week after injection and 35 days later [52].
Several studies address the influence of the route of cell administration on the clinical or preclinical outcome. In the regenerative field, ASCs have been shown to be able to enhance healing of mandibular defects in swine despite the way of administrations [57]: cells injected both systemically and locally into the defect completely repair the bone in 4 weeks, suggesting that a large number of ASCs reached and remained in the lesioned area. Among the studies describing the role of MSCs in pain relief, the elegant work of Guo et al. [58] about hypersensitivity after tendon injury in a rat model clearly shows that cells, injected i.v., act as the ones transplanted locally, suggesting that BMSCs act at the site of injury. In addition, the authors showed that the observed analgesic effect is both central and peripheral, and it seems to be related to their stemness. In our study, we believe that local and systemic hASCs are involved in the antihyperalgesic effect, and at the moment, we do not figure out which is the percentage of either circulating hASCs or recruited cells at the lesion site responsible of the described effect. Both cellular fractions might act synergistically; indeed, modulation of systemic endogenous cytokines is suggested by the data included in Table 2. Studies indicating that inflammation directs migration of transplanted MSCs to the inflamed site are consistent as the data on the plethora of molecules present on hASCs membrane able to affect their homing behavior [59].
Although the mechanism of action of these cells is still not completely elucidated, it is clear that the time course of the effects on pain definitely anticipated any possible effect on nerve repair, suggesting that their effect was likely due to factors locally carried and/or secreted by hASCs and/or by hASCs-stimulated resident cells, rather than a direct neuroregenerative action.
Indeed, hASCs are able to immediately downregulate, as fast as 24 h after their administration, the sciatic nerve levels of the proinflammatory cytokine IL-1β. Moreover, hASCs administration triggers a slower, but progressive, increase of the prototype anti-inflammatory cytokine IL-10, that, as previously reported, is decreased by the neuropathic pathology itself [27]. Seven days after hASCs administration, IL-10 concentrations are 10-fold higher than those observed in sciatic nerves obtained from neuropathic animals and 3 fold more elevated with respect to sham animals. This result is well beyond what we observed with NSCs administration and functionally very important. In fact it is now believed that, the sensitizing role of proinflammatory cytokines on pain transmission is one of the main components of the generation of neuropathic pain over time [22,28], and that the lack of a correct counterbalance response of anti-inflammatory/inhibitory cytokines can underline maintenance of pain [60]. Therefore, the IL-10 increase might explain the relevant effect of hASCs on allodynia and hyperalgesia. We and others demonstrated that the increase of proinflammatory cytokines such as IL-1β and tumor necrosis factor (TNF)-α well correlate with the presence of hyperalgesia and allodynia [61,62]. Moreover, when we previously tried to treat experimental neuropathic pain with pharmacological approaches, we always observed a parallelism between pain and cytokine modulation [22,23,38]. However, the early modulation of cytokines may induce/activate several endogenous mechanisms that at later time sustained the analgesic effect [52,53]. Consistently, the lower dose of hASCs that was only partially effective on pain symptoms also partially restored the normal cytokine levels. Indeed, we observed a significant increase of IL-10 serum concentrations, 1 day after hASCs treatment. These results suggest that already at early time point after hASCs administration, consistently with what reported by other groups [63–65], the hASCs posses a systemic immunomodulatory and anti-inflammatory activity that can blunt inflammatory cell infiltration and activation in the nervous tissue. However, the local effect at the injured site seems to be more efficacious and persistent, as demonstrated by the relevant suppression induced by hASCs on nerve IL-1β production. Furthermore, the known ability of MSCs to improve nerve blood flow by secreting VEGF and bFGF, playing a crucial role in neovascularization, might also be involved [16,66], together with other specific biochemical factors delivered by hASCs, such as glial cell-derived neurotrophic factor, IGF-1, brain-derived neurotrophic factor, IL-6, TNF-α, TNF-stimulated gene 6 [67], and also bFGF, shown to be a neuroprotective factor [68], which is able to improve sciatic nerve conduction in streptozotocin-induced diabetic rats [69].
Several authors have suggested that the effect on MSCs on hyperalgesia and allodynia could be sustained also by a central effect [22,26,53]. The upregulation of iNOS expression in CCI mice is completely reverted 1 week after administration of 106 hASCs, suggesting that systemic hASCs affect pain transmission at the central level, and not just at the lesion site, showing a modulation of iNOS at the level of the spinal cord, indicating that hASCs can also modulate the spinal cord nociceptive signaling pathways. In fact, we previously demonstrated that in CCI animals, the neurons overexpressing iNOS are located in the lamina II, specifically involved in pain transmission [22,23]. Based on the previous results [27] and the present ones, injecting hASCs, we feel confident in indicating both a local and systemic effect by ASCs acting as both direct immunomodulators and modulators of the process of host cell recruitment.
Here, we confirmed the rapid onset of hASCs effects, which are dose-related and can be prolonged for an extended period of time by recurrent treatments.
We conclude that the systemic administration of hASCs to normal immunecompetent mice is safe, that high doses, at least until 2.4×105 cells/g of b.w. are not toxic, and that 2×104 cells/g of b.w. are sufficient to promptly and completely revert neuropathic pain symptoms in a murine CCI model. Moreover, their effects are more potent than those elicited by murine NSCs, and we hypothesize that this difference can be due to the already-known relevant immunomodulatory potential of hASCs. The protection against painful symptoms in CCI mice exerted by systemic administration of hASCs shown here might be more a consequence of the paracrine action of mesenchymal progenitors that act as a drug store, rather than just directly contribute to the repair of the nerve injury [39].
Finally, the advantages of using hASCs rely on the large number of cells that can be easily obtained from subcutaneous adipose tissue allowing autologous transplantations, together with the absence of severe side effects. Therefore, we suggest that hASCs are worthy of further study as a valuable anti-inflammatory therapy in the treatment of chronic pain or other neuroinflammatory diseases.
Supplementary Material
Acknowledgments
This study was partially supported by the grants from the Italian Ministry of Health (2007-656853) and contract grant number Ricerca Corrente 2044 to A.T.B., and the Italian Ministry of Education, University, and Scientific Research to A.E.P.
The authors thank Dr. D. Lattuada and Dr. Lorena Maria Ferreira for their precious help in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Guilak F. Awad HA. Fermor B. Leddy HA. Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 3.Conforti E. Arrigoni E. Piccoli M. Lopa S. de Girolamo L. Ibatici A. Di Matteo A. Tettamanti G. Brini AT. Anastasia L. Reversine increases multipotent human mesenchymal cells differentiation potential. J Biol Regul Homeost Agents. 2011;25:S25–S33. [PubMed] [Google Scholar]
- 4.Ashjian PH. Elbarbary AS. Edmonds B. DeUgarte D. Zhu M. Zuk PA. Lorenz HP. Benhaim P. Hedrick MH. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003;111:1922–1931. doi: 10.1097/01.PRS.0000055043.62589.05. [DOI] [PubMed] [Google Scholar]
- 5.Safford KM. Safford SD. Gimble JM. Shetty AK. Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Choi J. Kim S. Jung J. Lim Y. Kang K. Park S. Kang S. Wnt5a-mediating neurogenesis of human adipose tissue-derived stem cells in a 3D microfluidic cell culture system. Biomaterials. 2011;32:7013–7022. doi: 10.1016/j.biomaterials.2011.05.090. [DOI] [PubMed] [Google Scholar]
- 7.de Girolamo L. Lopa S. Arrigoni E. Sartori MF. Baruffaldi Preis FW. Brini AT. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803. doi: 10.3109/14653240903079393. [DOI] [PubMed] [Google Scholar]
- 8.Quirici N. Scavullo C. de Girolamo L. Lopa S. Arrigoni E. Deliliers GL. Brini AT. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 2010;19:915–925. doi: 10.1089/scd.2009.0408. [DOI] [PubMed] [Google Scholar]
- 9.Yoo KH. Jang IK. Lee MW. Kim HE. Yang MS. Eom Y. Lee JE. Kim YJ. Yang SK, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Nasef A. Mazurier C. Bouchet S. Francois S. Chapel A. Thierry D. Gorin NC. Fouillard L. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253:16–22. doi: 10.1016/j.cellimm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Najar M. Raicevic G. Boufker HI. Fayyad-Kazan H. De Bruyn C. Meuleman N. Bron D. Toungouz M. Lagneaux L. Adipose-tissue-derived and Wharton's jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng Part A. 2010;16:3537–3546. doi: 10.1089/ten.TEA.2010.0159. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Rey E. Gonzalez MA. Varela N. O'Valle F. Hernandez-Cortes P. Rico L. Buscher D. Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 13.Tholpady SS. Ogle RC. Katz AJ. Adipose stem cells and solid organ transplantation. Curr Opin Organ Transplant. 2009;14:51–55. doi: 10.1097/MOT.0b013e328320d2cf. [DOI] [PubMed] [Google Scholar]
- 14.Constantin G. Marconi S. Rossi B. Angiari S. Calderan L. Anghileri E. Gini B. Bach SD. Martinello M, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 15.Choi EW. Shin IS. Park SY. Park JH. Kim JS. Yoon EJ. Kang SK. Ra JC. Hong SH. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 2012;64:243–253. doi: 10.1002/art.33313. [DOI] [PubMed] [Google Scholar]
- 16.Shibata T. Naruse K. Kamiya H. Kozakae M. Kondo M. Yasuda Y. Nakamura N. Ota K. Tosaki T, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunde K. Solheim S. Aakhus S. Arnesen H. Abdelnoor M. Egeland T. Endresen K. Ilebekk A. Mangschau A, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo ME. Zaffaroni N. Novara F. Cometa AM. Avanzini MA. Moretta A. Montagna D. Maccario R. Villa R, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 19.Ra JC. Shin IS. Kim SH. Kang SK. Kang BC. Lee HY. Kim YJ. Jo JY. Yoon EJ. Choi HJ. Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 20.MacIsaac ZM. Shang H. Agrawal H. Yang N. Parker A. Katz AJ. Long-term in-vivo tumorigenic assessment of human culture-expanded adipose stromal/stem cells. Exp Cell Res. 2012;318:416–423. doi: 10.1016/j.yexcr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treede RD. Jensen TS. Campbell JN. Cruccu G. Dostrovsky JO. Griffin JW. Hansson P. Hughes R. Nurmikko T. Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 22.Martucci C. Trovato AE. Costa B. Borsani E. Franchi S. Magnaghi V. Panerai AE. Rodella LF. Valsecchi AE. Sacerdote P. Colleoni M. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137:81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Valsecchi AE. Franchi S. Panerai AE. Sacerdote P. Trovato AE. Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008;107:230–240. doi: 10.1111/j.1471-4159.2008.05614.x. [DOI] [PubMed] [Google Scholar]
- 24.Franchi S. Giannini E. Lattuada D. Lattanzi R. Tian H. Melchiorri P. Negri L. Panerai AE. Sacerdote P. The prokineticin receptor agonist Bv8 decreases IL-10 and IL-4 production in mice splenocytes by activating prokineticin receptor-1. BMC Immunol. 2008;9:60. doi: 10.1186/1471-2172-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musolino PL. Coronel MF. Hokfelt T. Villar MJ. Bone marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett. 2007;418:97–101. doi: 10.1016/j.neulet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Siniscalco D. Giordano C. Galderisi U. Luongo L. Alessio N. Di Bernardo G. de Novellis V. Rossi F. Maione S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67:655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchi S. Valsecchi AE. Borsani E. Procacci P. Ferrari D. Zaffa C. Sartori P. Rodella LF. Vescovi A, et al. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012;153:850–861. doi: 10.1016/j.pain.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Sommer C. Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Achille V. Mantelli M. Arrigo G. Novara F. Avanzini MA. Bernardo ME. Zuffardi O. Barosi G. Zecca M. Maccario R. Cell-cycle phases and genetic profile of bone marrow-derived mesenchymal stromal cells expanded in vitro from healthy donors. J Cell Biochem. 2011;112:1817–1821. doi: 10.1002/jcb.23100. [DOI] [PubMed] [Google Scholar]
- 30.Gang EJ. Jeong JA. Hong SH. Hwang SH. Kim SW. Yang IH. Ahn C. Han H. Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- 31.Konno M. Hamazaki TS. Fukuda S. Tokuhara M. Uchiyama H. Okazawa H. Okochi H. Asashima M. Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem Biophys Res Commun. 2010;400:461–465. doi: 10.1016/j.bbrc.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Bodo M. Lilli C. Bellucci C. Carinci P. Calvitti M. Pezzetti F. Stabellini G. Bellocchio S. Balducci C. Carinci F. Baroni T. Basic fibroblast growth factor autocrine loop controls human osteosarcoma phenotyping and differentiation. Mol Med. 2002;8:393–404. [PMC free article] [PubMed] [Google Scholar]
- 33.Tullberg-Reinert H. Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999;112:271–276. doi: 10.1007/s004180050447. [DOI] [PubMed] [Google Scholar]
- 34.Guilak F. Lott KE. Awad HA. Cao Q. Hicok KC. Fermor B. Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 36.Bennett GJ. Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 37.Hargreaves K. Dubner R. Brown F. Flores C. Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 38.Valsecchi AE. Franchi S. Panerai AE. Rossi A. Sacerdote P. Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650:694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 39.Caplan AI. Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Wislet-Gendebien S. Wautier F. Leprince P. Rogister B. Astrocytic and neuronal fate of mesenchymal stem cells expressing nestin. Brain Res Bull. 2005;68:95–102. doi: 10.1016/j.brainresbull.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Mareschi K. Novara M. Rustichelli D. Ferrero I. Guido D. Carbone E. Medico E. Madon E. Vercelli A. Fagioli F. Neural differentiation of human mesenchymal stem cells: evidence for expression of neural markers and eag K+channel types. Exp Hematol. 2006;34:1563–1572. doi: 10.1016/j.exphem.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Uccelli A. Benvenuto F. Laroni A. Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24:59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 44.O'Connor AB. Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Scholz J. Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 46.Vallejo R. Tilley DM. Vogel L. Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 47.Siniscalco D. Giordano C. Rossi F. Maione S. de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9:523–529. doi: 10.2174/157015911798376208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iyer SS. Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 49.Jones BJ. McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K. Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 51.Beggs KJ. Lyubimov A. Borneman JN. Bartholomew A. Moseley A. Dodds R. Archambault MP. Smith AK. McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 52.Marconi S. Castiglione G. Turano E. Bissolotti G. Angiari S. Farinazzo A. Constantin G. Bedogni G. Bedogni A. Bonetti B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–1272. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]
- 53.Siniscalco D. Giordano C. Galderisi U. Luongo L. de Novellis V. Rossi F. Maione S. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. doi: 10.3389/fnint.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimble JM. Katz AJ. Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuo M. Neuronal mechanism for neuropathic pain. Mol Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colleoni M. Sacerdote P. Murine models of human neuropathic pain. Biochim Biophys Acta. 2010;1802:924–933. doi: 10.1016/j.bbadis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Wilson SM. Goldwasser MS. Clark SG. Monaco E. Bionaz M. Hurley WL. Rodriguez-Zas S. Feng L. Dymon Z. Wheeler MB. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg. 2012;70:e193–e203. doi: 10.1016/j.joms.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 58.Guo W. Wang H. Zou S. Gu M. Watanabe M. Wei F. Dubner R. Huang GT. Ren K. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells. 2011;29:1294–1303. doi: 10.1002/stem.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henschler R. Deak E. Seifried E. Homing of mesenchymal stem cells. Transfus Med Hemother. 2008;35:306–312. doi: 10.1159/000143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvo M. Dawes JM. Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- 61.Sacerdote P. Franchi S. Trovato AE. Valsecchi AE. Panerai AE. Colleoni M. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci Lett. 2008;436:210–213. doi: 10.1016/j.neulet.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Austin PJ. Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Mao F. Xu WR. Qian H. Zhu W. Yan YM. Shao QX. Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219–225. doi: 10.1007/s00011-009-0090-y. [DOI] [PubMed] [Google Scholar]
- 64.Caplan AI. Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 65.Horwitz EM. Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. Isr Med Assoc J. 2009;11:209–211. [PubMed] [Google Scholar]
- 66.Kim H. Park JS. Choi YJ. Kim MO. Huh YH. Kim SW. Han JW. Lee J. Kim S, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27:1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranganath SH. Levy O. Inamdar MS. Karp JM. Cell Stem Cell. A 2012 Elsevier Inc; United States: 2012. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease; pp. 244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson KJ. Dam D. Lee S. Cotman CW. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature. 1988;332:360–361. doi: 10.1038/332360a0. [DOI] [PubMed] [Google Scholar]
- 69.Nakae M. Kamiya H. Naruse K. Horio N. Ito Y. Mizubayashi R. Hamada Y. Nakashima E. Akiyama N, et al. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes. 2006;55:1470–1477. doi: 10.2337/db05-1160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.