Abstract
Although advances in cancer therapies continue to develop, the shortness of the survival of lung cancer patients is still disappointing. Therefore, finding new adjuvant strategies is within the focus of cancer cure. Based on observations that deuterium depletion inhibits the growth of cancer cell lines and suppresses certain proto-oncogenes, we have conducted a clinical study in 129 patients with small cell and nonsmall cell lung cancers who consumed deuterium-depleted drinking water (DDW) as a nontoxic agent in addition to conventional chemotherapy and radiotherapy. Median survival time (MST) was 25.9 mo in males and 74.1 mo in female patients; the difference between genders was statistically significant (p < 0.05). Median survival of subjects with brain metastasis was 27.1 mo. Cumulative 5-yr survival probabilities were 19%, 52%, and 33% in males, females, and all patients with brain metastasis, respectively. Gene expression analysis in mouse lung indicated that DDW attenuates 7,12-dimethylbenz(a)anthracene (DMBA)-induced expression of Bcl2, Kras, and Myc in females. In conclusion, DDW counteracts the DMBA-induced overexpression of Bcl2, Kras and Myc genes in mouse lung, and it may extend survival of lung cancer patients as a nontoxic anticancer dietary supplement, especially for women with tumors overexpressing cancer-related genes, because MST of DDW-consuming group was 2–4 times longer than it is generally observed in lung cancer patients.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide, and the incidence is rapidly increasing in developing countries (1,2). Median survival time (MST) strongly depends on the histological subtype of the cancer, localization of the metastasis, the quality of therapy, and other factors, such as gender (3,4) and age of the patient (5). The MST of small cell lung cancer (SCLC) is 8—10 mo (6). In advanced nonsmall cell lung cancers (NSCLC), such as adenocarcinoma and squamous cell carcinoma groups, the MST rarely exceeds 15 mo; however, adjuvant therapy can extend the survival of these patients (7). It has been observed that survival strongly depends on the available adjuvant therapy (8). Therefore, introduction of new adjuvant protocols associated with low toxicity is needed.
The first publications on deuterium-depleted water (DDW) as an adjuvant therapy appeared recently (9). Deuterium depletion can be obtained in living organisms through the intake of DDW, which has an inhibitory effect on the division of cancer cells and attenuates the expression of certain cancer-related genes (10). In living organisms, the deuterium concentration exceeds 10 mM, which is above the range of calcium, magnesium, or potassium (10), and concentrations of deuterium in body water correlate well with the deuterium level of the environment (11). In bottled water, concentrations of deuterium typically vary between 135 and 158 ppm (12).
Clinical data show extended survival of prostate, breast (13), and lung cancer patients (9) who took DDW that contained between 25 and 125 ppm of deuterium. In the studies of prostate cancer, the volume of the prostate was found to drop significantly in the DDW-consuming vs. control group, and urination problems ceased in some patients in the DDW group. In the studies of DDW in lung cancer, where four of the patients had brain metastasis, two of these patients showed a complete response (CR) and one a partial response (PR) (9); moreover, CR or PR was detected in all the primary tumors.
In the present study, we monitored the impact of DDW on the survival of 129 lung cancer patients. In addition, we examined the expression of Bcl2, Kras, and Myc genes in the lung tissue of carcinogen-treated mice.
MATERIALS AND CHARACTERIZATION OF SAMPLE
Patients and Specimens
One hundred twenty-nine lung cancer patients (51 women and 78 men) who received conventional chemo- and radiotherapy were included in this study. The follow-up period was from March 22, 1993 to December 2, 2010. Histopathology indicated that 90 of the patients (70%) had NSCLC, 24 (19%) had SCLC, and 15 (12%) had a mixed or uncharacterized lung cancer (Table 1). In total, 27 of all patients (21%) had brain metastasis (Table 1). Tumor staging was performed according to Duke's classification: 57 of the patients (44%) were in stage B, 45 (35%) in stage C without brain metastasis, and 27 (21%) in stage C with brain metastasis. Patients voluntarily consumed DDW that contained from 25 to 105 ppm deuterium as drinking water. They received available information on DDW and deuterium depletion. The commercially available DDW is under the regulations of foodstuffs at the present in Hungary.
TABLE 1.
Distribution of lung tumour subtypes (number of cases) according to gender and disease stagea among patients in the study
| Stadium |
|||||
|---|---|---|---|---|---|
| Gender | Tumour histological subtypes | B | C | C with brain metastasis | All |
| Male | NSCLC | 20 | 21 | 9 | 50 |
| NSCLC adenocarcinoma | 5 | 10 | 5 | 20 | |
| NSCLC squamous cell | 13 | 9 | 2 | 24 | |
| NSCLC mixed or other | 2 | 2 | 2 | 6 | |
| SCLC | 10 | 4 | 4 | 18 | |
| Mixed, or no data | 7 | 1 | 2 | 10 | |
| All subtypes | 37 | 26 | 15 | 78 | |
| Female | NSCLC | 14 | 16 | 10 | 40 |
| NSCLC adenocarcinoma | 12 | 11 | 7 | 30 | |
| NSCLC squamous cell | 2 | 4 | 1 | 7 | |
| NSCLC mixed or other | 0 | 1 | 2 | 3 | |
| SCLC | 2 | 2 | 2 | 6 | |
| Mixed, or no data | 4 | 1 | 0 | 5 | |
| All subtypes | 20 | 19 | 12 | 51 | |
| All | All subtypes | 57 | 45 | 27 | 129 |
NSCLC, nonsmall cell lung carcinoma; SCLC, small cell lung carcinoma.
Disease stage according to Duke's classification system.
Animal Experiment
The antiproliferative and gene silencing properties of DDW were studied in mice. Eight-week-old (20 ± 4 g weight) male and female CBA/Ca mice (n = 6 in each group, University of Pécs, Pécs, Hungary) were kept under standard conditions and fed a conventional dry rodent diet. Water was provided ad libitum, with control animals receiving normal drinking water (tap water) that contained 150 ppm deuterium (natural levels), and that of the treated animals contained 25 ppm deuterium. Some of the animals were treated with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA), which is known to activate cancer-related genes in CBA/Ca mice (14). The DMBA (Sigma Aldrich, Budapest, Hungary) was dissolved in corn oil and delivered by a single intraperitoneal injection at a dosage of 20 mg/kg body weight as it was described earlier (15). For 7 days prior to DMBA administration and 24 h thereafter, the test animals drank DDW, at this time point all animals were sacrificed and the lungs were removed. Samples of 100 mg tissue from each lung were collected and frozen immediately and stored on 80°C for less than 1 mo prior to homogenization.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Committee on Research of the University of Pécs (permit number: BA02/2000-24/2006).
Gene Expression Analysis
After homogenization the lungs of mice on ice with Ultra Turrax disperser (IKA, Staufen, Germany), total cellular RNA was isolated using TRIZOL reagent (Invitrogen, Paisley, UK). The A260/A280 absorbance ratio of the extracted RNA was greater than 1.8. After dilution, 10 μg RNA was dot-blotted onto Hybond N+ nitrocellulose membrane (Amersham, Little Chalfont, UK), then hybridized with specific labelled probes for Bcl2 (ATCC 79804, LGC Standards, Wesel, Germany), Kras (ATCC 41027), or Myc (ATCC 41010) and analyzed by chemilumi-nescence (ECL kit, Amersham, Little Chalfont, UK). Isolation of RNA, hybridization, and detection were performed according to the instructions of the manufacturers. The membranes were rehybridized with a probe for the constitutively expressed β-actin gene (ATCC 77644) as a control for sample normalization. The chemiluminescent signals were detected on X-ray films and scanned, and then the digital data were evaluated using the Quantiscan software (Biosoft, Cambridge, UK). The relative levels of Bcl, Kras, and Myc expression were calculated as percent of the corresponding β-actin values.
Statistical Analysis
The Epi Info software version 3.5.1 (Centers for Disease Control and Prevention, Atlanta, GA) and MedCalc for Windows, version 11.1.1.0 (MedCalc Software, Mariakerke, Belgium) were used for calculating Kaplan-Meier cumulative survival curves, and the log rank and Wilcoxon probes were used to calculate p values. Confidence intervals (CI) were expressed as ±1.96 of the standard error of the mean. Survival of patients was calculated from the date of diagnosis. For evaluating the significance of differences in gene expression data, Student's t test was used.
RESULTS
Clinical Study
The average age of the 51 women in the study was 58.1 ± 10.4 yr (range of 36–76 yr, and median of 58.5 yr) and of the 78 men was 58.7 ± 8.6 yr (range of 39–83 yr, and median of 58 yr). Younger patients had significantly (p < 0.01) shorter survival times than older patients (Fig. 1). Patients tended to have a longer MST in stage B, than in stage C with or without brain metastasis, but these differences were not statistically significant. The proportions of adenocarcinoma in stage B, squamous cell carcinomas, and of SCLC in stage C, were higher in females than in males (Table 1). The MST was 25.9 mo (CI 24.2–27.5) in males and 74.1 mo (CI 67.4–80.8) in females, and the difference between genders was statistically significant (p < 0.05; Table 2). The MST of subjects with brain metastasis was 27.1 mo (CI 24.3–29.8). The cumulative 5-yr survival probabilities were 19%, 52%, and 33% in males, females, and all patients with brain metastasis, respectively (Table 3).
FIG. 1.
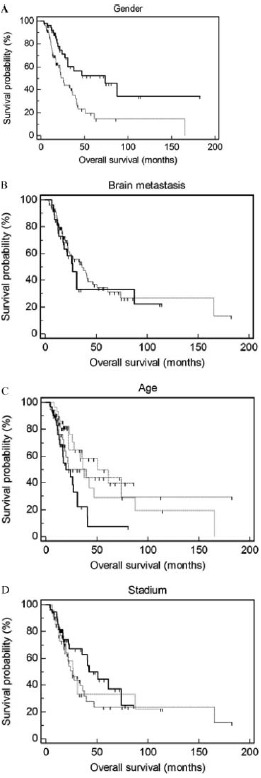
Cumulative survival probabilities among the different patient groups in the study. There are significant survival difference observed between genders (A; lines: male —, female —; Wilcoxon and log rank tests, p < 0.05), and between different age groups (panel C; lines: 36–51 years —, 52–58 years —, 59–65 years 66–83 years -••-; according to log rank test only p < 0.05). No significant differences in survival were observed on the basis of stage of the disease, as assessed by Duke's classification system (D; lines: stadium B —, stadium C without brain metastasis —, stadium C with brain metastasis …; Wilcoxon and log rank tests p > 0.05), or presence of brain metastasis (B; lines: with brain metastasis —, without brain metastasis —; Wilcoxon and log rank tests p > 0.05).
TABLE 3.
Cumulative survival (%) of patients in the study according to each of the main subtypes of lung cancer
| Patient | 1 yr | 2 yr | 3 yr | 5 yr |
|---|---|---|---|---|
| Males | ||||
| NSCLC | 87 | 57 | 48 | 14 |
| NSCLC adenocarcinoma | 95 | 71 | 47 | 16 |
| NSCLC squamous cell | 83 | 56 | 56 | 19 |
| NSCLC with brain metastasis | 89 | 78 | 52 | No dataa |
| SCLC | 57 | 25 | 17 | 17 |
| All subtypes | 77 | 51 | 38 | 19 |
| Females | ||||
| NSCLC | 92 | 72 | 58 | 53 |
| NSCLC adenocarcinoma | 97 | 81 | 66 | 59 |
| NSCLC squamous cell | 75 | 62 | 47 | 47 |
| NSCLC with brain metastasis | 89 | 63 | 32 | 32 |
| SCLC | 100 | 75 | 75 | 50 |
| All subtypes | 94 | 75 | 60 | 52 |
| Males and females | ||||
| NSCLC | 89 | 63 | 53 | 35 |
| NSCLC adenocarcinoma | 96 | 77 | 59 | 44 |
| NSCLC squamous cell | 83 | 60 | 55 | 31 |
| NSCLC with brain metastasis | 89 | 69 | 41 | 41 |
| SCLC | 69 | 40 | 33 | 25 |
| All subtypes | 84 | 60 | 47 | 33 |
NSCLC = nonsmall cell lung carcinoma; SCLC = small cell lung carcinoma.
There were no patients under observation in this group at 5 yr.
There was no significant difference in median survival between males and females with brain metastasis. The MST in this group was 26.4 mo for males (n = 15) and 31.0 mo for females (n = 12). Out of the total 27 patients, 19 had NSCLC (32.9 mo MST), and 6 had SCLC (14.9 mo MST). In the NSCLC group, the MST was 37.9 mo in males (n = 9) and 30.9 in females (n = 10). In the group aged ≥55 yr (n = 13), the MST was 32.9 yr, and it was 30.9 yr in patients < 55 yr of age (n = 14). The shortest MST (13.1 mo) was observed in the SCLC group of males, and the longest (87.5 mo) was seen in the NSCLC type adenocarcinoma group of females. The only group in which the MST was not greater in females than in males was that of patients with NSCLC type squamous cell carcinoma (Table 2).
TABLE 2.
Median survival time of patients classified according to subtypes of lung cancer and gender
| Gendera |
|||
|---|---|---|---|
| Tumour subtypes | Male | Female | All |
| NSCLC | 33.7 (30.8–36.6) | 74.1 (66.6–81.6) | 37.7 (35.7–39.6) |
| NSCLC adenocarcinoma | 33.7 (28.6–38.7) | 87.5 (75.3–99.6) | 37.7 (33.9–41.4) |
| NSCLC squamous cell | 39.7 (35.0–44.3) | 27.5 (22.8–32.3) | 39.7 (35.6–43.7) |
| NSCLC with brain metastasis | Not calculabled | 31.0 (25.6–36.5) | 31.1 (27.5–34.7) |
| SCLC | 13.2 (11.8–14.6) | 47.2 (35.6–58.7) | 16.7 (15.1–18.3) |
| All subtypes | 25.8 (24.2–27.5) | 74.1 (67.4–80.8) | 33.7 (31.9–35.5) |
NSCLC = nonsmall cell lung cancer; SCLC, small cell lung cancer.
The median survival times are expressed in months, and the 95% confidence interval is displayed in the brackets.
This value could not be calculated because the last survival probability was > 0.5.
Gene Expression
Analysis of the expression of the Bcl2, Kras, and Myc genes in lungs of mice 24 h after DMBA was administered indicates significantly increased levels of expression in tissue from female mice that had been given drinking water containing normal levels of deuterium (150 ppm). The levels were not elevated in females given a lower amount of deuterium (25 ppm) or in any of the male mice. In conclusion, DMBA administration failed to upregulate Bcl2, Kras, and Myc expression in the group of animals consuming DDW (Fig. 2). Upregulation of the proto-oncogene expression by DMBA is gender-specific (only seen in females), and it is attenuated by DDW (p < 0.05).
FIG. 2.
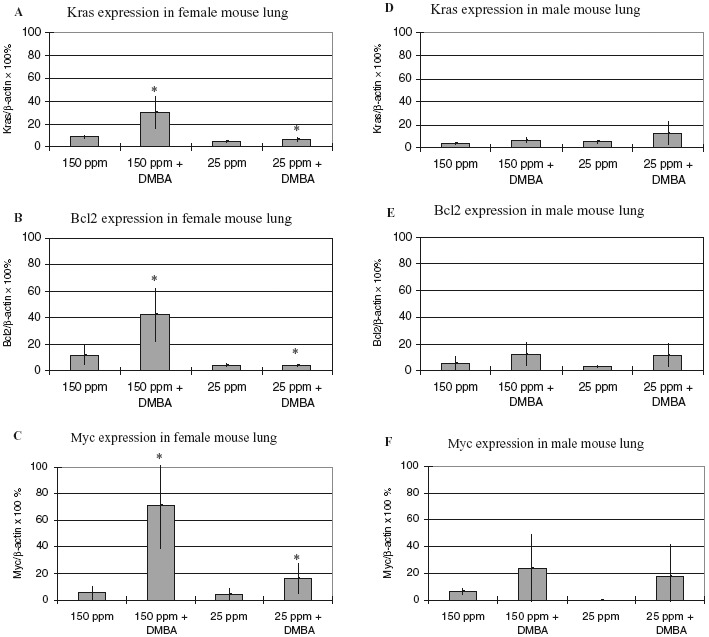
Expression of lung cancer-related proto-oncogenes in the lung tissue of experimental mice. The values given in ppm on the x-axes represent the concentration of deuterium in the deuterium-depleted drinking water (DDW) (25 ppm) and control drinking water (150 ppm). DMBA: 7,12-dimethylbenz(a)anthracene. Effects of levels of deuterium in drinking water on Kras gene expression in lungs of female and male mice are graphed in panels A and D; effects on Bcl2 expression in lungs of female and male mice are shown in panels B and E, whereas Myc expression in lungs of female and male mice are seen in panels C and F, respectively. There was significant (∗p < 0.05) difference in gene expression observed in DMBA-treated female animals consuming DDW or tap water at all the examined genes. (150 ppm = normal drinking water without DMBA-treatment, 150 ppm + DMBA = normal drinking water with DMBA-treatment, 25 ppm = deuterium-depleted drinking water without DMBA-treatment, 25 ppm + DMBA = deuterium-depleted drinking water with DMBA-treatment).
DISCUSSION
We investigated the survival of 129 patients with different lung cancer histological subtypes. The distribution of lung cancer subtypes in our study represents the population of lung cancer patients seen in Western countries (16). We observed longer survival of females and older patients; furthermore, patients with adenocarcinoma and squamous cell carcinoma could expect longer survival than those with SCLC. These findings are consistent with what is generally observed in lung cancer (17, 18), and this would suggest that DDW treatment in our study did not have a pronounced effect on certain expected survival patterns.
The MST of SCLC is 12–20 mo in the early stage disease, with a 5-yr survival of 6%-12%. This drops to a 7–12 month MST, with only a 2% 5-yr survival, in advanced disease (19, 20). In our study, the MST of SCLC patients was 16.7 mo, with a 5-yr survival of 25%. The MST of 47.2 mo in females was especially long, with a 50% 5-yr survival.
NSCLC represents the majority of human lung cancers (8). We analyzed our data from patients with adenocarcinoma and squamous cell carcinoma, the 2 main histological subtypes of NSCLC. In NSCLC patients of this study, the MST of 33.7 mo for males and 74.1 mo for females were both greater than the generally observed 15—20 mo values (7).
Brain is a common location of lung cancer metastasis. In NSCLC with cerebral metastasis, the MST ranges from 19 to 27 mo for the curative intent groups (bifocal therapy and adjuvant treatment), and the cumulative survival at 1, 2, and 5 yr ranges from 56%—69%, 28%—54%, and 11%—24%, respectively. In comparison, the median and 1-yr survivals of the palliative groups range from 7.1 to 12.9 mo and 33%-39.7%, respectively (21). In our study, the MST in both genders was 31.1 mo; the 1-, 2-, and 5-yr survival was 89%, 69%, and 41%, respectively. The MST of more than 30 mo for patients with brain metastasis is above the range of bifocal therapy and adjuvant treatment, although the patients were not selected for bifocal therapy in our study. These data confirm the results of previous clinical observations, in which DDW was observed to prolong the survival of 4 lung cancer patients with brain metastasis (9).
In the entire population of lung cancer patients in Hungary between 2002 and 2005, the MST of males was 7.5 mo, with a 10% 5-yr survival probability, and in females the MST was 11.3 mo, with a 5-yr survival probability of 20.5% (22). In our study, which lasted from 1993 to 2010, the MST was 25.8 mo in males, 74.1 mo in females, and 33.7 mo in both sexes overall. The 5-yr survival probabilities were 19%, 52%, and 33% in males, females, and both sexes, respectively. Thus, we observe relatively expanded survival characteristics in patients in our study, who were also administered DDW.
The patients in the study voluntarily consumed DDW, and on this basis they can be subcategorized as cancer patients who willingly take supplements during therapy. Jatoi et al. reported a doubled survival of NSCLC patients who voluntarily take supplements (takers) vs. those who do not (nontakers) (23). In general, 80% of cancer patients take supplements (24); on this basis, the survival advantage of takers, compared to the total population of cancer patients, is only 11%. Therefore, the higher survival rates of patients in our study could not only be attributed to this additional advantage.
The cell cycle machinery is modified by DDW, but little is known about the mechanisms involved. Decreased deuterium concentrations in the environment of a cell slow its division cycle and may induce stress signals (25). This stress factor could play a role in the mechanism of action of deuterium depletion. In response to a stress stimulus, mRNA abundance of a large fraction of the transcribed genome can change (26) because of modified levels of synthesis and degradation of mRNA (27). Inhibitory effects of DDW have been observed on the expression of H-Ras, p53, and c-Myc in different organs of animals, including the lungs (28). Mutated Kras in tumors can worsen the prognosis because of its permanently high level of activation. The occurrence of Kras mutations predominates in adenocarcinomas, being rare in SCLC (29). We observed that DDW significantly diminishes DMBA-induced expression of Kras in the lungs of mice. Thus, the knock-down of Kras that we observed in mouse lung, which is most pronounced in female animals, may correlate with the longer survival of patients having lung adenocarcinoma, especially because of the elongation of survival we observed was most pronounced in NSCLC.
The frequency of high expression of Bcl2 is greater in SCLC (in 75%—95% of cases) than in NSCLC (in 25% of squamous cell carcinomas and 12% of adenocarcinomas) (30). According to the available data, the role of Bcl2 in lung cancer prognosis is not clear. On one hand, advanced NSCLC patients with high Bcl2 expression were reported to have a better prognosis (31); but on the other hand, repression of Bcl2 through lysyl oxidase (LOX) inhibits the transformed phenotype of NSCLC cells (32).
Increased expression of Myc suggests greater proliferation in damaged alveolar cells (33). Furthermore, suppression of Myc expression induces apoptosis in lung cells bearing active Kras (34). In our experiments, Myc expression was also elevated by DMBA and silenced in female mice that drank DDW. This result confirms the putative anticancer properties of DDW in females.
Inhibition of gene expression by DDW results in cell cycle arrest and a 5—10 h delay in the start of the cell cycle in cancer cells. The most striking inhibitory effect on cell division was reported to occur when the concentration of deuterium was decreased in several steps, compared to a single step, in PC-3 prostate, MCF-7 breast, and A4 melanoma cell lines (35). This type of treatment mimics the human and animal experiments, where the consumption of DDW is continuous, and the level of deuterium gradually decreases by depletion over the course of the treatment. Transplanted MDA, MCF-7 breast (36), PC-3 prostate (37), uterine, cervical, and Lewis lung (38) cell-derived tumors in animals grew slower, or disappeared, in host animals who were given DDW treatment. In the most recent publication of Cong et al., evidence is presented that DDW inhibits the growth of lung cancer cells in vitro and in vivo (39).
Because DDW extends the life of lung cancer patients, it is a promising nontoxic agent for therapy in lung cancer patients. The advantage in longer survival for females, and the gender-specific regulation of proto-oncogene expression by DDW, may require further investigation in the future.
REFERENCES
- 1.Toh CK. The changing epidemiology of lung cancer. Methods Mol Biol. 2009;472:397–411. doi: 10.1007/978-1-60327-492-0_19. [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 3.Stabile LP, Siegfried JM. Sex and gender differences in lung cancer. J Gend-Specif Med. 2003;6:37–48. [PubMed] [Google Scholar]
- 4.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291:1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 5.Hansen HH, Rørth M. Lung cancer. Cancer Chemother Biol Response Modif. 1997;17:444–463. [PubMed] [Google Scholar]
- 6.Kurup A, Hanna NH. Treatment of small cell lung cancer. Crit Rev Oncol Hem. 2004;52:117–126. doi: 10.1016/j.critrevonc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK. Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care: First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260–274. doi: 10.1097/JTO.0b013e3181c6f035. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 9.Krempels K, Somlyai I, Somlyai G. A retrospective evaluation of the effects of deuterium depleted water consumption on 4 patients with brain metastases from lung cancer. Integr Cancer Ther. 2008;7:172–181. doi: 10.1177/1534735408322851. [DOI] [PubMed] [Google Scholar]
- 10.Somlyai G, Molnár M, Laskay G, Szabó M, Berkényi T, et al. Biological significance of naturally occurring deuterium: the antitumor effect of deuterium depletion. Orv Hetil. 2010;151:1455–1460. doi: 10.1556/OH.2010.28865. [DOI] [PubMed] [Google Scholar]
- 11.Hobson KA, Bowen GJ, Wassenaar LI, Ferrand Y, Lormee H. Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia. 2004;141:477–488. doi: 10.1007/s00442-004-1671-7. [DOI] [PubMed] [Google Scholar]
- 12.Bowen GJ, Winter DA, Spero HJ, Zierenberg RA, Reeder MD, et al. Stable hydrogen and oxygen isotope ratios of bottled waters of the world. Rapid Commun Mass Spectrom. 2005;19:3442–3450. doi: 10.1002/rcm.2216. [DOI] [PubMed] [Google Scholar]
- 13.Somlyai G, Kovács A, Guller I, Gyöngyi Z, Krempels K, et al. Deuterium has a key role in tumour development: new target in anticancer drug development. Eur J Cancer. 2010;208(Suppl 8) [Google Scholar]
- 14.Li XL, Eckard J, Shah R, Malluck C, Frenkel K. Interleukin-1 alpha up-regulation in vivo by a potent carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and control of DMBA-induced inflammatory responses. Cancer Res. 2002;62:417–423. [PubMed] [Google Scholar]
- 15.Szanyi I, Bauer M, Gerlinger I, Járai T, Gobel G, et al. Changes in expression of oncogenes and TP53 tumour suppressor gene as biomarkers in head and neck cancers. Eur Arch Otorhinolaryngol. 2011;268:1041–1046. doi: 10.1007/s00405-010-1425-6. [DOI] [PubMed] [Google Scholar]
- 16.Bennett VA, Davies EA, Jack RH, Mak V, Møller H. Histological subtype of lung cancer in relation to socio-economic deprivation in South East England. BMC Cancer. 2008;8:139. doi: 10.1186/1471-2407-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang EA, Wynder EL. Differences in lung cancer risk between men and women: Examination of the evidence. J Nat Cancer Inst. 1996;3–4:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 18.Shugarman LR, Mack K, Sorbero ME, Tian H, Jain AK, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–781. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A, Maione P, Palazzolo G, Sacco PC, Ferrara ML, et al. New targeted therapies and small-cell lung cancer. Clin Lung Cancer. 2008;9:271–279. doi: 10.3816/CLC.2008.n.042. [DOI] [PubMed] [Google Scholar]
- 20.Toh CK, Hee SW, Lim WT, Leong SS, Fong KW, et al. Survival of small-cell lung cancer and its determinants of outcome in Singapore. Ann Acad Med Singapore. 2007;36:181–188. [PubMed] [Google Scholar]
- 21.Modi A, Vohra HA, Weeden DF. Does surgery for primary non-small cell lung cancer and cerebral metastasis have any impact on survival? Interact Cardiovasc Thorac Surg. 2009;8:467–473. doi: 10.1510/icvts.2008.195776. [DOI] [PubMed] [Google Scholar]
- 22.Tusnády G, Gaudi I, Rejtö L, Kásler M, Szentirmay Z. Survival chances of Hungarian cancer patients in the National Cancer Registry. Magyar Oncol. 2008;52:339–349. doi: 10.1556/MOnkol.52.2008.4.2. [DOI] [PubMed] [Google Scholar]
- 23.Jatoi A, Williams B, Nichols F, Marks R, Aubry MC, et al. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients? Results from the Mayo Clinic lung cancer cohort. Lung Cancer. 2005;49:77–84. doi: 10.1016/j.lungcan.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Kumar NB, Hopkins K, Allen K, Riccardi D, Besterman-Dahan K, et al. Use of complementary/integrative nutritional therapies during cancer treatment: implications in clinical practice. Cancer Control. 2002;9:236–243. doi: 10.1177/107327480200900307. [DOI] [PubMed] [Google Scholar]
- 25.Salomonsson L, Brändén G, Brzezinski P. Deuterium isotope effect of proton pumping in cytochrome c oxidase. Biochim Biophys Acta. 2008;1777:343–350. doi: 10.1016/j.bbabio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalem O, Dahan O, Levo M, Martinez MR, Furman I, et al. Transient transcriptional responses to stress are generated by opposing effects of mRNA production and degradation. Mol Syst Bio. 2008;4:223. doi: 10.1038/msb.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyöngyi Z, Somlyai G. Deuterium depletion can decrease the expression of C-myc Ha-ras and p53 gene in carcinogen-treated mice. In Vivo. 2000;14:437–439. [PubMed] [Google Scholar]
- 29.Modrek B, Ge L, Pandita A, Lin E, Mohan S, et al. Oncogenic activating mutations are associated with local copy gain. Mol Cancer Res. 2009;7:1244–1252. doi: 10.1158/1541-7786.MCR-08-0532. [DOI] [PubMed] [Google Scholar]
- 30.Duarte RL, Paschoal ME. Molecular markers in lung cancer: prognostic role and relationship to smoking. J Bras Pneumol. 2006;32:56–65. doi: 10.1590/s1806-37132006000100012. [DOI] [PubMed] [Google Scholar]
- 31.Shibata Y, Hidaka S, Tagawa Y, Nagayasu T. Bcl-2 protein expression correlates with better prognosis in patients with advanced non-small cell lung cancer. Anticancer Res. 2004;3:1925–1928. [PubMed] [Google Scholar]
- 32.Wu M, Min C, Wang X, Yu Z, Kirsch KH, et al. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 33.Adamson A, Perkins S, Brambilla E, Tripp S, Holden J, et al. Proliferation, C-myc, and cyclin D1 expression in diffuse alveolar damage: potential roles in pathogenesis and implications for prognosis. Hum Pathol. 1999;30:1050–1057. doi: 10.1016/s0046-8177(99)90222-8. [DOI] [PubMed] [Google Scholar]
- 34.Fukazawa T, Maeda Y, Matsuoka J, Yamatsuji T, Shigemitsu K, et al. Inhibition of Myc effectively targets KRAS mutation-positive lung cancer expressing high levels of Myc. Anticancer Res. 2010;10:4193–4200. [PubMed] [Google Scholar]
- 35.Somlyai G, Laskay G, Berkényi T, Jákli G, Jancsó G. Naturally oc-curing deuterium may have a central role in cell signalling. In: Heys JR, editor; Mellilo DG, editor. Synthesis and Application of Isotopically Labelled Compound, Chichester, West Sussex, UK: John Wiley & Sons Ltd.; 1998. pp. 137–141. [Google Scholar]
- 36.Somlyai G, Jancsó G, Jákli G, Vass K, Barna B, et al. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993;317:1–4. doi: 10.1016/0014-5793(93)81479-j. [DOI] [PubMed] [Google Scholar]
- 37.Somlyai G, Laskay G, Berkényi T, Galbács Z, Galbács G, et al. The biological effects of deuterium-depleted water, a possible new tool in cancer therapy. J Oncol. 1998;30:91–94. [Google Scholar]
- 38.Sinyak Y, Turusov V, Grigroriev A, Yaridze D, Gaidadimov V, et al. Possibility of deuterium free water using as antitumoral means with reference to conditions of Martian expedition. 34th COSPAR Scientific Assembly, The Second World Space Congress, Houston, TX, October 10–19, 2002.
- 39.Cong FS, Zhang YR, Sheng HC, Ao ZH, Zhang SY, et al. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp Ther Med. 2010;1:277–283. doi: 10.3892/etm_00000043. [DOI] [PMC free article] [PubMed] [Google Scholar]


