Abstract
Failed therapies directed against MMPs in cancer patients may be attributed, in part, to lack of diagnostic tools to differentiate between pro- and active MMPs, which indicate whether a treatment is efficacious or not. Since galectin-3 is cleavable in vitro by MMPs, we have developed differential antibodies recognizing its cleaved and non-cleaved forms and tested their clinical utilization as a surrogate diagnostic marker for the presence of active MMPs in growing breast cancers.
Wild type and cleavage resistant galectin-3 were constructed and expressed in galectin-3 null human breast carcinoma cells (BT-549). Tumorigenic and angiogenic potential of the clones was studied by injections into nude mice. MMP-2, -9, full length and cleaved galectin-3 were localized in the xenografts by immunohistochemical analysis of paraffin embedded sections using specific antibodies. Activities of MMP-2/9 were corroborated by in situ zymography on frozen tissue sections. Galectin-3 cleavage was demonstrated in vivo by differential antibody staining and co-localized with predicted active MMPs both in mouse xenografts and human breast cancer specimens. In situ zymography validated these results. In addition, BT-549 cells harboring non-cleavable galectin-3 demonstrated reduced tumor growth and angiogenesis as compared with the wild type.
We conclude that galectin-3 cleavage is an active process during tumor progression and could be used as a simple, rapid and reliable surrogate marker for MMPs’ activities in growing breast cancers.
Keywords: Galectin-3, MMP activity, breast cancer, diagnostic tool
Introduction
Matrix metalloproteinases (MMPs) are a family of Zn–dependent proteinases that can be divided into 5 groups based on substrate specificity. They remodel extra-cellular matrix (ECM) components and cleave a broad range of cell surface proteins resulting in substrate degradation in areas of cell-matrix contact, thus affecting various cellular activities. Certain aspects of MMPs involvement in tumor metastasis such as angiogenesis, invasion, and establishment of metastatic foci, have received extensive attention resulting in an overwhelming amount of data concerning critical roles of MMPs in cancer (1). The gelatinases MMP-2 and-9, which specifically degrade collagen IV, are important for initiation and development of tumor vascularization (2,3). Dependency of tumor angiogenesis on the activity of these MMPs renders this step a likely target of synthetic MMP inhibitors.
The search for MMP inhibitors with possible anti-cancer efficacy is a nearly three-decade endeavor and an ideal effective inhibitor is yet to be found (Reviewed by (4,5). Possible reason(s) for this failure include broad MMP sub-type selectivity and toxicity as well as the diversity of MMP biology. MMPs have been shown to enhance angiogenesis by recruiting pericytes (6), releasing ECM bound angiogenic growth factors (7), exposing cryptic pro-angiogenic integrins binding sites (8,9), and cleaving endothelial cell-cell adhesion molecules (8,10). MMPs can also contribute negatively to angiogenesis through the generation of endogenous angiogenesis inhibitors by proteolytic cleavage of collagen and plasminogen and by modulating cell receptor signaling by cleaving off their ligand-binding domains (11,12). MMPs are synthesized as inactive pro-enzymes, which are activated by proteolytic cleavage of the pro-peptide domain (13). However, to date, there is no simple diagnostic tool to distinguish between active and non-active MMPs in vivo. To search for an anti-cancer inhibitor, which must possess selectivity against the MMP subtype critically important in relation to temporal progression of metastasis as well as with degradation of the matrix, the foremost requirement is to be able to differentiate between the active and the proactive form of MMP in the tissue. Two techniques are currently being used to evaluate MMP activity in tumors: MMP targeting probes for in vivo imaging (14) and in situ zymography (15–17). The results obtained from in vivo imaging have yet to be validated by biochemical or functional methodologies (14). In situ zymography is difficult to analyze due to imprecise localization, broad range of targets and need of adequate controls to validate specificity and efficacy. Moreover, it is only applicable to fresh frozen specimens. In the present manuscript we provide evidence that cleavage of galectin-3 can be used as a novel surrogate diagnostic marker for the activity of MMP-2/9 in cancer tissues, which can be easily analyzed by differential immuno-staining on paraffin-embedded specimens as well as fresh frozen sections providing a wider range and ease of use.
Galectin-3, a ~30 kDa chimera carbohydrate-binding protein belonging to the galectin gene family is composed of three distinct structural motifs, an amino terminal domain consisting of 12 amino acid residues, preceding an amino terminal half of collagen-like sequence containing Pro-Gly-Tyr tandem repeats and a sugar-binding carboxy terminal half (18–20). The collagen-like domain of galectin-3 is susceptible to rapid and efficient cleavage by MMPs (enzyme/substrate 1/10-100) in particular MMP-2, MMP-9 and MT1-MMP at the Ala62-Tyr63 peptide bond, resulting in the generation of a ~22kDa cleaved product (19).
Although the N-terminal region of human galectin-3 contains five Ala-Tyr repeats, the Ala62-Tyr63 peptide bond is the only site that is sensitive to MMP-2/9 cleavage (19). The Ala62-Tyr63 is followed by histidine whereas the other sites are followed by proline. This suggests that structure around the MMP cleavage site may be important in determining its availability for enzymatic degradation. Galectin-3 is implicated in different cell lineages at different developmental and pathological stages and is involved in cell growth, apoptosis resistance, adhesion, differentiation, inflammation, transformation, angiogenesis, invasion and metastasis (21,22). Galectin-3, although present in the cytoplasm, nucleus and the cell surface, is also secreted into the extra-cellular matrix, where it binds to the ECM proteins laminin, fibronectin and collagen IV. An additional band of ~22kda was observed in the cell lysates and a ~27kDa from the conditioned medium from the three-dimensional co-cultures of epithelial and endothelial cells (23). The 22 kDa polypeptide is also observed in the conditioned medium from various cell lines occasionally (unpublished data). It has been presumed that the active form of MMP may be responsible for the cleavage of galectin-3 in the extra-cellular environment. No experimental evidence was provided so far.
In this manuscript, we show for the first time, the cleavage of galectin-3 by MMPs in vivo and its co-localization with active MMPs. It is expected that this study will assist in monitoring MMP activity in cancer tissues and eventually in predicting the efficacy of MMP inhibitors in vivo. In addition, the approach described here may aid in the diagnosis and prognosis of the diseases involving MMP activity due to its ease of use, reliability and cost effectiveness.
Materials and Methods
Cell Lines, Antibodies and Recombinant Enzymes
The human breast cancer cell line BT-549 was a gift from Dr. Eric W. Thompson (St. Vincent’s Institute of Medical Research and University of Melbourne, Melbourne, Australia). The cells were maintained in Dulbecco’s Minimal Essential Medium (Invitrogen Corporation, Carlsbad, CA) containing 10% fetal calf serum, essential and non-essential amino acids (Invitrogen), vitamins, and antibiotics (Mediatech Cellgro Inc., Herndon, VA). 11-9-1-4 is a clone obtained by the transfection of wild type galectin-3 in BT-549 as described earlier (24). MCF10DCIS.com cells were developed at the Karmanos Cancer Institute (25) and maintained in DMEM/F12 (1:1) with 5% horse serum, 0.029M sodium bicarbonate, and 10mM HEPES. All cells were maintained in a humidified chamber with 95% air and 5% CO2 at 37°C. The cells were grown to near confluence and detached from the monolayer with 0.25% trypsin and 2mM EDTA for 1–2 min at 37°C. The use of cell lines was approved by the Human Investigation Committee, Wayne State University, Detroit, MI. A monoclonal antibody specific for full-length galectin-3 was isolated from the hybridoma TIB166 clone (ATCC, Manassas, VA). A custom made polyclonal antibody (anti-hL31) was prepared against the whole molecule, which recognized the full length as well as fragments of galectin-3 (19,26). Human recombinant pro-MMP-2 and pro-MMP-9 were expressed in HeLa cells infected with the appropriate recombinant vaccinia viruses and purified to homogeneity, as previously described (27). The zymogen were activated by incubation with 1mM p-aminophenylmercuric acetate (APMA) in a buffer containing 0.02% Brij-35, 5mM Tris HCl pH 7.5, 0.15mM NaCl, 5mM CaCl2 at 37oC for 30 min. Anti-CD34 antibodies were from Cell Sciences, (Canton, MA), Anti matrix-metalloprotease-9 and 2 antibodies were from Oncogene (Cambridge, MA).
Site Directed Mutagenesis
To generate various point mutations on galectin-3 human cDNA, Quick Change Site Directed Mutagenesis Kit (Strategene, LaJolla, CA) was employed using the primer pairs sense 5′-CTGCTGGGGGAGGGGGCTACCCAGG-3′ and antisense 5′-CCTGGGTAGCCCCCTCCCC CAGCAG-3′ for A33G; and sense 5′-GCGCCTACCCTGGAGCACCTGGAGC-3′ and antisense 5′-GCTCCAGGTGCTCCAGGGTAGGCGC-3′ for H64P. Briefly, pGEX-6P-2 vector containing human wild type galectin-3 cDNA fused with Glutathione-S-transferase (GST) was used as a template for PCR to generate A33G and H64P point mutations. After amplification, the template DNA was cleaved with Dpn-1 restriction enzyme and transformed into Escherichia coli (E.coli) XL1-Blue super-competent cells. Recombinant pGEX-6P-2/gal-3 mutant plasmids were purified and sequenced at the Macromolecular Core Facility of Wayne Sate University. Double mutations including both A33G and H64P were generated using the A64P primer pair on plasmid containing the A33G mutation.
Protein Purification and Cleavage by MMP-2 and-9
The mutant and wild type galectin-3 proteins were isolated as GST fusion proteins using the manufacturer’s instructions (GE Healthcare Biosciences Corp., Piscataway, NJ). Briefly, E. coli containing the desired plasmid were grown to log phase and protein expression was induced by adding 0.1mM IPTG. After 4hr, the bacteria were centrifuged and the pellet was sonicated in 1xPBS. After solubilization of the proteins with 1% Triton X-100, the extract was centrifuged and the supernatant was incubated with slurry of Glutathione Sepharose 4B with gentle agitation to bind the fusion protein to the slurry. Galectin-3 was separated from the fusion protein by incubation with Prescission Protease (GE Healthcare Biosciences Corp.) and isolated by centrifugation.
The purified protein was incubated with activated recombinant MMP-2 and MMP-9 at a molar ratio of 1:10 for 30 min and separated on a 12.5% polyacrylamide gel and analyzed by Western blot analysis using polyclonal anti-galectin-3 antibodies to detect full length as well as cleaved fractions of galectin-3.
Stable Transfection of Galectin-3 Mutants
To analyze the biological significance of these substitutions in the context of galectin-3 mediated functions, the coding sequence of galectin-3 containing mutations at A33G, H64P and A33G/H64P was removed from the pGEX-6P-2 vector by restriction digestion and placed into pCNC10 expression plasmid containing the cytomegalovirus early promoter and a dominant selection marker, G418 (24). The orientation of the insert was determined by restriction mapping and transfected in the non-galectin-3 expressing non-tumorigenic breast cancer cell line BT-459 with either the control (pCNC10 vector) or the pCNC10-mutated galectin-3 construct by LipofectAMINE reagent (Life Technologies Inc., Gaithersburg, MD) according to the manufacturer’s protocol. After 48 hr 500 μg/ml G418 (Invitrogen, Carlsbad, CA) was added to the cultures for 14 days to obtain stable transfected clones. Single cell clones were expanded and galectin-3 expression was determined by Western blot analysis. From each transfection, the clone with highest galectin-3 expression was selected. The resulting clones were given the nomenclature of M33, M64, M33+64, vector and 11-9-1-4 for A33G, H64P, A33G+H64P, vector alone and wild type galectin-3 transfections respectively.
Western Blot Analysis
1×106 cells were seeded in 100 mm petri dishes. The cells were trypsinized, lysed and equivalent numbers of cells (1×105) or equal amounts of total protein were subjected to SDS-PAGE and Western blot analysis with a 1:500 dilution of TIB166 or 1:2000 dilution of anti-galectin-3 polyclonal antibody. Blots were also immunoreacted with a 1:5000 dilution of anti-tubulin mouse polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) to normalize for variation in protein loading.
Tumor Growth in Nude Mice
2×106 cells suspended in Matrigel were injected into NCR nu/nu mice, obtained from Taconic (Germantown, NY) into the mammary fat pad region subcutaneously on both sides in two groups of 6 mice each, respectively. Tumor growth was measured twice a week and the tumor volumes were calculated using the formula: volume= length × width × width/2. The xenografts were harvested at 35 or 56 days as described. The tumors were weighed, fixed with 10% buffered formalin, and processed for immunohistochemical staining. As the BT-549 cells transfected with galectin-3 formed undifferentiated tumors in nude mice, MCF10 DCIS.com xenografts and human DCIS were used to analyze if differential distribution of galectin-3 could also be detected in differentiated tumors. The DCIS xenografts were obtained similarly by injecting MCF10 DCIS.com cells (25,28). After 28 days the xenografts were harvested, half of the tumor was fixed with buffered formalin, while the other half was fixed with 2-methylbutane in liquid nitrogen. The human DCIS serial sections were obtained from Karmanos Cancer Institute tissue core. The infiltrating ductal carcinoma sections were part of a breast cancer progression tissue array (BR480) from U.S. Biomax (Rockville, MD). The animal experiments were performed according to the guidelines provided by the Animal Investigation Committee, Wayne State University.
Immunohistochemical Analysis
Four μm tissue sections were deparafinized, rehydrated and microwaved on high 2 times for 5 min each in 1mM sodium citrate buffer, pH 6.0. The sections were washed three times in PBS and blocked with Super Block (Skytek Laboratories, Logan, UT) for 10 min. Sequential sections were incubated with primary antibodies (anti-CD34, anti-galectin-3 monoclonal, anti-galectin-3 polyclonal, and anti MMP-2 and -9) at 4°C overnight at the suitable dilution. The sections were washed 3 times for 10 min each in PBS and linked with the appropriate host secondary antibodies (Vector Laboratories, Burlingame, CA). The secondary antibodies were tagged with Avidin biotinylated horseradish peroxidase, colorized with 3′-3′-diaminobenzidine and counterstained with hematoxylin. Visualization, and documentation were accomplished with an OLYMPUS BX40 microscope supporting a Sony DXC-979MD 3CCCD video camera and stored with the M5+ micro-computer imaging device (Interfocus, Cambridge, UK).
In situ Zymography
In situ zymography was performed on the fresh frozen DCIS xenografts as described by Mook et. al.(29). In brief, 8 micron thick cryosections were air dried, rehydrated with PBS for 5 min and overlaid with a solution of 50ug/ml fluorescein-labeled gelatin (DQ gelatin; Molecular Probes, Eugene, OR), 1% w/v low melting temperature agarose (BioWhittaker Molecular Applications, Rockland, ME) and 5ug/ml DAPI (Molecular Probes) in PBS in the presence or absence of 2mM EDTA and incubated on ice for 15 min followed by incubation in a humidified chamber at 37°C for 2 hr. Protease catalyzed hydrolysis of the heavily labeled and totally quenched DQ Gelatin relieves the intra-molecular self quenching, yielding brightly fluorescent peptide, which was visualized by using imaging microscope.
TUNEL Assay
TdT mediated dUTP Nick End Labeling (TUNEL) assay was performed to visualize the fragmented DNA directly by fluorescence microscopy in paraffin embedded sections using DeadEnd Fluorometric TUNEL system (Promega, Madison, WI). Briefly, the paraffin sections were deparafinized and permeabilized with proteinase K. Fluorescein 12-dUTP was then catalytically incorporated into the 3′-hydroxyl ends, which are exposed in fragmented DNA of the apoptotic cells using the enzyme terminal deoxynucleotidyl transferase (TdT). The sections were then counterstained with propidium iodide, which, in contrast to flourescein-12-UTP stains both apoptotic and non-apoptotic cells.
Statistical Analysis
The experiments conducted to measure growth of the tumors were repeated twice with multiple animals. We used one-way ANOVA with Tukey’s multiple comparison test to calculate the statistical significance when the number of readings was the same and Dunnett’s multiple comparison test using Prism software in the experiments where the number of readings was not the same. All statistical tests were two sided, and p values less than 0.05 were considered statistically significant.
Results
Cleavage of Galectin-3 Mutants by MMP-2 and –9
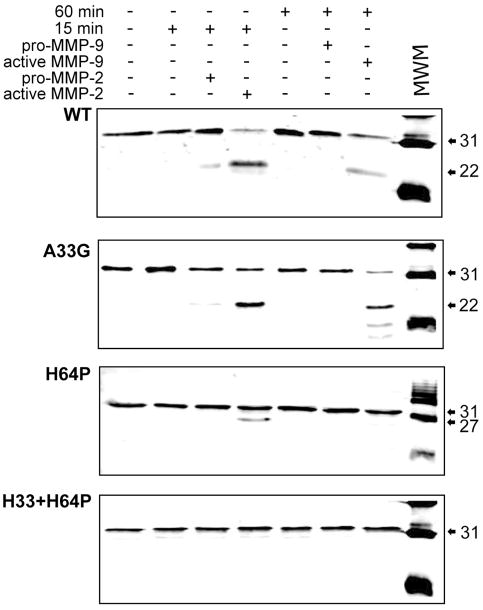
First we identified the amino acids that were important in determining the susceptibility of galectin-3 to MMPs cleavage and mutated them. Five galectin-3 mutants A63G, A62V, Y63H, Y63P and H64P were generated by amino acid substitution around the MMP cleavage site. Only the two mutants Y63P and H64P showed resistance to cleavage at the Ala62-Tyr63 site, while exposing a new cleavage site producing a ~27-kDa polypeptide. N-terminal sequencing revealed that the 27-kDa product displays an N-terminus starting with Ala33 indicating that the alternate cleavage site occurs at the Gly32-Ala33 peptide bond. We performed amino acid substitutions at this new site and obtained clones displaying A33G or H64P substitutions alone or in combination. Recombinant proteins were incubated with purified active MMP-2 or MMP-9 for 15 and 60 min respectively (Fig 1). Wild type and A33G galectin-3 produced a polypeptide of ~22 kDa consistent with cleavage at Ala62-Tyr63. The H64P mutant produced a ~27-kDa polypeptide upon incubation with MMP-2 indicating that it is cleaved at Gly32-Ala33. Interestingly, MMP-9 did not cleave the H64P mutant. Double mutation at A33G and H64P showed complete resistance to cleavage by both MMP-2 and MMP-9. The cleavage products were not seen when galectin-3 was incubated alone or with pro-MMPs.
Figure 1.
Cleavage of recombinant galectin-3 by MMP-2 and -9: One ug protein was incubated at 37°C for the indicated time with activated or pro enzyme and separated on a 15% polyacrylamide gel.
Expression of Galectin-3 in the Mutant Clones
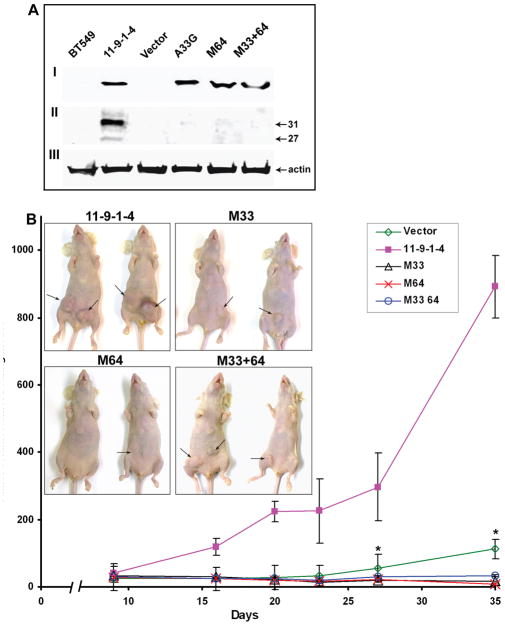
Galectin-3 was detected in the total cell lysates (Fig 2AI) of all the BT-549 clones transfected with wild type and mutant galectin-3. Galectin-3 expression was also seen in the nucleus and cell surface (not shown). However, secretion of full length as well as the ~27-kDa polypeptide was detected only in 11-9-1-4 (Fig 2AII).
Figure 2.
A: Expression of galectin-3 in BT-549 cell transfectants: I: Galectin-3 expression in total cell lysates. Fifty μg total protein was loaded per lane. II: Galectin-3 expression in the conditioned medium. 50μg protein was loaded per lane. Note the presence of an additional band of ~27 kDa in 11-9-1-4. III: Actin was used as a loading control for cell lysate.
B: Tumorigenicity of various BT-549 transfected cell clones in nude mice: Each point is the average of the tumor volume in tumor bearing mice. The bars represent standard error. p values with respect to 11-9-1-4 on day 35 were 0.0071, 0.0036, 0.0036 and 0.0042 and on day 27 were 0.0099, 0.0048, 0.0050, and 0.0060 respectively for vector, M33, M64 and M33+64 respectively. Inset: pictures of representative mice at the time of sacrifice. Arrows indicate tumors.
Tumorigenicity of Mutant Clones
2X106 cells were from each clone were injected in the mammary fat pad region of nude mice to study the effect of galectin-3 mutation on tumor take and growth. By 5 weeks there was ~60% tumor take in the wild type transfected cell clone (11-9-1-4) and only 10, 3, 6, and 23% in M33, M64, M33+64, and vector transfected cells respectively (average of two experiments). Average tumor volume in tumor bearing mice of the representative experiment through day 35 is depicted in Figure 2B. Due to increased tumor burden, mice injected with 11-9-1-4 cells were sacrificed after 5 weeks, other groups were sacrificed after 8 weeks. The average tumor weight at the time of sacrifice in 11-9-1-4 was 1.7 +/−1.44 gm and 0.7+/−0.07, 0.6+/−1.13, 0.05+/−0.03 and 0.5+/−0.98 gm with p values 0.020, 0.018, 0.0006 and 0.011 respectively for vector, M33, M64 and M33+64.
Immunohistochemical Analysis of Xenografts
Tumor specimens were sectioned and stained with anti-CD34 antibody to visualize angiogenesis (Fig. 3A). The 5 week tumor of the 11-9-1-4 cell clone depicted many fully formed blood vessels with lumens (top right), whereas vector and M33+64 mutant cells’ tumor of 8 weeks showed a few endothelial precursor cells (fibrocytes) that were positive for CD34, indicating the slow initiation of angiogenesis (top left and bottom right). No tumors could be obtained from M64 mutant cell clone. No blood vessels or precursor cells were seen in M33 xenograft even after 8 weeks (bottom left).
Figure 3.
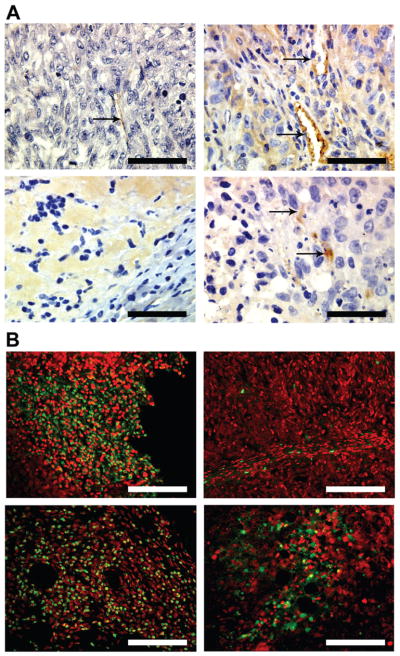
Figure 3A: Angiogenesis in the xenografts: The sections were stained with anti-CD34 to identify the blood vessels. Top left: Vector; Top right: 11-9-1-4; Bottom left: M33; Bottom right: M33+64. Arrows indicate positive staining. Bar: 200 micron.
B: TUNEL assay in the xenografts: Top left: Vector; Top right: 11-9-1-4; Bottom left: M33; Bottom right: M33+64. Green color represents the apoptotic nuclei. Bar: 100 micron
The xenografts obtained from vector alone, M33 and M33+64 mutant cell clones (Fig 3B top left, bottom right and bottom left) showed a very high incidence of apoptosis using TUNEL assay, whereas no significant apoptosis could be detected in BT-549 wild type 11-9-1-4 tumors (Fig 3B top left).
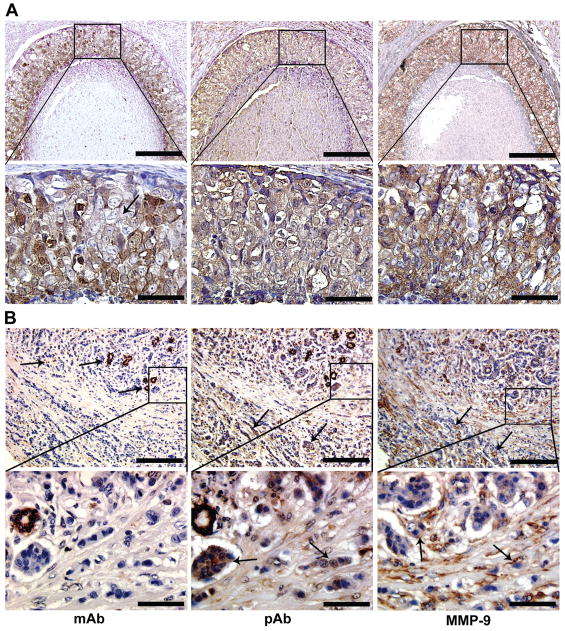
Galectin-3 Cleavage as Surrogate Marker for MMP Activity in Tumor Xenografts
Anti-MMP-2/9 and anti-galectin-3 monoclonal and polyclonal antibodies were used to visualize whether cleaved galectin-3 could be identified in the xenografts (Fig 4). In 11-9-1-4 xenograft, full length galectin-3 (using monoclonal antibody) is present in tumor cells localized in the periphery of the tumor mass (arrow), whereas cleaved galectin-3 (using polyclonal antibody) as well as MMP-9 are localized in tumor cells present in the center of the xenograft (arrowheads). M33+64 xenograft, on the other hand did not show differences in the distribution of galectin-3 using either monoclonal or polyclonal antibody. Because the mutant galectin-3 is resistant to cleavage, there was no indication of its cleavage despite of the presence of MMP-9 throughout the section. MMP-2 also showed a staining pattern similar to MMP-9 (not shown). Immuno-staining with mouse, rat and rabbit IgG as negative controls for MMP-2/-9, mono and polyclonal galectin-3 respectively did not show any staining (not shown).
Figure 4.
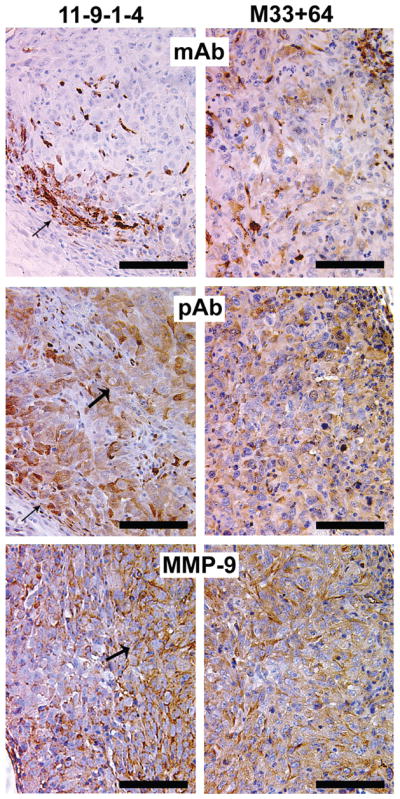
Distribution of full length and cleaved galectin-3 in the xenografts: Full length galectin-3 is localized on the peripheral cells of the 11-9-1-4 xenograft (arrow), cleavage products are seen in peripheral (arrow) as well as luminal (wider arrow) cells of the section, MMP-9 is localized more intensely in the luminal cells of the section (wider arrow), where cleaved form of galectin-3 is predominant. In M33+64, monoclonal (mAb), polyclonal antibody (pAb) as well as anti-MMP-9 showed a more uniform distribution throughout the section. Bar: 100 micron
Galectin-3 Cleavage as Surrogate Marker for MMP Activity in Ductal Carcinoma in Situ (DCIS) xenografts and Human Breast Cancer Tissue
In DCIS xenografts, intact galectin-3 was localized mainly in the cytoplasm of epithelial cells in focally intense clusters (Fig. 5 top left) as recognized by the monoclonal antibody. Some cells in the stroma also stained positive with this antibody (arrow). Cleaved galectin-3 (top right) and MMP-9 (bottom left) was distributed throughout the ducts and the stroma. In situ zymography on fresh frozen DCIS xenograft sections showed no gelatinolytic activity in the epithelial ducts, whereas positive activity was seen in the stroma (Fig 5 bottom middle), which was abrogated when incubated with EDTA, a non-specific MMP inhibitor (bottom right).
Figure 5.
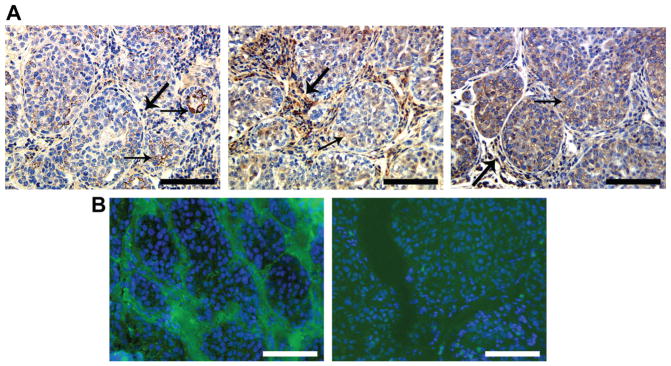
Distribution of full length and cleaved galectin-3 in MCF10 DCIS.com xenograft: A: paraffin embedded; B: fresh frozen. A: left: monoclonal anti galectin-3 antibody shows mainly epithelial and some stromal labeling (arrows); middle: polyclonal anti-galectin-3 antibody shows some epithelial (arrow) and strong stromal labeling (wider arrows); right: anti-MMP-9 antibody showing epithelial (arrow) and stromal (wider arrow) labeling. B: left: In situ zymography in the fresh frozen section showing stromal activity (green color), nuclei were stained with DAPI (blue color); right: In situ zymography in the presence of 20mM EDTA. Bar: 100 micron.
In the human DCIS, full-length galectin-3 was more intensely localized towards the luminal side, and many cells on the stromal side of the duct were devoid of the protein (Fig 6A left). Cleaved protein (middle) and MMP-9 (right) were distributed uniformly in all the cells indicating that many cells adjacent to stroma have cleaved galectin-3 and active MMP-2 and -9. In the infiltrating ductal carcinoma the presence of full-length galectin-3 was seen in the ductules that still maintained a normal morphology (Fig 6B left) and a positive reactivity for the cleaved galectin-3 in normal ductules as well in the invasive cells and stroma (middle). MMP-9, on the other hand, was not expressed in the normal ductules, but localized in the stroma and clusters of invasive cells (co-localizing with the cleaved galectin-3) (right) implying that it is in the active form.
Figure 6.
Distribution of full-length and cleaved galectin-3 in human breast cancer: A: DCIS; B: Infiltrating ductal carcinoma. In human DCIS (A), full-length galectin-3 is concentrated more towards the lumen of the duct, many cells towards the stromal end are devoid of the protein (mAb; arrow). The cleaved galectin-3 fragments (pAb) as well as MMP-9 could be seen in all cells. In infilterating ductal carcinoma (B), full-length galectin-3 is seen in ductules that maintained a normal morphology (arrow) and in some invasive cells (mAb); cleaved galectin-3 (pAb) and MMP-9 are seen in invasive cell clusters and stroma (arrows). Lower panels in A and B represent box in the upper panels. Bar: 50 micron (upper panels); 200 micron (lower panels).
DISCUSSION
The strong causal relationship between MMPs over-expression and a wide range of tumorigenic events, including early carcinogenesis, tumor growth, tumor invasion, angiogenesis and metastasis makes them attractive therapeutic targets. Consequently, several broad range inhibitors (MMPI) advanced to phase III clinical trials in patients with advanced cancer. Unfortunately, the trials failed to reach their end points of increased survival (5,30,31), probably due to lack of adequate target validation and identification of in vivo substrate(s), among other factors. Indeed, to date, no diagnostic marker is available to distinguish between latent and active MMP(s) to monitor tumor MMPs’ response to treatment. Our results demonstrate that cleaved galectin-3 co-localized with active MMP-2 and MMP-9 and therefore, could be used as a novel diagnostic marker for MMP activity. In paraffin embedded DCIS.com xenograft, epithelial cells stained for intact galectin-3, while the stromal cells stained only for cleaved galectin-3, where it is localized following its secretion and cleavage. This hypothesis was validated using in situ zymography on fresh frozen tissue. Whereas total MMP-2/9 showed positive staining in epithelial and stromal cells, the active MMPs were identified only in the stroma by in situ zymography and co-localized with cleaved galectin-3 attesting to the validity of the use of galectin-3 cleavage as a surrogate marker for MMPs activities. The localization of MMP activity in the stroma of the xenografts confirms the earlier observation of Stuelton et al in CA1A xenografts and co-cultures of fibroblasts and CA1A cells (17). The differential staining of full length versus cleaved galectin-3 could also predict the activity of MMP in human DCIS and infilterating ductal carcinoma. Many cells adjacent to the stroma exhibited the presence of cleaved galectin-3 and probably active MMP-2 and -9 in DCIS indicating their possible invasive phenotype. Consistent with this observation, the invasive cell clusters and stroma were positive for cleaved galectin-3 and active MMP-9 in the infilterating ductal carcinoma, whereas the ductules that maintained a normal morphology expressed only full-length protein detected by monoclonal as well polyclonal anti-galectin-3 antibodies.
The diverse effects of MMP-2 and -9 cleavage on many proteins have been reported e.g. MMP-9 cleaves the pro-angiogenic cytokine IL-8, increasing its activity ten-fold, as well as degrading and inactivating the angiogenic inhibitor platelet factor-4 (32). On the other hand, MMP-2 cleaves the FGF receptor 1 (FGFR1), releasing the soluble ectodomain of FGFR1 that can still bind FGFs, but lacks signaling capacity (33). Proteolytic processing of some ECM substrates such as laminin 5 exposes cryptic epitopes (34,35) and new molecules with properties that are distinct from their precursor protein (36). In vitro cleavage of galectin-3 by MMP-2/-9 resulting in a ~22kDa product has been reported, but its occurrence in vivo, and its biological significance have yet to be elucidated.
It was reported that loss of its N-terminus 62 amino acids leads to increased binding of the ~22kDa fragment to endothelial cells (23) and laminin (26). Injection of the cleaved galectin-3 peptide containing 108-250 amino acids into mice bearing MDA-MB-435 tumors resulted in loss of tumor growth and metastasis because of competitive inhibition of carbohydrate binding (37). It was suggested that loss of the N-terminus reduces self association of galectin-3 and thereby abrogates the biological properties dependent on such association e.g. formation of tumor cell emboli in vivo and hemagglutination in vitro (26). Yang et. al., however showed that only the C-terminus of galectin-3 could self-associate in the absence of its saccharide ligand (38), raising more questions about role of the collagen-like domain in galectin-3 and how its interaction with MMP-2/-9 affects biological functions of galectin-3. To answer these questions, we created cleavage resistant galectin-3. The mutations rendered the recombinant protein resistant to cleavage at that particular site, but the protein cleaved at the remaining site.
Single or double mutations at the MMP cleavage sites did not alter cellular distribution of galectin-3, but inhibited its secretion. Intracellular galectin-3 regulates pathways including mRNA splicing reactions, cell growth, cell cycle and apoptosis (38–40); reviewed by (41), while extra-cellular galectin-3 modulates cellular adhesion and signaling, immune response, angiogenesis and tumorigenesis (21,42–46) by binding to cell surface glycoproteins such as integrin subunits (12,26) or to extra-cellular matrix glycoproteins such as laminin, fibronectin and collagen IV (21,47).
We have shown earlier that over-expression of galectin-3 in non-tumorigenic breast or colon cancer cell lines induced tumorigenicity and metastasis, whereas its suppression resulted in loss of tumorigenicity and metastasis (24,48,49). When the clones were injected in nude mice, the wild type clone showed a rapid increase in tumor volume over 35 days, but cleavage resistant clones or vector transfected cell clones showed a significantly low tumor take and tumor growth. The galectin-3 mutant clones showed lack of angiogenesis and induction of apoptosis in the xenografts. It was proposed earlier that secreted galectin-3 binds to cell surface receptors on endothelial cells inducing their migration and morphogenesis leading to angiogenesis (50). Absence of secreted protein in the mutated clones may be the reason for inability of these cells to induce blood vessel formation. The xenografts from cleavage resistant cells also showed many more apoptotic cells compared to BT-549 11-9-1-4 cells. Even though the cellular localization of galectin-3 was not affected in mutant clones, on the cell surface however, the mutant proteins could not be cleaved and affect processes like chemo-invasion, chemotaxis, tumor growth and angiogenesis. It is possible that the cleaved fragment is responsible for the tumorigenic potential of the cells. Once the surface protein is cleaved, the carbohydrate binding domain remains attached to the surface receptor, and the cleaved product is released into the extra-cellular matrix, where it may either interact with other extra-cellular proteins or may be internalized and interact with various signal transduction pathways.
To summarize, we report here that galectin-3 is cleaved in vivo by MMPs and this phenomenon could be used to distinguish between active and latent MMPs in the tumor, which could affect decision regarding therapeutic strategies and anti-MMP drugs efficacy.
Acknowledgments
Financial Support:
National Institute of Health R37CA46120-19 to A.R., RO1 CA-61986-11 and RO1 CA100475 to RF and Karmanos Cancer Institute’s Strategic Research Grant to A.R. and P.N.M.
References
- 1.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 2.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–51. [PubMed] [Google Scholar]
- 3.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsey ML. Novel strategies to delineate matrix metalloproteinase (MMP)-substrate relationships and identify targets to block MMP activity. Mini Rev Med Chem. 2006;6:1243–8. doi: 10.2174/138955706778742777. [DOI] [PubMed] [Google Scholar]
- 5.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–50. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 6.Chantrain CF, Shimada H, Jodele S, et al. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–86. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Rodriguez D, Petitclerc E, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–8. [PubMed] [Google Scholar]
- 10.Herren B, Levkau B, Raines EW, Ross R. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell. 1998;9:1589–601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9) J Biol Chem. 1997;272:28823–5. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 12.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–10. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 13.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 14.Van de Wiele C, Oltenfreiter R. Imaging probes targeting matrix metalloproteinases. Cancer Biother Radiopharm. 2006;21:409–17. doi: 10.1089/cbr.2006.21.409. [DOI] [PubMed] [Google Scholar]
- 15.Chiappori AA, Eckhardt SG, Bukowski R, et al. A phase I pharmacokinetic and pharmacodynamic study of s-3304, a novel matrix metalloproteinase inhibitor, in patients with advanced and refractory solid tumors. Clin Cancer Res. 2007;13:2091–9. doi: 10.1158/1078-0432.CCR-06-1586. [DOI] [PubMed] [Google Scholar]
- 16.Wright JL, Tai H, Wang R, Wang X, Churg A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L125–33. doi: 10.1152/ajplung.00539.2005. [DOI] [PubMed] [Google Scholar]
- 17.Stuelten CH, DaCosta Byfield S, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J Cell Sci. 2005;118:2143–53. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 18.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 19.Ochieng J, Fridman R, Nangia-Makker P, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 20.Gong HC, Honjo Y, Nangia-Makker P, et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–45. [PubMed] [Google Scholar]
- 21.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Nangia-Makker P, Akahani S, Bresalier R, Raz A. The role of Galectin-3 in tumor metastasis. Harwood Academic Publishers; 2000. [Google Scholar]
- 23.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–41. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangia-Makker P, Thompson E, Hogan C, Ochieng J, Raz A. Induction of tumorigenicity by galectin-3 in a non-tumorigenic human breast carcinoma cell line. Int J Oncol. 1995;7:1079–87. doi: 10.3892/ijo.7.5.1079. [DOI] [PubMed] [Google Scholar]
- 25.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS. com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92:1185–6. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- 26.Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 27.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272:29975–83. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 28.Tait LR, Pauley RJ, Santner SJ, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer. 2007;120:2127–34. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 29.Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–9. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- 30.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 31.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 32.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–9. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 33.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93:7069–74. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenk S, Hintermann E, Bilban M, et al. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirila E, Sharabi A, Salo T, et al. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem Biophys Res Commun. 2003;303:1012–7. doi: 10.1016/s0006-291x(03)00452-2. [DOI] [PubMed] [Google Scholar]
- 36.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–60. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 37.John CM, Leffler H, Kahl-Knutsson B, Svensson I, Jarvis GA. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin Cancer Res. 2003;9:2374–83. [PubMed] [Google Scholar]
- 38.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995;92:1213–7. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–6. [PubMed] [Google Scholar]
- 41.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 42.Liu FT, Frigeri LG, Gritzmacher CA, Hsu DK, Robertson MW, Zuberi RI. Expression and function of an IgE-binding animal lectin (epsilon BP) in mast cells. Immunopharmacology. 1993;26:187–95. doi: 10.1016/0162-3109(93)90034-n. [DOI] [PubMed] [Google Scholar]
- 43.Hughes RC. The galectin family of mammalian carbohydrate-binding molecules. Biochem Soc Trans. 1997;25:1194–8. doi: 10.1042/bst0251194. [DOI] [PubMed] [Google Scholar]
- 44.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–8. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinovich GA, Riera CM, Landa CA, Sotomayor CE. Galectins: a key intersection between glycobiology and immunology. Braz J Med Biol Res. 1999;32:383–93. doi: 10.1590/s0100-879x1999000400002. [DOI] [PubMed] [Google Scholar]
- 46.Nangia-Makker P, Baccarini S, Raz A. Carbohydrate-recognition and angiogenesis cancer and metastasis reviews. 2000;19:51–7. doi: 10.1023/a:1026540129688. [DOI] [PubMed] [Google Scholar]
- 47.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246:788–91. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 48.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–8. [PubMed] [Google Scholar]
- 49.Bresalier RS, Mazurek N, Sternberg LR, et al. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology. 1998;115:287–96. doi: 10.1016/s0016-5085(98)70195-7. [DOI] [PubMed] [Google Scholar]
- 50.Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]