Abstract
Obesity is a disease of oxidative stress (OS). Acute hyperoxia (breathing 100% O2) can evoke coronary vasoconstriction by the oxidative quenching of nitric oxide (NO). To examine if weight loss would alter the hyperoxia related-coronary constriction seen in obese adolescents, we measured the coronary blood flow velocity (CBV) response to hyperoxia using transthoracic Doppler echocardiography before and after a 4-week diet-and-exercise regimen in 6 obese male adolescents (age 13–17 yrs, BMI, 36.5 ± 2.3 kg/m2). Six controls of similar age and BMI were also studied. The intervention group lost 9 ± 1% body weight, which was associated with a reduced resting heart rate (HR), reduced diastolic blood pressure (BP), and reduced rate pressure product (RPP, all P<0.05). Before weight loss, hyperoxia reduced CBV by 33 ± 3%. After weight loss, CBV only fell by 15 ± 3% (P <0.05). In the control group, CBV responses to hyperoxia were unchanged during the two trials. Thus weight loss: 1) reduces HR, BP, and RPP; and 2) attenuates the OS related-coronary constrictor response seen in obese adolescents. We postulate that: 1) the high RPP before weight loss led to higher myocardial O2 consumption, higher coronary flow and greater NO production, and in turn a large constrictor response to hyperoxia; and 2) weight loss decreased myocardial oxygen demand and NO levels. Under these circumstances, hyperoxia induced vasoconstriction was attenuated.
Keywords: obesity, weight loss, coronary circulation
Introduction
In the Western world, obesity is taking on epidemic proportions. Data from the Center for Disease Control and Prevention indicates that 34.4% of the American adults are overweight and 33.9% are obese (Ogden and Carroll 2010). Alarmingly, childhood obesity is increasing (Strauss and Pollack 2001) and are associated with impaired blood vessel function (Steinberger and Daniels 2003; Woo et al. 2004). The magnitude of this problem is amplified by the fact that obesity in childhood frequently continues into adulthood (Magarey et al. 2003) where cardiovascular and respiratory morbidity became a major health care challenge (Eisenmann et al. 2005; Singhal 2005).
Overweight and obese children and adolescents have marked endothelial dysfunction, which is an early step in the development of atherosclerosis (McGill et al. 2002). It is often inferred that the reduction in endothelial function is the result of a decrease in nitric oxide (NO) (Higashi et al. 2001). Decreased NO would result in impaired vasodilation and an increase in vascular resistance that could predispose individuals to ischemia and perhaps cardiac disease (Sundell 2005). In obesity, it has been found that decreased NO is related to an increase in oxidative stress. Evidence is mounting that obesity is a state of chronic oxidative stress that plays a central role in endothelial dysfunction (Davi et al. 2002; Roberts et al. 2002). Oxidative stress is an imbalance between tissue reactive oxygen species and antioxidants, and may be a major mechanism underlying obesity-related comorbidities (Higdon and Frei 2003).
The function of the endothelium is usually assessed in either the coronary or peripheral circulation. Coronary artery endothelial function is most commonly determined by the intra-coronary infusion of acetylcholine, which can evoke the release of NO and hence coronary artery vasodilation (Quyyumi et al. 1995). Recent studies from this lab have shown that breathing 100% O2 can evoke coronary vasoconstriction by the oxidative quenching of NO (McNulty et al. 2005; Momen et al. 2009). Thus, the administration of 100% oxygen for short periods of time may be a useful method to test the endothelial cell’s capacity to physiologically produce NO. Technological advances in transthoracic Doppler echocardiography have provided measurement of coronary blood flow velocity at the distal portion of the left anterior descending coronary artery (LAD) and are useful in the noninvasive coronary assessment of vascular tone. Coupling 100% O2 with Doppler echocardiography makes it possible to noninvasively assess coronary NO mediated endothelial function in response to hyperoxia in human subjects.
Obesity is associated with increases in blood pressure (BP) and heart rate (HR), which determine myocardial O2 consumption (Duncker and Bache 2008; Feigl 1983). This increase in myocardial oxygen demand must be met primarily with a rise in coronary blood flow since coronary bed oxygen extraction is relatively fixed. We suspect that this increase in flow leads to long term adjustments that augment vascular NO production (Duffy et al. 1999). Weight loss may lead to a fall in HR and BP, which in turn would lead to a reduction in the NO quenchable flow component. Accordingly, we hypothesized that hyperoxia-mediated coronary vasoconstriction would be attenuated after weight loss in obese adolescents. The results of this study confirm these hypotheses.
Methods
A total of twelve obese (BMI >95th percentiles for age) male adolescents 13–17 years of age were enrolled. To be eligible, children had to have no known medical illness and no medical cause for their obesity, no medication or vitamin supplementation. All of the subjects self-reported that they were weight stable (±2 kg) for the previous 6 months. The experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine. Informed consent was obtained from all subjects prior to testing. Subjects were divided into intervention and control groups.
Intervention (weight loss) group
Six subjects agreed to participate in a summer camp program, which was a four-week University sponsored session of weight management (Camp LION). The camp program was held by the Department of Pediatrics, Penn State Milton S. Hershey Medical Center. The program consisted of dietary management and exercise training. Dietary modification included personal dietary prescriptions that were monitored by a registered dietitian. These prescriptions were designed to provide an intake of 1800–2000 cal/day with fat constituting <30% of the calories. The exercise program included at least one-hour of structured aerobic activity per day (could be split up throughout the day). Additionally, the participants walked from their residence to the dining hall (1/2 mile each way or 3 miles/day).
Control (body weight stable) group
Six age-and weight-matched male adolescents comprised a control group.
Study Design
This study employed a 2 visit (pre-, post-intervention) by 2 time point (baseline, hyperoxia) repeated measures design with group (control, intervention) serving as a between-subjects factor. All subjects came to the laboratory on two occasions, separated by ~28 days (Visit 1 was Week 0, Visit 2 was Week 4). On these two experimental visits they underwent baseline measures and then a 10 min period of breathing 100% oxygen through a tight sealing facemask (hyperoxia).
Measurements
Anthropometric assessment
Body weight, BMI, waist circumference and hip circumference were measured on both Visit 1 and 2.
Coronary blood flow velocity (CBV) assessment
CBV measurements were performed using transthoracic echo/Doppler with a digital ultrasound system (iE33, Philips Ultrasound, Bothell, WA, USA). A variable frequency phased-array transducer (S8-1) was employed. As previously described (Gao et al. 2012a; Gao et al. 2012b; Muller et al. 2011), the transducer was placed in the midclavicular line in the fourth and fifth intercostal spaces in the left lateral decubitus position, from the apical 4-chamber view with the transducer rotated counterclockwise and angulated anteriorly, the distal potion of the LAD was first identified with color flow mapping of the LV apex. For color Doppler flow mapping, the velocity range was set at ±19cm/s. The color gain was adjusted to provide optimal imaging; after the distal portion of the LAD was identified, care was taken to place the transducer in a fashion that allowed for the acquisition of a long axis view of the LAD. With a sample volume (2.0 mm) positioned over the color signal in the LAD, we recorded CBV at the end of expiration before and during hyperoxia. BP and HR were recorded simultaneously. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv®3.0 to obtain coronary diastolic blood velocity. Beat-to-beat BP was recorded and analyzed with a PowerLab (ADInstruments). For each cardiac cycle, diastolic velocity and the corresponding diastolic BP were obtained. Subsequently, an index of CVR was calculated by dividing corresponding diastolic BP by CBV (cm/s). CVR was expressed in arbitrary units (Momen et al. 2009).
Echocardiographic examination
Complete echocardiography at rest was performed on all subjects in the initial study and at the end of the program. Parasternal long-and short-axis and apical 2 and 4 chamber views were used for evaluating left atrial and left ventricular dimension and function. Measurements were obtained from M- mode tracking: left ventricular end-diastolic (LVEDD) and end-systolic diameter (LVESD), septum and posterior wall diastolic thickness, fractional shortening (FS) = (LVEDD−LVEDSD)/LVEDD, and ejection fraction (EF) (Teichholz et al. 1976). Left ventricular mass was calculated by the Devereux formula (Devereux and Reichek 1977); conventional mitral inflow Doppler was used to measure transmitral E and A peak velocities, and E/A ratio was calculated.
HR was measured by electrocardiogram and BP using a Finometer (FMS, Arnhem, The Netherlands). Baseline BP was established by an automated sphygmomanometer (DinampCritikon, Tampa, FL) and the Finometer was adjusted to match that pressure. Rate-pressure product (RPP), as an index for myocardial oxygen consumption, was calculated as HR x systolic BP.
Data Analysis and Statistics
All statistical analyses were conducted using IBM SPPS 19.0. Normality was confirmed by the Kolmogorov-Smirnov test (i.e. P> 0.05 for all measurements). Anthropometric and baseline echocardiography data were compared between groups with unpaired t-tests (Table 1). For each dependent variable (HR, SBP, DBP, MAP, RPP, CBV), a 2 visit (pre-, post-intervention) by 2 time point (baseline, hyperoxia) repeated measures ANOVA was conducted and partial eta squared (ηp2) is presented. Group (control, intervention) was entered as a between-subjects factor. When significant interactions were present, post-hoc pairwise comparisons were performed and those that were clinically meaningful were reported. In order to compare RPP (an index of myocardial oxygen demand) to CBV (an index of myocardial oxygen supply), percent changes (baseline to hyperoxia) were also calculated (Muller et al. 2011). Intra- and inter-observer variability for measurements of coronary flow velocity was compared by simple linear regression. Data are presented as mean (SEM) and a P-value of < 0.05 was considered statistically significant.
Table 1.
Anthropometric measurement in control and intervention group
| Variables | Control group (N=6) | Intervention group (N=6) | |||
|---|---|---|---|---|---|
| Week 0 (Visit 1) | Week 4 (Visit 2) | Week 0 (Visit 1) | Week 4 (Visit 2) | % Decrease | |
| Age | 15 ± 1 | - | 16 ± 1 | - | - |
| Body weight (kg) | 105.3 ± 8.2 | 105.1 ± 8.7 | 115.4 ± 7.8 | 105.2 ± 6.7** | 8.7 |
| BMI | 32.5 ± 1.5 | 33.6 ± 1.4 | 36.4 ± 2.3 | 33.2 ± 2.0** | 8.8 |
| Waist circumference (cm) | 109 ± 5 | 109 ± 3 | 115 ± 4 | 109 ± 3** | 5 |
| Hip circumference (cm) | 119 ± 6 | 118 ± 4 | 124 ± 3 | 120 ± 3 | 2.6 |
| Waist-to-hip ratio | 0.93 ± 0.01 | 0.92 ± 0.02 | 0.93 ± 0.01 | 0.90 ± 0.01** | 2.4 |
Values are means±SE,
P< 0.05,
P< 0.01 week 0 vs. week 4.
Results
Baseline characteristics and anthropometric measurements of both the Intervention and Control groups are shown in Table 1. The groups were similar in age, weight, BMI, waist and hip circumferences. In the weight loss intervention group, the 4-week diet and exercise program significantly reduced body weight (P< 0.01) and body mass index (BMI; P< 0.01; see Table 1). There were no significant differences in echocardiographic measurements between the Intervention group and Control group; however, the weight loss/exercise program significantly decreased the baseline stroke volume, and cardiac output (Table 2; P< 0.01).
Table 2.
Echocardiography measurement in control and intervention group
| Control group (N=6) | Intervention group (N=6) | |||
|---|---|---|---|---|
| Week 0 (Visit 1) | Week 4 (Visit 2) | Week 0 (Visit 1) | Week 4 (Visit 2) | |
| LAD (cm) | 3.28 ± 0.15 | 3.32 ± 0.12 | 3.28 ± 0.10 | 3.37 ± 0.07 |
| IVSD (cm) | 0.98 ± 0.07 | 1.01 ± 0.06 | 1.07 ± 0.03 | 1.05 ± 0.03 |
| LVDD (cm) | 5.17 ± 0.13 | 5.08 ± 0.10 | 5.29 ± 0.08 | 5.13 ± 0.07 |
| LVPW (cm) | 1.04 ± 0.04 | 1.10 ± 0.03 | 1.07 ± 0.06 | 1.11 ± 0.03 |
| LV Mass (g) | 206.50 ± 12.19 | 205.83 ± 12.53 | 222.77 ± 9.52 | 213.62 ± 2.99 |
| FS (%) | 37.00 ± 2.37 | 35.83 ± 1.49 | 33.55 ± 1.04 | 32.49 ± 1.63 |
| EF (%) | 74.67 ± 3.04 | 74.00 ± 1.46 | 69.17 ± 3.15 | 66.83 ± 2.63 |
| SV (ml) | 103.33 ± 7.36 | 97.67 ± 3.94 | 111.18 ± 6.59 | 89.08 ± 6.51** |
| CO (L/min) | 7.38 ± 0.62 | 6.81 ± 0.47 | 7.29 ± 0.60 | 5.40 ± 0.43** |
| E (cm) | 97.17 ± 5.92 | 100.17 ± 4.94 | 108.83 ± 4.80 | 105.17 ± 4.35 |
| A (cm) | 49.33 ± 3.59 | 50.67 ± 2.87 | 52.67 ± 4.81 | 44.33 ± 3.87 |
| E/A | 2.04 ± 0.25 | 2.02 ± 0.19 | 2.14 ± 0.20 | 2.47 ± 0.23 |
Values are means ± SE, LAD= left atrial end-systolic dimension; IVSD= interventricular septum dimension; LVDD= left ventricular diastolic diameter; LVPW=left ventricular posterior wall dimension; LV mass= left ventricular mass. FS= fractional shortening; EF= ejection fraction; SV= stroke volume; CO=cardiac output; E=early diastolic mitral flow; A=late diastolic mitral flow.
P< 0.05,
P< 0.01 week 0 vs. week 4.
For HR, a 2 visit (pre-, post-intervention) by 2 time point (baseline, hyperoxia) repeated measures ANOVA was conducted. Group (Control, Intervention) was entered as a between-subjects factor. The analysis showed a main effect for time [F(1,10) = 27.12, P ≤ 0.001, ηp2= 0.731] and a time by group interaction [F(1,10) = 9.76, P = 0.011, ηp2= 0.494]. Compared to Control subjects, the Intervention group had lower HR at Visit 2 baseline (56 ± 3 vs. 67 ± 4, P=0.032), indicating that weight loss exercise program per se reduced resting HR.
SBP revealed a main effect for time [F(1,10) = 6.25, P = 0.031, ηp2= 0.385] such that hyperoxia tended to increase SBP, regardless of group or visit. However, there were no group differences at any specific timepoint. DBP and MAP did not show any significant main effects across visit, time, or group.
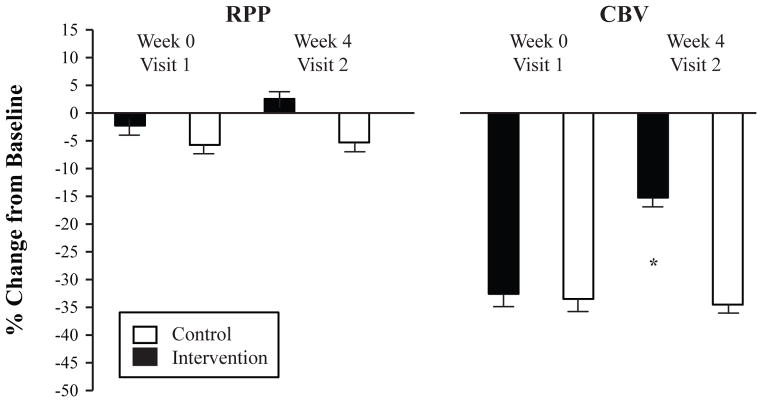
The derived quantity RPP was similarly analyzed with a 2 visit (pre-, post-intervention) by 2 time point (baseline, hyperoxia) repeated measures ANOVA with group as the between-subjects factor. RPP demonstrated a main effect for visit [F(1,10) = 5.65, P = 0.039, ηp2= 0.361] and a time by group interaction [F(1,10) = 6.388, P = 0.030, ηp2= 0.390]. As shown in Figure 2, the groups had a differential RPP response to the hyperoxia paradigm. There was also a non-significant trend for RPP to be lower at Visit 2 baseline in the Intervention group compared to the Control group (P =0.085).
Figure 2.
Percent change (baseline to hyperoxia) in rate pressure product (RPP, an index of myocardial oxygen demand) and coronary blood flow velocity (CBV, an index of myocardial oxygen supply) in obese adolescents. Both the Intervention (black bars) and Control (white bars) groups underwent baseline measurements and then 100% oxygen breathing on Week 0 (Visit 1) and Week 4 (Visit 2). *indicates significantly attenuated coronary vasoconstriction in the Intervention group compared to Control group at Week 4. Values are mean ± SEM (N = 6 in each group).
CBV revealed a main effect for time [F(1,10) = 131.05, P< 0.001, ηp2= 0.928] such that hyperoxia dramatically reduced CBV regardless of visit or group. In fact, all 24 experiments that we conducted (twelve subjects completed both visit 1 and visit 2) resulted in a decrease in CBV (Figure 3). There was also a significant 3-way interaction between visit, time, and group [F(1,10) = 13.48, P = 0.004, ηp2= 0.574]. As shown in Figure 2, the percent change in CBV (baseline to hyperoxia) was significantly smaller in the Intervention group at Visit 2 (P< 0.001, posteriori power analysis = 0.908). Individual CBV data are shown in Figure 3. The derived quantity CVR similarly showed a main effect for time [F(1,10) = 200.20, P< 0.001, ηp2= 0.952] and a group by time interaction [F(1,10) = 8.24, P = 0.017, ηp2= 0.452]. Thus, the weight loss paradigm employed in this study was able to reduce the hyperoxia-mediated coronary vasoconstriction in obese adolescents.
Figure 3.
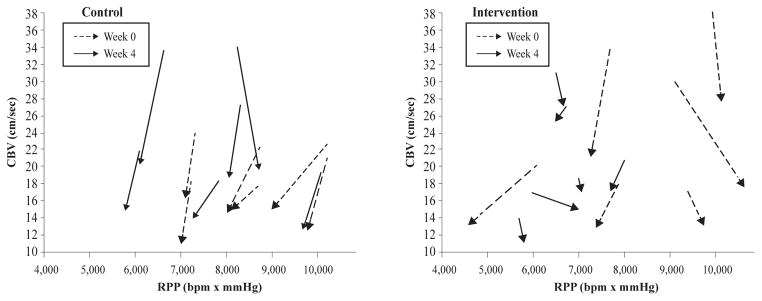
Individual coronary blood flow velocity (CBV) responses to 100% oxygen breathing (hyperoxia) plotted with respect to rate pressure product (RPP) for both Control (left panel) and Weight Loss Intervention (right panel) group at Week 0 (Visit 1, dashed lines) and Week 4 (Visit 2, solid lines). The arrows point from baseline to hyperoxia. A decrease in CBV at a relatively stable RPP is considered to be evidence of coronary vasoconstriction; a shorter arrow is therefore blunted vasoconstriction.
Reproducibility
To account for inter-observer variability, CBV was measured by two independent observers in six subjects at rest. For intra-observer variability assessment, CBV was measured twice by the same observer with at least one month between measurements. Linear regression analysis was performed and correlations of r = 0.95 (P< 0.05) and r = 0.98 (P< 0.05) were calculated for inter- and intra- variability respectively.
Discussion
This study indicates that hyperoxia-mediated coronary vasoconstriction can be significantly reversed in young obese males by a combined 4-week diet and exercise protocol. Data from our lab suggests that acute coronary vasoconstriction during hyperoxia is a consequence of oxidative stress (McNulty et al. 2005), which is a hallmark of obesity (Keaney et al. 2003; Khan et al. 2006). To our knowledge, this is the first study to demonstrate an improvement in coronary vascular function in obese adolescents as a result of weight loss intervention. Several other groups have suggested that exercise and/or a diet program improves peripheral arterial endothelial function in adults (Kingwell et al. 1997) and children (Woo et al. 2004). Our current study extends these findings, documenting a benefit of weight loss on coronary vascular tone related to oxidative stress in obese adolescents. These data underscore the potential importance of body weight management in improving obesity related coronary vascular reactivity, even commencing from an early age.
As an independent and modifiable risk factor for cardiovascular disease, obesity is increasing to epidemic proportions in the United States. Primary prevention may be the key to decreasing obesity, especially because its prevalence is increasing in childhood and adolescents (Eckel and Krauss 1998; Schonfeld-Warden and Warden 1997). It is known that during the pediatric years, obese youngsters demonstrate a higher incidence of cardiovascular dysfunction as well as autopsy evidence of coronary atherosclerosis compared to their non obese peers (Berenson et al. 1998; Tounian et al. 2001). The onset of cardiovascular disease lies in childhood (McGill et al. 2002). Impairment of endothelial function is an important early observation in the atherosclerotic process (Pahkala et al. 2008). Although the public awareness of beneficial effects of weight management in adult obesity is growing, the data on the interaction between weight loss and endothelial function in children are lacking and this fact was the impetus for us to perform this study.
It has long been known that hyperoxia, as used in our study, may result in a decrease in coronary blood flow, which is independent on the activation of alpha-adrenoceptors and alterations of cardiac oxygen consumption (Feigl 1983). The exact mechanisms responsible for hyperoxia-related coronary vasoconstriction is not certain although recent work suggests that high arterial oxygen tension may increase coronary vascular tone via the oxidative quenching of NO in the vessel wall (Lee and Choi 2003). Specifically, data from our lab have found that administering supplemental oxygen as an acute oxidative stress substantially and acutely increases coronary resistance in patients with coronary artery disease. The mechanism is sensitive to the antioxidant vitamin C, which supports this concept that acute coronary vasoconstriction during hyperoxia is a consequence of oxidative stress (McNulty et al. 2005; Momen et al. 2007). Based on the magnitude of vessel constriction, acute and short term administration of 100% oxygen, as applied in our study, may be used as a stress test to assess capacity reserve of antioxidants in regional vessels.
Human obesity is a state of chronic oxidative stress (Vincent et al. 2007). Both obesity and oxidative stress can manifest even within the first two decades of life. The resulting excess of reactive oxygen species can react with NO and reduce its bioavailability and impair endothelium-dependent dilation. In this study, we found that, compared with coronary vasoconstriction to hyperoxia after weight loss, there was a significantly enhanced coronary vasoconstriction before weight loss, which implies a high contribution of NO-induced vasodilation in the coronary circulation. After weight loss, coronary vasoconstriction to hyperoxia was attenuated, which suggests that exercise and diet induced weight loss increased the antioxidant capacity, by either increasing NO production or decreasing oxidative stress, in the coronary circulation.
Several studies have provided convincing evidence of the positive effects of diet and/or exercise on the balance between pro-oxidants and anti-oxidants. Caloric restriction, the increased intake of fruits and vegetables, and low-fat foods may reduce body weight and free radical formation in the obese, which in turn lowers oxidative stress (Lopes et al. 2003). Exercise interventions significantly improve NO availability, which could be due to either an increase in NO production or a decrease in NO metabolism by reactive oxygen species. Roberts et al. (Roberts et al. 2002) have examined the effects of a short-term, combined dietary and exercise intervention on NO availability. They found that this intervention significantly decreased serum 8-isoprostaglandin F2α, a biomarker of systemic oxidative stress, which suggests a reduction in reactive oxygen species. They also found that a high fat diet can limit NO availability via enhanced ROS-mediated inactivation and scavenging of NO in animal studies (Roberts et al. 2000).
The result from this group of adolescents is consistent with the previous adult studies, which have suggested that, on average, a weight-reduction of 1 kg lowers systolic BP by 1 mmHg (Blumenthal et al. 2000). It is interesting to note that prior work in obese adults suggests that a weight loss of >10% may be required before endothelial function improves (Brook et al. 2004; Raitakari et al. 2004). It is possible that the effects of weight loss on oxidative stress are particularly offset by the greater flow mediated release of NO likely seen with this condition. In obese adolescents, the threshold for the magnitude of weight loss at which there is an alteration in vascular function is unclear. To our knowledge, this is the first time hyperoxia has been employed as a physiologic probe of NO quenching in the obese.
It is also known that hyperoxia leads to the production of 20-hydroxyeicosatetraenoic acid (20-HETE) and this is a potent vasoconstrictor (Miyata and Roman 2005). Indeed, some believe that 20-HETE may be the key determinant of the constrictor response seen with O2 (Frisbee et al. 2001). Thus, an alternate explanation for our findings is that the increases in 20-HETE concentrations seen with hyperoxia are augmented in the presence of obesity (or some other linked factors such as insulin resistance, hyperlipidemia, on greater BP levels) (Laffer et al. 2004). Future studies will be necessary to test these hypotheses.
Lifestyle modification including diet and/or exercise is widely recognized as the cornerstone of modern obesity treatment. “Weight-loss camps” are a relatively new approach as a short-term lifestyle modification in treating obese subjects, mostly in children (Gately et al. 2005; Rossner et al. 2008). Compared with previous weight-loss studies, weight reduction in the current intervention group was robust and rapid.
Most investigations in obese adults have found that weight loss achieved via life-style modification or though bariatric surgeries have both been consistently associated with improvement in cardiac structure and function (Abel et al. 2008; Alpert et al. 1995a; Alpert et al. 1995b; Ashrafian et al. 2008; Pasanisi et al. 2001). Using traditional echocardiographic measurements, we did not find any change in cardiac function after weight loss. Several studies have employed newer, more sensitive non-invasive imaging techniques (i.e. tissue Doppler imaging). These studies suggest that substantial weight loss in morbidly obese adults produces improvement in LV diastolic filling and favorable alterations in myocardial function (Di Bello et al. 2008; Willens et al. 2005).
Study Limitation and Future Research
There are several limitations to the present report. First, this study was not randomized (i.e. subjects self-selected to be in the Intervention group) and a relatively small sample of male adolescents age 14–17 were studied. Both the gender and age may limit the generalization of our findings. Second, this was a short-term study, whether these findings would be seen months and years after a sustained weight loss is unclear. Along-term follow-up experiment could yield different findings. Future studies are essential to evaluate these issues.
Clinical Implication
Sedentary habits or a lifestyle lacking in exercise is becoming increasingly prevalent worldwide. Almost half of young people (12 to 21 years of age) fail to engage in vigorous activity on a regular basis in the United States, and one in four children get no physical education in school at all. Currently 1 in 10 American and British children are obese, and the prevalence is rapidly increasing in the developing world (Martorell et al. 2000). The results from the current study suggest that constrictor mechanisms seen in adolescents are reversible during short-term life style modification. Whether the physiologic findings noted in this report have implications for the long-term health and prognosis of the obese awaits further study.
Conclusion
In the present study, we observed that exercise and diet induced weight loss led to a significant attenuation in coronary vasoconstrictive response to hyperoxia, a stress test to assess capacity reserve of antioxidants. The data provides indirect evidence to support that weight loss might improve coronary microcirculation by increasing antioxidant capacity in obese adolescents.
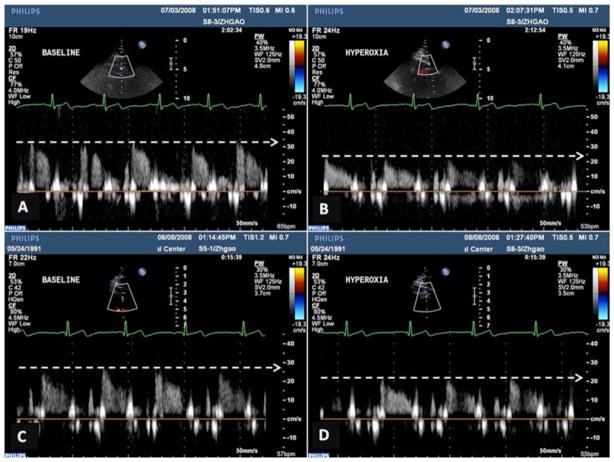
Figure 1.
Original coronary blood velocity (CBV) in left anterior descending coronary artery (LAD) from one obese subject with 130 kg body weight at the baseline and 119 kg after weight loss. A and B show coronary blood flow signals during rest (A) and hyperoxia (B) before weight reduction; C and D show coronary blood flow response to hyperoxia after weight loss. Note that hyperoxia induced a lower CBV before weight loss than after weight loss.
Table 3.
Hemodynamic measurements of hyperoxia protocol in the intervention group before and after weight loss
| Variables | Week 0 (Visit 1) | Week 4 (Visit 2) | ||
|---|---|---|---|---|
| Baseline | Hyperoxia | Baseline | Hyperoxia | |
| HR (bpm) | 65 ± 3 | 63 ± 5 | 56 ± 23* (13 ± 3%↓) | 55 ± 3 |
| SBP (mmHg) | 127 ± 5 | 129 ± 8 | 121 ± 5 (5 ± 3%↓) | 126 ± 5# |
| DBP (mmHg) | 63 ± 3 | 63 ± 5 | 56 ± 2** (11 ± 3%↓) | 56 ± 3 |
| MAP (mmHg) | 90 ± 4 | 90 ± 5 | 78 ± 4** (13 ± 4%↓) | 79 ± 5 |
| RPP (mmHg·beats/min) | 8240 ± 578 | 8186 ± 921 | 6647 ±318* (19 ± 4 %↓) | 6785 ± 265 |
| CBV (cm/s) | 26.17 ± 3.67 | 17.47 ± 2.49## (33 ± 3%↓) | 21.47 ± 2.57 | 18.40 ± 2.56# (15 ± 3%↓) |
| CVR | 2.7 ± 0.5 | 4.0 ± 0.6## | 2.8 ± 0.4 | 3.4 ± 0.5# |
Significantly lower than Baseline Visit 1 (P<0.05, P<0.01);
Significantly different from corresponding baseline value (P< 0.05, P < 0.01).
SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP =mean arterial blood pressure; HR= heart rate; RPP= rate-pressure product; CBV = coronary blood flow velocity; CVR = coronary vascular resistance. Values are mean ± SEM (N = 6).
Acknowledgments
We are thankful to Cheryl Blaha and Jessica Mast for their expert study coordination and invaluable technical assistance during the studies. The authors also express gratitude to Dr. Stephen E. Cyran and Jennie Stoner for consulting assistance and outstanding secretarial skills. Supported by R01 HL070222 (LS), M01 RR010732 (GCRC Grant), C06 RR016499 (Construction Grant) from the National Institutes of Health (LS) and in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds (LS). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, Hashimi MW, Mukerji V. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995a;76:1194–1197. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- Alpert MA, Lambert CR, Terry BE, Cohen MV, Mulekar M, Massey CV, Hashimi MW, Panayiotou H, Mukerji V. Effect of weight loss on left ventricular diastolic filling in morbid obesity. Am J Cardiol. 1995b;76:1198–1201. doi: 10.1016/s0002-9149(99)80339-7. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, le Roux CW, Darzi A, Athanasiou T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–2102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. The New England journal of medicine. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Craighead LW, Tweedy D, Feinglos M, Appelbaum M, Hayano J, Hinderliter A. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–1958. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Glazewski L, Kehrer C, Bodary PF, Eitzman DL, Rajagopalan S. Effect of short-term weight loss on the metabolic syndrome and conduit vascular endothelial function in overweight adults. Am J Cardiol. 2004;93:1012–1016. doi: 10.1016/j.amjcard.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA : the journal of the American Medical Association. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- Di Bello V, Santini F, Di Cori A, Pucci A, Talini E, Palagi C, Delle Donne MG, Marsili A, Fierabracci P, Valeriano R, Scartabelli G, Giannetti M, Anselmino M, Pinchera A, Mariani M. Effects of bariatric surgery on early myocardial alterations in adult severely obese subjects. Cardiology. 2008;109:241–248. doi: 10.1159/000107787. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999;100:1951–1957. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation. 1998;97:2099–2100. doi: 10.1161/01.cir.97.21.2099. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Wickel EE, Welk GJ, Blair SN. Relationship between adolescent fitness and fatness and cardiovascular disease risk factors in adulthood: the Aerobics Center Longitudinal Study (ACLS) Am Heart J. 2005;149:46–53. doi: 10.1016/j.ahj.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Krishna UM, Falck JR, Lombard JH. Role of prostanoids and 20-HETE in mediating oxygen-induced constriction of skeletal muscle resistance arteries. Microvasc Res. 2001;62:271–283. doi: 10.1006/mvre.2001.2341. [DOI] [PubMed] [Google Scholar]
- Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012a;112:483–492. doi: 10.1007/s00421-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol. 2012b;302:H312–H318. doi: 10.1152/ajpheart.00297.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately PJ, Cooke CB, Barth JH, Bewick BM, Radley D, Hill AJ. Children’s residential weight-loss programs can work: a prospective cohort study of short-term outcomes for overweight and obese children. Pediatrics. 2005;116:73–77. doi: 10.1542/peds.2004-0397. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens. 2001;14:1038–1045. doi: 10.1016/s0895-7061(01)02191-4. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol. 2003;23:365–367. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Khan NI, Naz L, Yasmeen G. Obesity: an independent risk factor for systemic oxidative stress. Pak J Pharm Sci. 2006;19:62–65. [PubMed] [Google Scholar]
- Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- Laffer CL, Laniado-Schwartzman M, Nasjletti A, Elijovich F. 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension. 2004;43:388–392. doi: 10.1161/01.HYP.0000112224.87290.3a. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35:341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–430. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. 2003;27:505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- Martorell R, Kettel Khan L, Hughes ML, Grummer-Strawn LM. Overweight and obesity in preschool children from developing countries. Int J Obes Relat Metab Disord. 2000;24:959–967. doi: 10.1038/sj.ijo.0801264. [DOI] [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, Chambers CE, Demers LM, Sinoway LI. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol. 2007;102:735–739. doi: 10.1152/japplphysiol.00618.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296:H854–H861. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol. 2011;111:1694–1702. doi: 10.1152/japplphysiol.00909.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD. National Center for Health Statistics. CDC; 2010. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. http://www.cdc.gov/nchs/fastats/overwt.htm. [Google Scholar]
- Pahkala K, Heinonen OJ, Lagstrom H, Hakala P, Simell O, Viikari JS, Ronnemaa T, Hernelahti M, Sillanmaki L, Raitakari OT. Vascular endothelial function and leisure-time physical activity in adolescents. Circulation. 2008;118:2353–2359. doi: 10.1161/CIRCULATIONAHA.108.791988. [DOI] [PubMed] [Google Scholar]
- Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–406. [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Vaziri ND, Wang XQ, Barnard RJ. Enhanced NO inactivation and hypertension induced by a high-fat, refined-carbohydrate diet. Hypertension. 2000;36:423–429. doi: 10.1161/01.hyp.36.3.423. [DOI] [PubMed] [Google Scholar]
- Rossner S, Hammarstrand M, Hemmingsson E, Neovius M, Johansson K. Long-term weight loss and weight-loss maintenance strategies. Obes Rev. 2008;9:624–630. doi: 10.1111/j.1467-789X.2008.00516.x. [DOI] [PubMed] [Google Scholar]
- Schonfeld-Warden N, Warden CH. Pediatric obesity. An overview of etiology and treatment. Pediatr Clin North Am. 1997;44:339–361. doi: 10.1016/s0031-3955(05)70480-6. [DOI] [PubMed] [Google Scholar]
- Singhal A. Endothelial dysfunction: role in obesity-related disorders and the early origins of CVD. Proc Nutr Soc. 2005;64:15–22. doi: 10.1079/pns2004404. [DOI] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. JAMA : the journal of the American Medical Association. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- Sundell J. Obesity and diabetes as risk factors for coronary artery disease: from the epidemiological aspect to the initial vascular mechanisms. Diabetes Obes Metab. 2005;7:9–20. doi: 10.1111/j.1463-1326.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillon JC, Lowery MH. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:1521–1524. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]