Abstract
Ocimum sanctum (OS) is a traditionally used medicinal herb, which shows anti-oxidant, anti-carcinogenic, radio-protective and free radical scavenging properties. So far no detailed studies have been reported on its effects on human cancers. Thus, we analyzed its effects on human breast cancer utilizing in vitro and in vivo methodologies. Aqueous extracts were prepared from the mature leaves of Ocimum sanctum cultivated devoid of pesticides. Tumor progression and angiogenesis related processes like chemotaxis, proliferation, apoptosis, 3-dimensional growth and morphogenesis, angiogenesis, and tumor growth were studied in the presence or absence of the extract and in some experiments a comparison was made with purified commercially available eugenol, apigenin and ursolic acid. Aqueous OS leaf extract inhibits proliferation, migration, anchorage independent growth, three dimensional growth and morphogenesis, and induction of COX-2 protein in breast cancer cells. A comparative analysis with eugenol, apigenin and ursolic acid showed that the inhibitory effects on chemotaxis and three dimensional morphogenesis of breast cancer cells were specific to OS extract. In addition, OS extracts also reduced tumor size and neoangiogenesis in a MCF10 DCIS.com xenograft model of human DCIS. This is the first detailed report showing that OS leaf extract may be of value as a breast cancer preventive and therapeutic agent and might be considered as additional additive in the arsenal of components aiming at combating breast cancer progression and metastasis.
Keywords: Ocimum sanctum, Breast Cancer, DCIS, Medicinal herb
Introduction
Ocimum sanctum (OS) also known as holy basil or tulsi, is one of the most popular herbs used in European and Asian countries for the treatment of various ailments since ancient times. It is associated with chemopreventive, anticarcinogenic, free radical scavenging, radioprotective and numerous others pharmacological uses (1). The aqueous leaf extract and seed oil showed anti-proliferative and chemopreventive activity on Hela cells (2). Oral supplementation of seed oil against 20-methylchloranthrene (MCA)-induced fibrosarcoma tumors and against 7,12-dimethylbenz(a) anthracene (DMBA) induced papillomagenesis in Swiss albino mice reduced the cumulative tumor incidence and tumor volume (2, 3). OS significantly reduced the incidence of benzo(a)pyrene induced neoplasia of stomach and 3′-methyl-4-dimethylaminoazobenzene induced hepatomas in rats (4). Topical treatment of OS leaf extract in DMBA-induced papillomagenesis significantly reduced the tumor incidence, average number of papillas per mouse, and the cumulative number of papillomas in mice (5). Incidence of papillomas and squamous cell carcinomas were significantly reduced when OS in the form of fresh leaf paste, aqueous and ethanolic extract was topically applied or the extracts were orally administered to buccal pouch mucosa of hamsters exposed to 0.5% DMBA. The aqueous extract showed a more profound effect than the other two forms (6). Ethanolic leaf extract also had significant modulatory influence on carcinogen metabolizing enzymes (cytochrome P450, cytochrome b5 and aryl hydrocarbon hydroxylase, glutathione-s-transferase) and glutathione levels in mice (7).
A number of active constituents are thought to be responsible for the medicinal action of OS. The leaves contain 0.7% volatile oils comprising ~ 70% eugenol and 20% methyl eugenol (8). Eugenol (4-allyl-1-hydroxy-2-methoxybenzene) is also present in other aromatic plants like clove, cinnamon and nutmeg (9). In a recent study eugenol, isolated from the cortex of Eugenol caryophyllata (clove) inhibited the activity and gene expression of inducible cyclooxygenase (COX-2) in lypopolysacharide (LPS) activated mouse macrophage cells (10). COX–2 catalyzes the conversion of arachidonic acid to prostaglandins (11) and is an early response gene when stimulated by serum, tumor promoters, mitogens, endotoxin, cytokines, and hormones (12). It is considered an important therapeutic target in breast cancer progression, angiogenesis and metastasis (13–15). Studies have shown that selective COX-2 inhibitors reduce the human breast cancer risk (16). Kelm et al demonstrated inhibition of COX-1 and COX-2 levels by eugenol and six other phenolic compounds extracted from the leaves and stems of OS (17), one of these compounds is Apigenin (17). Apigenin (4′,5,7,-trihydroxyflavone) has low toxicity, is nonmutagenic and is widely distributed in many fruits and vegetables including parsley, onions, oranges, tea, camomile and wheat sprouts (18) and has recently been shown to possess antitumor properties (19–22). It has also been shown to inhibit the activity of aromatase (estrogen synthase) (23, 24), which is a cytochrome P450 hemoprotein-containing enzyme complex that plays key role in the conversion of C19 androgens to C18 estrogens (25). Aromatase activity has been demonstrated in breast tissue in vitro, and its expression is highest in or near breast tumor sites and is an important therapeutic target for hormone refractory breast cancer (26).
Ursolic acid (3β-hydroxy-urs-12-en-28-oic-acid), a pentacyclic triterpenoid, is another compound exhibiting clinical value that has been isolated from OS leaves and also from berries, leaves, flowers and fruits of Rosemarinus officinalis, Eriobotrya japonica, Calluna vulgaris and Eugenia jumbolana (27). Ursolic acid has been shown to suppress nuclear factor-κB (NF-κB), which regulates the expression of a number of genes whose products are involved in tumorigenesis (28,29). Thus, agents that can suppress NF-κB activation have the potential to suppress carcinogenesis and have therapeutic potential (29, 30).
Based on the above and lack of any reported scientific data on the role of OS on human cancers, we questioned whether OS extract has an effect on breast cancer. In the current study, we have analyzed the role of OS on tumor growth, angiogenesis and metastasis related properties. A comparative analysis was also performed between eugenol, apigenin and ursolic acid, which are commercially available, on tumor cell migration and 3-dimensional tube formation. As total OS leaf extract was found to be more effective than its separated constituents in these studies, it was used in remainder experiments.
Material and Methods
Preparation of OS Extract
Seeds of Ocimum sanctum were purchased from Horizon Herbs, Williams, OR. The plants were grown devoid of pesticides in the greenhouse at 78°F under a 12 hr photoperiod, watered daily and fertilized once a week with 20:20:20 (NPK). The matured leaves were harvested, dried in shade and extracted with water (1gm dry weight /100ml water). The extract was boiled for 5 min, centrifuged, pH was adjusted to 7.0 and sterilized by filtering through 0.22μm pore size Steritop filters (Millipore, Bedford, MA). Enough extract was prepared to last for the duration of all the experiments and stored at −20°C as a 1% solution (w/v). The activity was rechecked after 6 months and 1 year by performing chemotaxis assay. Leaf extracts prepared similarly from iceberg lettuce Lactuca sativa (family Asteraceae) were used as control.
Cell Lines and Culture
Human breast cancer cell lines MDA-MB-435 and MDA-MB-231 were a gift from Dr. Eric W. Thompson (St. Vincent’s Institute of Medical Research and University of Melbourne, Melbourne, Australia). The cells were maintained in Dulbecco’s Minimal Essential Medium (Invitrogen Corporation, Carlsbad, CA) containing 10% heat-inactivated fetal calf serum (FCS), essential and non-essential amino acids, (Invitrogen), vitamins, and antibiotics (Mediatech Cellgro Inc., Herndon, VA). MCF10AT1 EIII8 cells (referred as EIII8) (31) were maintained in DMEM-F12 medium (Invitrogen Corporation) supplemented with 0.1μg/mL cholera toxin (Sigma Chemical Co., St. Louis, MO), 10 μg/mL insulin (Sigma), 0.5 μg/mL hydrocortisone (Sigma), 0.02μg/mL epidermal growth factor (BD Biosciences, Bedford, MA), 100 IU/mL penicillin and 100μg/mL streptomycin (Mediatech), and 10% horse serum (Invitrogen). MCF10DCIS.com (referred as DCIS.com) cells were a gift from Dr. F. Miller (32) and were cultured in DMEM/F12 medium supplemented with 10% horse serum. EIII8 and DCIS.com cells are derived from the human preneoplastic breast cancer cells MCF10A. Bovine Adrenal Medulla Endothelial Cells (BAMEC) were a gift from Dr. D. Banerjee (University of Puerto Rico, San Juan, PR) (33) and cultured in medium consisting of minimal essential medium with Earle’s salts and L-glutamine (EMEM) (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), and antibiotics (Mediatech). All the cells were maintained in a humidified chamber with 95% air and 5% CO2 at 37°C. The cells were grown to near confluence and detached from the monolayer with 0.25% trypsin and 2mM EDTA for 2 min at 37°C. The use of cell lines was approved by the Human Investigation Committee, Wayne State University, Detroit, MI.
Chemotaxis
This assay was performed as described earlier (34, 35) using a Boyden chamber (Neuroprobe Inc., Cabin John, MD). In the lower chamber, various chemo-attractants e.g. Matrigel (BD Biosciences, Bedford, MA) or fibronectin (Sigma), alone or mixed with various concentrations of OS extract, eugenol, apigenin or ursolic acid (Sigma) were added. MDA-MB-435, MDA-MB-231 or DCIS.com (5×104) cells were loaded in the upper chamber. The two chambers were separated by a polycarbonate filter of 8μ pore size and incubated in a 37°C tissue culture incubator for 5 hr, after which the filter was removed, the cells on top of the filter were wiped off and the migrated cells were fixed, stained using Protocol Hema 3 stain set (Fisher Scientific Company, Pittsburgh, PA), scanned and the cell density was calculated using NIH Image Version 1.62. Each assay was carried out in triplicate.
Cell Viability
Cell viability and cell death of MDA-MB-231 cells were detected using Annexin V-FITC Apoptosis detection kit (Oncogene, San Diego, CA). The cells (5×105) were seeded in 100 mm dishes, serum starved for 24 hr, incubated with the extracts at various concentrations for 48 hr and counted, taking care that none of the floating cells were lost during washes and suspended at a density of 1×106 cells/ml binding buffer. The cells were first stained with Annexin V-FITC and later incubated with propidium iodide (PI). The quantification of the PI and FITC signals was performed using fluorescence activated cell sorter (FACScalibur and Cell Quest (Beckton Dickinson, San Jose, CA)) and data were plotted and analyzed using WinMDI 2.8 software.
Cell Proliferation
MDA-MB-231 cells (2×103) were seeded in triplicates onto 24 well culture dishes. After 24 hr, fresh medium containing various concentrations of the aqueous extract was added. In one set of experiments, fresh medium supplemented with OS extract was added every third day, whereas in another set of experiments OS was not replenished in the medium. Cell proliferation was determined by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) to formazan over a period of 10 days. Briefly, the medium was removed and the cells were incubated at 37°C with MTT (0.5mg/mL) for 4 hr. The medium was aspirated and the cells were solubilized in 0.04N HCl in isopropanol. The optical density (OD) was measured at 570 and 630 nm.
Three-Dimensional Growth and Morphogenesis Assay
Formation of tubular network by EIII8 cells or BAMEC was performed as described (34, 35). Matrigel (200μl) was added to each chamber of a pre-chilled eight chamber slide and gelled by 15 min incubation at 37°C. After which, 5×104 EIII8 or BAMEC cells were plated onto the gel in 200 μl of the medium. In some chambers Ocimum leaf extract or apigenin, eugenol or ursolic acid was added. After 2, 3 or 7 days, tubular structures were observed under phase contrast microscope and photographed. Co-cultures of BAMEC and EIII8 cells (5×104 each) were performed to analyze in vitro angiogenesis and interactions between epithelial and endothelial cells.
Induction of COX-2 Expression and Western Blot Analysis
MDA-MB-231 (5×105) cells were seeded in 60 mm petri dishes. After 24 hr of serum starvation, the cells were incubated with 25nM TPA alone or in combination with different concentrations of the OS extract or the components. After 4 hr, the cells were trypsinized, lysed and equivalent number of cells (1×105) were subjected to SDS-PAGE and Western blot analysis with a 1:1000 dilution of anti COX-2 mouse monoclonal antibody (Cayman Chemical Co., Ann Arbor, MI). Blots were also immunoreacted with a 1:5000 dilution of anti-tubulin mouse polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) to normalize for variation in protein loading. Following washes the blots were reacted with secondary antibody mix containing 1:5000 dilutions of IR Dye 680 or IR Dye 800 conjugated anti-mouse antibody (Molecular Probes, Eugene, OR) and scanned by Odyssey infra red imaging system (LI-COR Biosciences, Lincoln, NE) to locate the COX-2 and tubulin proteins.
Angiogenesis Assay
MDA-MB-231 (1×106 cells) suspended in 400 μl of Matrigel were injected subcutaneously in the dorsolateral region near shoulder of nude mice (34, 35). To determine the effects of OS, in parallel experiments, 0.1% (w/v) OS leaf extract was included in cell Matrigel suspensions prior to injections into the animals. After one week, the Matrigel plugs along with overlaying skin were removed, fixed in 10% buffered formalin, embedded in paraffin, and sectioned longitudinally, using the skin as the reference point. Sections were stained immuno-histochemically with markers for neoangiogenesis (CD34; Cell Sciences, Canton, MA), cell proliferation (PCNA; Dako, Carpinteria, CA) and apoptosis (TUNEL assay; Promega, Madison, WI).
Tumor Growth in Nude Mice
2×106 DCIS.com cells were injected into NCR nu/nu mice obtained from Taconic, Germantown, NY, in the mammary fat pad region subcutaneously on both sides (32) in two groups of 6 mice each, respectively. Three days after the injections, one group received water supplemented with 1% OS extract, whereas the remaining group received regular drinking water. Tumor volumes were measured twice a week. The xenografts were harvested at 3 weeks. The tumors were weighed, fixed with 10% buffered formalin and processed for immunohistochemical staining using anti CD34, vascular endothelial growth factor (VEGF; Santa Cruz), COX-2, matrix-metalloprotease-9 (MMP9; Oncogene, Cambridge, MA) and proliferating cell nuclear antigen (PCNA) antibodies as described. The animal experiments were performed according to the guidelines provided by Animal Investigation Committee, Wayne State University.
Immunohistochemical Analysis
Four μm tissue sections were deparafinized, rehydrated and microwaved on high for 5 min twice in 1mM sodium citrate buffer, pH 6.0. The sections were washed three times in PBS and blocked with Super Block (Skytek Laboratories, Logan, UT) for 10 min. Sequential sections were incubated with primary antibodies against CD34, COX-2, PCNA, VEGF and MMP-9 at 4°C at the suitable dilution. The sections were washed 3 times for 10 min each in PBS and linked with the appropriate host avidin biotinylated horseradish peroxidase tagged secondary antibodies (Vector Laboratories, Burlingame, CA). Color development was obtained with 3′-3′-diaminobenzidine and counterstaining was performed with hematoxylin. Visualization and documentation were accomplished with an OLYMPUS BX40 microscope supporting a Sony DXC-979MD 3CCCD video camera and stored with the M5+ microcomputer imaging device (Imaging Research Inc., Brock University, St Catherine, ONT, Canada).
TUNEL Assay
TdT mediated dUTP Nick End Labeling (TUNEL) assay was performed to visualize the fragmented DNA directly by fluorescence microscopy in paraffin embedded sections using DeadEnd Fluorometric TUNEL system (Promega, Madison, WI). Briefly, the paraffin sections were deparafinized, and permeabilized with proteinase K. Fluorescein 12-dUTP was then catalytically incorporated into the 3′-hydroxyl ends, which are exposed in fragmented DNA of the apoptotic cells using the enzyme terminal deoxynucleotidyl transferase (TdT). The sections were then labeled using the apoptosis detection system, fluorescein and analyzed by fluorescence microscopy.
Statistical Analysis
Microsoft Excel software was used for calculating statistical significance by two sample t test: using unequal variance. P values of < .05 were considered statistically significant.
Results
Inhibition of Chemotaxis by Ocimum Extract
MDA-MB-231 and -435 cells migrate strongly towards 50μg/ml Matrigel. No migration was seen in DCIS.com cells towards 100μg/ml fibronectin or 50 and 100 μg/ml Matrigel. However, when 100μg/ml Matrigel was supplemented with 100μg/ml fibronectin, the cells showed migration after an overnight incubation (not shown). There was a dose dependent decrease in migration of MDA-MB-231 (Fig. 1A) as well as MDA-MB-435 (Fig. 1B) cells towards Matrigel in the presence of OS extract, with a significant inhibition of ~ 60–75% at 0.2 and 0.1% (w/v) in MDA-MB-231 cells and 90–98% in MDA-MB-435 cells. An inhibition of approximately 38% in MDA-MB-231 cells was observed in the presence of 0.05% OS. At lower concentrations of OS extract (0.01, and 0.02% w/v) the inhibition was not statistically significant in both the cell lines. Migration of MDA-MB-231 cells towards fibronectin was not as pronounced as towards Matrigel, but it was inhibited even with low concentrations of OS extract (Fig. 1A). The experiment was repeated three times and representative data are shown. OS extract also inhibited the migration of DCIS.com cells towards Fibronectin supplemented Matrigel by about 60% (not shown). In order to determine the specificity of inhibition by OS leaf extract, extract of iceberg lettuce (Lactuca sativa) leaves was tested on MDA-MB-231 cells migration towards 50μg/ml Matrigel. However, no inhibition in migration was observed (Fig. 1C).
Figure 1.
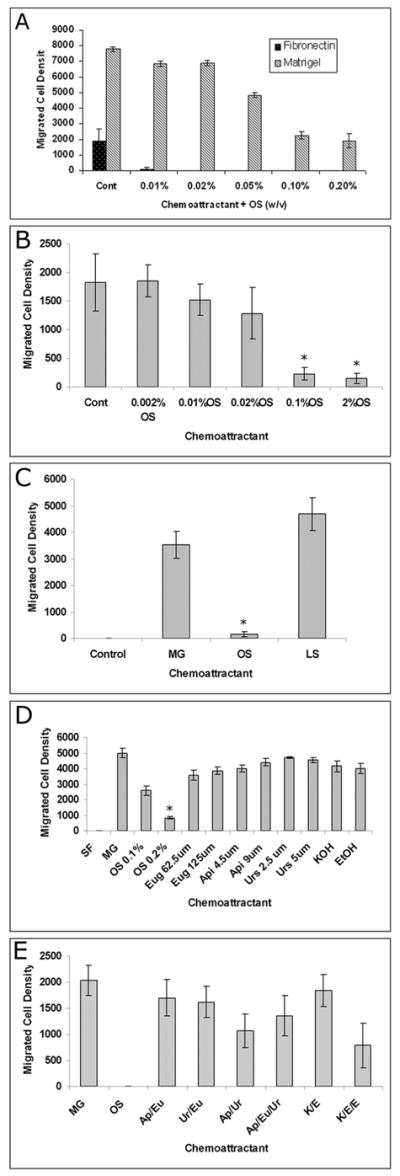
Boyden chamber analysis of MDA-MB-231 and –435 human breast cancer cell lines using Matrigel or Fibronectin as a chemo-attractant. A: OS leaf extract was mixed with 100 μg /ml Fironectin or 50μg/mL Matrigel in serum free medium at concentrations ranging from 0.01–0.2% (w/v). Note the dose dependent inhibition of migration by leaf extract. B: Chemotaxis of MDA-MB-435 cells towards 50 μg /ml Matrigel in the absence or presence of 0.2–0.002% OS (w/v). C: Chemotaxis of MDA-MB-231 cells. Control: towards serum free medium; MG: towards 50μg/ml Matrigel; OS: towards Matrigel in the presence of 0.1% OS extract; LS: towards Matrigel in the presence of 0.1% Lactuca extract.
D: Migration of MDA-MB-231 cells towards Matrigel. SF: serum free medium; MG: 50μg/ml Matrigel; Eug: Eugenol; Api: Apigenin; Urs: Ursolic acid. All components and extract were mixed with MG.
E: Migration of MDA-MB-231 cells towards Matrigel: MG: 50μg/ml Matrigel; OS: 0.2% OS extract (w/v); Ap/Eu: 4.5μM apigenin and 62.5μM eugenol; Ur/Eu: 2.5 μM ursolic acid and 62.5μM eugenol; Ap/Ur: 4.5μM apigenin and 2.5μM ursolic acid; Ap/Eu/Ur: 4.5μM apigenin, 2.5μM ursolic acid and 62.5μM eugenol; K/E: 0.125 μM KOH and .001% ethanol; K/E/E: 0.125μM KOH and 0.002% ethanol. All reagents were mixed with 50μg/ml Matrigel. * P <0.001; *′ P< 0.005
To test whether chemotaxis inhibition was due to the known components present in the OS leaf extract, we used 62.5 and 125 μM eugenol, 4.5 or 9 μM apigenin and 2.5 or 5 μM ursolic acid alone or in various combinations in chemotaxis studies (Fig. 1D&E). None of these components suppressed migration.
Effect of Ocimum Extract on Viability and Apoptosis in Breast Cancer Cells
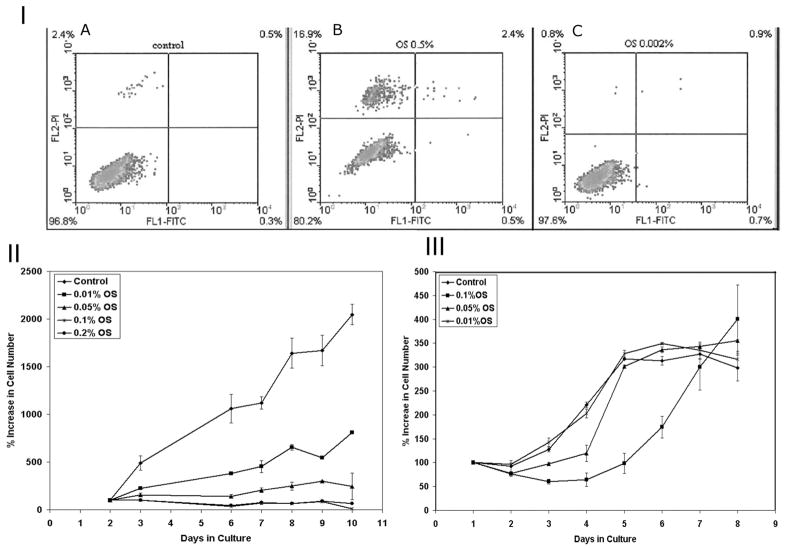
To rule out the possibility that inhibition in cell migration was due to cytotoxic effects, we analyzed the viability, necrosis and apoptosis of MDA-MB-231 cells treated with OS extract by flow cytometric analysis (Fig. 2I) using PI and Annexin V FITC staining. The untreated control cells showed 96.8% viable cells that were negative for FITC as well as PI (Fig. 2IA). At a concentration of 0.5% (w/v), 80.2% cells were viable and 16.9 % cells stained with PI indicating cell death. Only 2.4% cell in this group stained with PI as well as FITC indicating apoptosis (Fig. 2IB). Because no differences in cell numbers were observed in lower concentrations, we have chosen 0.5 and 0.002% (w/v) concentrations to prepare density plot. The results indicate that OS does not induce apoptosis or necrosis at lower concentrations ranging from 0.1 to 0.005% (w/v), but at a very high concentration of 0.5%, it causes cell death in 16% cells because of toxicity and in 3% due to apoptosis.
Figure 2.
I: Cell viability and toxicity curve of MDA-MB-231 cells treated with OS extract using AnnexinV-FITC staining. A: Density plot control; B: Density plot 0.5% OS; C: Density plot 0.002% OS. II&III: Growth curve of MDA-MB-231 cells in the presence of OS extract. To account for the differences in seeding capacity of various cell lines, the OD at day 2 was given a value of 100 and % increase was calculated relatively. Note a dose dependent inhibition in the cell proliferation in the continuous presence of OS extract in II and a dose dependent delay in cell number increase in III after only the initial treatment with OS extracts. Each time point in II showed a statistical significance of <1×10−5, which is a highly significant inhibition compared to the untreated control.
Inhibition of Cell Proliferation
After having established that OS extracts do not affect cell viability, we questioned if it affects cell proliferation. Fig. 2II indicates a significant dose dependent decrease in cell proliferation in the continuous presence of Ocimum extract. There was a complete inhibition of cell proliferation at 0.1 and 0.2% OS (w/v), cell number stayed constant over the 10 day period, though the cells were viable. A slight increase in cell numbers was seen in 0.05 and 0.01% (w/v) with increasing time, the total number reaching approximately 10 and 40% respectively of the control values by day 10. In the absence of fresh OS extract, however, the cell numbers reached the values equivalent to controls with a delay that was dose dependent (Fig. 2III).
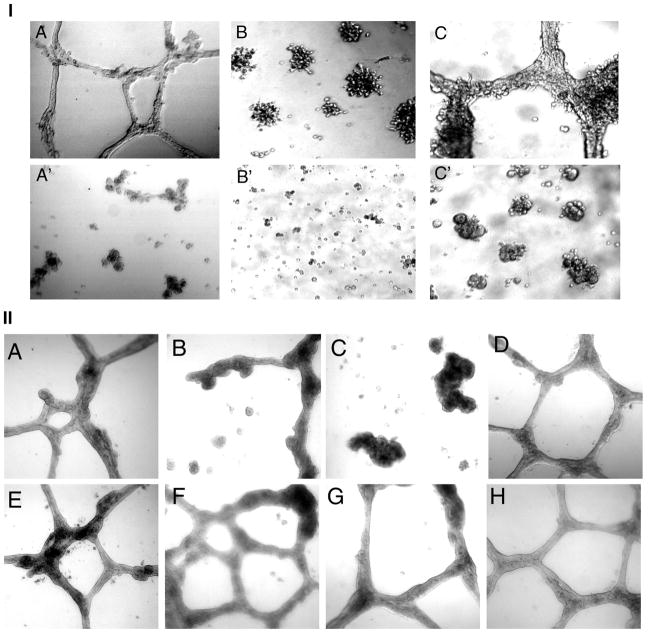
Inhibition of Three-Dimensional Growth by Ocimum Extract
When seeded as monotypic cultures on Matrigel, EIII8 cells organize into tubular network (Fig. 3IA). BAMEC on the other hand, do not form very well organized tubes on Matrigel, instead they organize into actively dividing aggregates (Fig. 3IB). When BAMEC and EIII8 cells are co-cultured on Matrigel, the cells organize into branching structures indicative of in vitro angiogenesis (Fig. 3IC) (36). In the presence of OS extract, there was a disrupted tube formation in EIII8 cells and the interconnecting network was lacking (Fig. 3IA′), and BAMEC did not form aggregates (Fig. 3IB′). The co-cultures did not form tubes, but aggregates were seen (Fig. 3IC′). We also did not see many floating cells, which indicates that the OS extract did not inhibit cell-cell interaction, it may be homotypic or heterotypic interaction, but inhibited the morphogenesis of cells into interconnected tube like structures.
Figure 3.
I: 3-Dimensional growth and capillary tube formation. A,A′: EIII8 cells; B,B′: BAMEC; C,C′: Co-cultures of the two cell lines. A–C: control; A′–C′: 0.1% OS extract (40X) II: Effect of OS extract and components on morphogenesis of EIII8 cells. A: control untreated; B: 0.05% OS extract; C: 0.1% OS extract; D: 625 μM eugenol, E: 10μm ursolic acid; 18μm apigenin, G: 0.01% ethanol; H: 1.25mM KOH
To analyze if various components could also inhibit tubular network formation, we seeded EIII8 cells in the presence of eugenol (125, 250 and 625 μM), apigenin (4.5, 9 and 18 μM) and ursolic acid (2.5, 5 and 10 μM) and their corresponding vehicles KOH and ethanol. The tubes were observed after 7 days. The highest tested concentrations (625 μM eugenol, 18 μM apigenin and 10 μM ursolic acid) have been depicted in Fig. 3II. No reagent other than the total leaf extract inhibited tube formation of EIII8 cells.
Inhibition of COX-2 Induction by Ocimum Extract
Since studies have shown that eugenol is present in appreciable quantities in OS extracts and it inhibits the induction of COX-1 and COX-2 (17), we questioned whether OS extract could also inhibit COX-2 expression. Our results indicate an appreciable increase in the levels of COX-2 by treating the MDA-MB-231 cells with 25nm TPA, which was abolished in the presence of 0.05 or 0.1% w/v OS extract as well as by 62.5 μM eugenol, 2.5μM apigenin or 2.5μM ursolic acid. The vehicle DMSO did not induce COX-2 (Fig. 4). Higher concentrations of components (5 μM each of apigenin and ursolic acid, 125 μM eugenol) were toxic to the cells in the presence of TPA.
Figure 4.
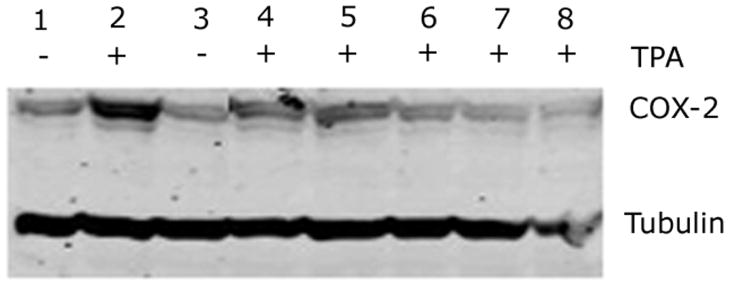
Western blot analysis of COX-2 induction. 1: Control untreated cells; 2: 25 nM TPA; 3: DMSO at 1:200 dilution; 4: 0.05% OS extract; 5: 0.1% OS extract; 6: 62.5 μm eugenol; 7: 2.5μm apigenin; 8: 2.5μm ursolic acid. 4–8 were mixed with 25nM TPA. Tubulin was used as loading control.
Inhibition of Angiogenesis by Ocimum Extract
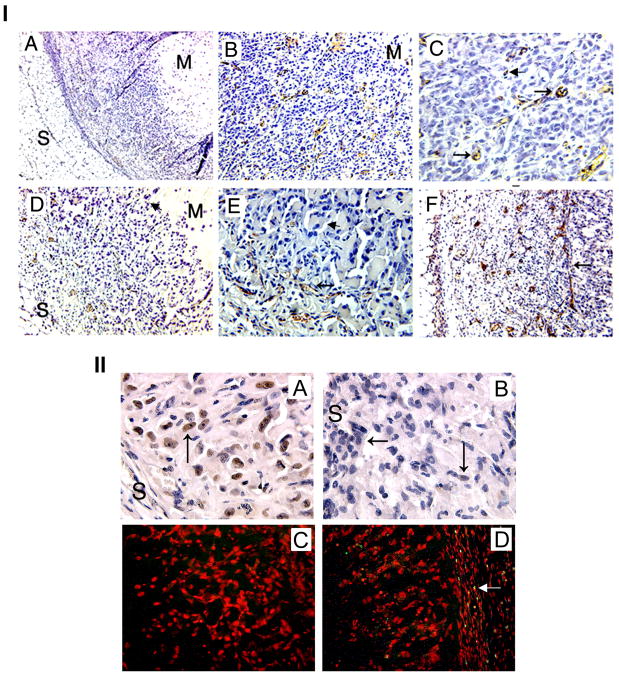
The formation of blood vessels in the Matrigel plugs was analyzed by staining the sections with anti CD34 monoclonal antibody. Our results show that the plugs formed by MDA-MB-231 cells are more dense, and show the presence of blood vessels throughout the plug (Figs. 5IB&C), whereas in the presence of OS extract, the plugs showed a reduced cell number per microscopic field (Fig. 5ID compared with B) and the blood vessels were restricted to the periphery (Figs 5IE&F) of the plug.
Figure 5.
I: Effect of OS extract on MDA-MB-231 cell induced angiogenesis: A: Lower magnification (40X) to show the overall orientation of the plugs. Subcutaneous fat can be seen on one side (S) and Matrigel (M) on the other side of the section. In-between are the tumor and stromal cells infiltrated from the host tissue. B: Section from a control plug staining neovessels (brown) with CD34 antibodies and nuclei (blue) with haematoxylin. The cells are spread over a large area of the section; blood vessels are distributed uniformly over the entire cellular area (100X). C: Higher magnification (200X) to show the blood vessels with small lumen (arrows), some vessels have not yet developed the lumen (arrowhead). D, E &F: Sections from the plugs formed in the presence of OS extract. Note that fat cells as well as Matrigel are visible in D indicating that cells fill up a relatively smaller area (compare with B). The cells towards center of the section did not show any vasculogenesis, the vessels were only peripheral i.e. closer to the skin (100X). E: 100X showing the vessels (arrow) and the nonvascular area (arrowhead). F: Restriction of blood vessels at the interface (arrow). The older vessels (with lumen) can be seen on the subcutaneous side and new vessels (no lumen) are bordering the interface between angiogenic and non-angiogenic areas. 100X II: Angiogenesis in MDA-MB-231 plugs. A & C: sections from control plug and B&D: sections from OS extract treated plugs showing PCNA (A&B 400X) and TUNEL assay (C&D: 200X). Arrows indicate positive staining in PCNA. Red color indicates intact DNA with propidium iodide staining (C&D) yellow color indicates fragmented DNA in early apoptosis (arrow) in the treated section (D).
To check if the reduced number of cells and blood vessels in OS treated plugs could be due to either an induction of apoptosis or an inhibition in cell proliferation, we stained some sections with anti PCNA antibodies and performed TUNEL assay on some sections. There was a significant decrease in the levels of PCNA (Fig. 5IIA&B) in the treated sections. However, using TUNEL assay, the yellow green stain indicating fragmented DNA was observed only in the mouse stromal cells in the OS treated plugs (Fig. 5IID compared to 5IIC), indicating induction of apoptosis in the stroma by OS extract. Only 1–2 cells in the whole field showed apoptotic nuclei in the untreated control section (Fig. 5IIC).
Inhibition of Tumor Growth and Angiogenesis by Ocimum Extract
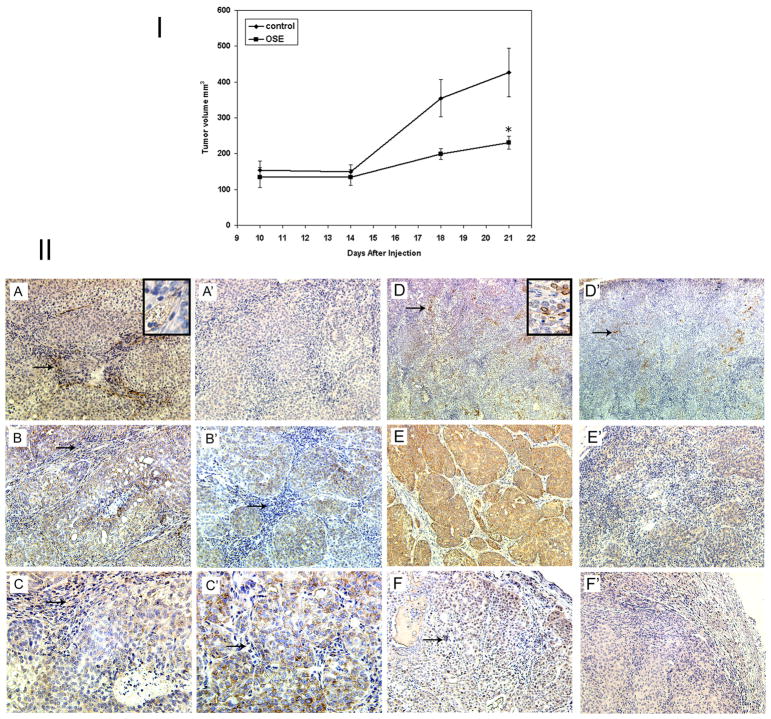
The anti-tumorigenic potential of the extract was tested using the DCIS xenograft mouse model. Although tumorigenic efficiency was not affected by OS extracts, but the tumor growth rate slowed after the first 14 days when the tumors reached a volume of 150mm3(Fig. 6I) in mice that were orally supplemented with OS extract. The average tumor weight of DCIS.com xenografts on day 21 was 662 ± 89 mg in control group and 252 ± 31mg in the OS fed group.
Figure 6.
I: Growth of DCIS.com xenografts in nude mice fed with OS extracts: Mice were divided into two groups. One group was administered OS extract orally and the other one was fed on water. The mice were injected with 2×106 DCIS.com cells. *P <.05. II: Immunohistochemical analysis of the DCIS.com xenografts. A&A′: CD34 (100x) Inset 200x; B&B′: Lack of VEGF in the stroma of the OS treated xenografts (arrows) 100x; C&C′: VEGF 200x D&D′: Cox-2 expression restricted to the peripheral cells of the DCIS (arrows) 40x; inset 200x: E&E′: MMP9 100x; F&F′: PCNA arrows indicate positive staining 100x. A–F: control; A′–F′: OS treated.
The reduction in tumor size was accompanied by many changes in protein distribution (Fig. 6II). Treatment with OS resulted a reduction in the number of neo-vessels (reduced angiogenesis indicated by CD34 staining) (A, A′). A redistribution of VEGF from epithelial and stromal to only epithelial compartment was also observed in the treated xenografts (B,B′&C,C′). A reduced expression of COX-2 (D,D′), MMP-9 (E,E′) and PCNA (F,F′) was also observed in OS treated xenografts.
Discussion
The use of dietary components such as traditionally used spices and herbs having protective and/or preventive effects on cancer progression is an important emerging field of research. It is generally assumed that the use of these food supplements is safe and efficacious, given that they have been used for human consumption for centuries. However, understanding their mechanisms of action as a cancer preventive and therapeutic modality is one of the main challenges for modern science.
In the present study we have shown that OS extracts significantly reduced tumor cell proliferation when the medium was replaced with fresh OS supplemented medium every third day. The inhibition in cell growth was dose dependent and it was not due to cytotoxicity or apoptosis as analyzed by Annexin V FITC and propidium iodide staining after 48 hr of incubation. However, OS extracts induce apoptosis in the stromal cells, which was detected after 7 days in Matrigel plug assay. Interestingly, the extracts seem to have a reversible cytostatic effect on growth. When the extracts were not replenished after their first effect wore off, cells reentered the growth phase after an initial delay period, the length of which was proportional to the concentration of the extract used. At the highest concentration used (0.1% w/v), even though the delay was of 7 days, nevertheless cells entered the growth phase and by 10 days, reached numbers higher than the controls, which had already reached a peak level by day 7. Inhibition of tumor cell proliferation by OS extracts has been reported earlier (2), though its reversible cytostatic effects have been reported for the first time to the best of our knowledge. The inhibition of cell proliferation was accompanied with a reduced ability of cells to grow in anchorage independent conditions (not shown). Ocimum also affected the 3-dimensional growth and morphogenesis of epithelial and endothelial cells on Matrigel as monotypic as well as heterotypic cultures.
Chemotaxis, an in vitro assay to study the directed motility of tumor cells, is another important phenomenon affected by OS extract. Using this assay we have shown that OS extracts inhibit the migration of MDA-MB-231, -435 and DCIS.com cells towards Matrigel and fibronectin in a dose dependent manner, whereas the similarly prepared extract from the leaves of Lactuca sativa did not inhibit the migration. We used Lactuca as a control, which consists of 85% water and very few other components. To test whether inhibition of chemotaxis was due to the presence of some previously known component of the leaf extract, we used eugenol, apigenin, ursolic acid and their corresponding vehicles ethanol and KOH at comparable dilutions and compared the results with total extract. The concentrations of the components were selected based on the viability studies and the amounts present in Ocimum leaves. Based on available data a 1% solution (w/v) of Ocimum leaves contains about 29.8 μM eugenol (1) and 3.7 μM apigenin (17). Concentration of ursolic acid in Ocimum leaves could not be found in literature, but when used in the range of at 5–20 μM on various cell lines it induced apoptosis (37). We used eugenol at 62.5 or 125 μM, apigenin at 4.5 or 9 μM and ursolic acid at 2.5 or 5 μM for chemotaxis studies compared with 5.96 μM eugenol and 0.74 μM apigenin present in 0.2% w/v OS extract. No inhibition in migration was observed (Fig. 1D) by any of the components in the given concentrations. In separate experiments, higher concentrations of eugenol (6.25 and 25mM), apigenin (9 and 18 μM) and ursolic acid (10 and 25 μM) showed no inhibition in chemotaxis (not shown). However, when they were used in various combinations, a mixture of 4.5μM apigenin and 2.5 μM ursolic acid showed an inhibition of approximately 40% in chemotaxis towards Matrigel. Similarly morphogenesis of the EIII8 cells as 3-dimensional cultures on Matrigel was not suppressed by eugenol, apigenin or ursolic acid at concentrations as high as 625μM, 18μM and 10μM respectively, whereas 0.1 and 0.05% (w/v) extracts inhibited the interconnecting tubular network very effectively. Our results indicate that the total leaf extract of OS is a more potent inhibitor of the MDA-MB-231 cell migration and EIII8 morphogenesis compared to any of the tested components alone or in combination even though they were used at much higher concentrations than those present in the extract. This could be due to presence of a component(s) different from these three, or the combined effect of more than one component. Another reason for the inability of purified components to inhibit migration and morphogenesis could be the inactivating effect of purification procedures on the biological activities. Therefore, we studied the induction of COX-2 in MDA-MB-231 cells. Fig. 4 shows that all components as well as OS extract inhibit the TPA induced COX-2 in MDA-MB-231 cells, indicating that biological activity of the purified extracts is not affected by the purification procedures and secondly, COX-2 may be one of the proteins involved with migration and morphogenesis as reported by (38), but not solely responsible for these processes.
Since chemotaxis, morphogenesis and cell proliferation are crucial steps of angiogenesis and our 3-dimensional co-cultures indicate an inhibition of in vitro angiogenesis by OS extracts, we performed Matrigel plug assay to study the effect of OS extract on angiogenesis. When compared with the untreated plug, the OS containing plugs showed fewer cells per microscopic field and localization of blood vessels only to the periphery of the plug. The treated plugs also showed highly reduced cell proliferation in these sections compared to untreated controls. We hypothesize that the OS extract diffuses from the plug and inhibits the angiogenic and growth factors secreted by tumor and stromal cells. The inhibitory effect is stronger in the Matrigel end (center) of the plug and forms a gradient towards the subcutaneous end (periphery), where an interface is formed between the tumor cells in the plug and the infiltrated mouse stromal cells, restricting the blood vessel formation beyond this point. Also, we demonstrated apoptosis in the mouse stroma cells using TUNEL assay, which could be responsible for the failure of angiogenic response from the host stroma in the OS treated plugs. Using the 3 dimensional co-culture of EIII8 and BAMEC cells on Matrigel, we have shown that lack of angiogenesis may not be due to failure of epithelial endothelial interactions, but because these cells failed to elongate and morphogenize into tube like structures.
Neoangiogenesis, the growth of new capillaries in response to pro-angiogenic cytokines synthesized by tumor cells deprived of oxygen and nutrients plays a crucial role in tumor growth and metastasis (39, 40). We selected the DCIS.com xenograft model for tumorigenic studies because these cells develop into human DCIS like xenograft in 3 weeks with extensive angiogenic response (unpublished data). When OS extracts were fed to nude mice injected with DCIS.com cells, there was a reduction in tumor weight and volume in the treated mice. Substitution of drinking water with OS extract did not affect the welfare of the mice. The extract was not distasteful to the animals, as the water consumption by both the groups was the same. No side effects of toxicity like dry skin, weight loss, lethargy or any other abnormal behavior were observed in the treated mice, indicating that OS extract was well tolerated by mice at this concentration. Immunohistochemical comparison between the water fed and OS fed DCIS.com xenografts indicates absence of neoangiogenesis in OS fed xenografts using anti CD34 antibody (Fig. 6IIA, A′). There was also a redistribution of the angiogenesis marker VEGF from stromal and epithelial compartments to the epithelial compartment alone (Fig. 6II B, B′). VEGF is known to increase vascular permeability and induce endothelial cell proliferation and migration through high affinity binding to its receptor at the endothelial cell surface (41). Presence of VEGF in epithelial cells may not be of consequence to the formation of blood vessels, which were mainly seen in the stromal areas (Fig. 11E,E′). It was shown that functional interference of VEGF-signaling impaired angiogenic switch and the association of VEGF to its receptors was shown only in angiogenic islets and tumors and not in normal or preangiogenic pancreatic islets (42). Failure to form angiogenesis was accompanied with a reduced expression of COX-2 (Fig. 16IIC,C′), which is another protein associated with angiogenesis and shows a significant correlation with VEGF & HER2/neu (43). COX-2 was expressed in the xenografts in the peripheral epithelial cells, but was absent from the stroma. The distribution of COX-2 protein was unchanged in the OS treated xenografts, but overall expression was significantly low (Fig. 6IID,D′). There was also a significant inhibition in PCNA expression indicating a reduced cell proliferation (Fig. 6IIF,F′), which could be responsible for slower tumor growth rate in treated mice. Cell migration is dependent upon local proteolysis of extra-cellular membrane by MMPs (44). MMPs not only facilitate the migration of the endothelial cells through ECM, but also that of tumor cells (41, 42, 45). MMP9 was shown to be a functional component of the angiogenic switch during multistage pancreatic carcinogenesis in RIP1-Tag2 transgenic mice and was reported to increase the availability of VEGF (42). Levels of MMP-9, were drastically reduced in OS treated xenografts (Fig. 11D,D′). To summarize, the xenografts from the OS fed animals showed a reduced level of angiogenesis, which could be due to reduced cell proliferation, migration and invasion in the tumor cells and induction of apoptosis in the stromal cells.
The inhibitory effects of OS could be due to one or multiple components present in the extracts utilizing various pathways. It has been shown that induction of COX-2 leads to conversion of arachidonic acid to postaglandin PG2 (11). It was recently shown that increased secretion of PGE2 from constitutive COX-1 and inducible COX-2 isozymes present in breast epithelium and stromal cell compartments results in both autocrine and paracrine actions to increase aromatase expression in the tissues (25, 46). Eugenol inhibits the induction of COX-2 (10), and apigenin has been shown to inhibit aromatase as well as COX-2 expression (23, 24). Ursolic acid was reported to inhibit NF-kB activity, which controls the expression of COX-2 and MMP-9 (47) along with many more proteins (28, 29). Thus all three recognized components could act synergistically to affect these interrelated pathways, act independently, or act in combination with other components present in the extract. However, our data indicate that none of the tested compounds alone or in combination was as effective as the total extract in inhibiting chemotaxis of MDA-MB-231 cells or 3-dimensional growth and morphogenesis of EIII8 cells. Our results support the earlier assumptions that the total herbal extracts contain a number of chemical constituents that interact in a complex way to elicit their pharmacological response.
In summary, our data demonstrate that aqueous extract of Ocimum leaves inhibits tumor growth and angiogenesis by affecting tumor cell proliferation, migration, morphogenesis, stromal apoptosis and induction of COX-2. These data stress the importance of validating the use of traditional medicinal herbs in combination with the modern medicine in tumor prevention and therapy. More experiments are in progress to understand the molecular targets and pathways affected by this herb.
Acknowledgments
Grant Support: NIH/ National Cancer Institute RO1 CA46120 (A.R.).
The authors would like to thank Dr. Fred Miller and Dr. Dipak Banerjee for the gift of DCIS.com and BAMEC cells respectively, and to Dr. Fred Miller for the critical evaluation of the manuscript.
References
- 1.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–773. [PubMed] [Google Scholar]
- 2.Prakash J, Gupta SK, Singh N, Kochupillai V, Gupta YK. Antiproliferative and chemopreventive activity of Ocimum sanctum Linn. Int J Med Biol Environ. 1999;27:165. [Google Scholar]
- 3.Prakash J, Gupta SK. Chemopreventive activity of Ocimum sanctum seed oil. J Ethnopharmacol. 2000;72:29–34. doi: 10.1016/s0378-8741(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 4.Aruna K, Sivaramakrishnan VM. Anticarcinogenic effects of some Indian plant products. Food Chem Toxicol. 1992;30:953–956. doi: 10.1016/0278-6915(92)90180-s. [DOI] [PubMed] [Google Scholar]
- 5.Prashar R, Kumar A, Banerjee S, Rao AR. Chemopreventive action by an extract from Ocimum sanctum on mouse skin papillomagenesis and its enhancement of skin glutathione S-transferase activity and acid soluble sulfydryl level. Anticancer Drugs. 1994;5:567–572. doi: 10.1097/00001813-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Karthikeyan K, Ravichandran P, Govindasamy S. Chemopreventive effect of Ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–119. doi: 10.1016/s1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Prashar R, Kumar A, Rao AR. Modulatory influence of alcoholic extract of Ocimum leaves on carcinogen-metabolizing enzyme activities and reduced glutathione levels in mouse. Nutr Cancer. 1996;25:205–217. doi: 10.1080/01635589609514443. [DOI] [PubMed] [Google Scholar]
- 8.Shah CSaQ, JS . A Textbook of Pharmacognosy. Ahmedabad: B.S.Shah Prakashan; 1988. Volatile oils. [Google Scholar]
- 9.Rompelberg CJ, Evertz SJ, Bruijntjes-Rozier GC, van den Heuvel PD, Verhagen H. Effect of eugenol on the genotoxicity of established mutagens in the liver. Food Chem Toxicol. 1996;34:33–42. doi: 10.1016/0278-6915(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, Nam Han Y, Lee SK. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 11.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, Dannenberg AJ. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 13.Tan KB, Yong WP, Putti TC. Cyclooxygenase-2 expression: a potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology. 2004;44:24–28. doi: 10.1111/j.1365-2559.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- 14.Gately S, Kerbel R. Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res. 2003;37:179–192. doi: 10.1159/000071373. [DOI] [PubMed] [Google Scholar]
- 15.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 16.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 18.Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000;3:447–451. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 21.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–420. [PubMed] [Google Scholar]
- 22.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wahala K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 23.Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004;82:70–79. doi: 10.1093/toxsci/kfh257. [DOI] [PubMed] [Google Scholar]
- 24.Jeong HJ, Shin YG, Kim IH, Pezzuto JM. Inhibition of aromatase activity by flavonoids. Arch Pharm Res. 1999;22:309–312. doi: 10.1007/BF02976369. [DOI] [PubMed] [Google Scholar]
- 25.Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002;80:203–212. doi: 10.1016/s0960-0760(01)00187-x. [DOI] [PubMed] [Google Scholar]
- 26.Fentiman IS. Aromatase inhibitors and breast cancer: time for a change? Int J Clin Pract. 2004;58:1152–1158. doi: 10.1111/j.1742-1241.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 28.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 29.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 30.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 31.Shekhar MP, Nangia-Makker P, Wolman SR, Tait L, Heppner GH, Visscher DW. Direct action of estrogen on sequence of progression of human preneoplastic breast disease. Am J Pathol. 1998;152:1129–1132. [PMC free article] [PubMed] [Google Scholar]
- 32.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92:1185–1186. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee DK, Ornberg RL, Youdim MB, Heldman E, Pollard HB. Endothelial cells from bovine adrenal medulla develop capillary-like growth patterns in culture. Proc Natl Acad Sci U S A. 1985;82:4702–4706. doi: 10.1073/pnas.82.14.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 36.Shekhar MP, Werdell J, Tait L. Interaction with endothelial cells is a prerequisite for branching ductal-alveolar morphogenesis and hyperplasia of preneoplastic human breast epithelial cells: regulation by estrogen. Cancer Res. 2000;60:439–449. [PubMed] [Google Scholar]
- 37.Harmand PO, Duval R, Delage C, Simon A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int J Cancer. 2005;114:1–11. doi: 10.1002/ijc.20588. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005;90:2563–2570. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 39.Harvey K, Siddiqui RA, Sliva D, Garcia JG, English D. Serum factors involved in human microvascular endothelial cell morphogenesis. J Lab Clin Med. 2002;140:188–198. doi: 10.1067/mlc.2002.126827. [DOI] [PubMed] [Google Scholar]
- 40.Harvey K, Welch Z, Kovala AT, Garcia JG, English D. Comparative analysis of in vitro angiogenic activities of endothelial cells of heterogeneous origin. Microvasc Res. 2002;63:316–326. doi: 10.1006/mvre.2002.2406. [DOI] [PubMed] [Google Scholar]
- 41.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 42.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A, Tonini G, Rabitti C. COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology. 2005;46:561–568. doi: 10.1111/j.1365-2559.2005.02132.x. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 45.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 46.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, Hla T, Hudis C, Dannenberg AJ. HER-2/neu Status Is a Determinant of Mammary Aromatase Activity In vivo: Evidence for a Cyclooxygenase-2-Dependent Mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 47.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]